Abstract
This study describes the risk of delayed chronic kidney disease (CKD) in 1190 adult hematopoietic cell transplantation (HCT) survivors who underwent HCT for hematologic malignancies or aplastic anemia between 1976 and 1997, and survived at least one year. CKD was defined as a sustained elevation of serum creatinine inferring a glomerular filtration rate of <60 mL/min/1.73 m2 for three months or longer. Median age at HCT was 35.0 years (range, 18.1 to 68.6 years) and median length of follow-up, 7.1 years from HCT (range, 1 to 24.3 years). Sixty patients with CKD were identified, resulting in a cumulative incidence of 4.4% at 5 years (autologous HCT: 3.8%; matched sibling HCT: 4.5%; unrelated donor HCT: 10.0%; p=0.09 compared to autologous HCT). Older age at HCT (relative risk [RR] per five-year increment, 1.33; 95% CI, 1.2-1.5); exposure to cyclosporine without tacrolimus (RR=1.90, 95% CI, 1.1 to 3.4) or with tacrolimus (RR=4.59, 95% CI, 1.8 to 11.5); and a primary diagnosis of multiple myeloma (RR=2.51, 95% CI, 1.1 to 5.6) were associated with an increased risk of delayed CKD. This study identifies sub-populations of HCT at increased risk for CKD, setting the stage for appropriate long-term follow-up of vulnerable patients.
INTRODUCTION
Hematopoietic cell transplantation (HCT) is an established effective therapeutic modality for a variety of malignant and nonmalignant disorders. The number of patients undergoing this procedure has increased steadily over the last decade.1 Improved transplantation techniques, and supportive care strategies have resulted in a growing population of long-term survivors, who may potentially be at risk for treatment-related complications adversely affecting their long-term survival as well as their health and well-being. One such late complication of HCT is chronic kidney disease (CKD), which may lead to progressive loss of renal function and could potentially terminate in end-stage renal disease.2 Previously reported risk factors for developing renal insufficiency within the first year after HCT include exposure to a variety of nephrotoxic agents, such as pre-HCT conditioning with high-dose chemotherapy and total body irradiation (TBI);3-9 post-HCT use of aminoglycoside antibiotics and antifungal agents for management of sepsis;9-11 exposure to calcineurin inhibitors for graft versus host disease (GvHD) prophylaxis and treatment;9, 12-20 and the development of chronic GvHD21. Although delayed CKD after HCT (defined as CKD developing one of more year after HCT) was recognized 15 years ago, inconsistent reporting of magnitude of risk and associated risk factors stem from widely differing definitions used for describing CKD22, varying periods of post-HCT follow-up of the cohort at risk4, 6, 21, 23, and limited sample sizes3, 8, 14, 17, 18, 20, 21.
Using a standardized definition of CKD24, we followed 1190 patients who underwent HCT at age 18 years or older at the City of Hope National Medical Center (COH) and survived at least one year, in an attempt to gain a better understanding of the incidence and risk factors associated with the development of delayed CKD in long-term survivors of HCT.
PATIENTS AND METHODS
A retrospective cohort study design was used. Eligible participants had undergone autologous or allogeneic HCT at COH between 1976 and 1997 for a hematologic malignancy or severe aplastic anemia, were at least 18 years or older at the time of transplantation, survived at least one year post-HCT, and had not developed CKD during the first year after HCT. The COH Long-term Follow-up program actively follows patients who have undergone HCT at COH and survived at least one year. A Long-term Follow-up (LTFU) data collection form is completed for all patients meeting eligibility criteria. The form captures information beginning one year post-transplantation through the date of last contact. Medical records maintained at COH are the primary source of data for completion of the LTFU form. If the date of last hospital/clinic visit recorded in the medical records is not recent (exceeds 18 months from the date of data abstraction), or if there are any unexpected gaps in the patients’ history within the time window of interest (exceeding a period of 18 months), a standard protocol is used to identify and contact physicians taking care of patients outside COH to obtain the pertinent information. If the physician is not available or unable to provide recent information, the patient is called directly. The Human Subjects Committee at City of Hope approved the protocol. Informed consent was provided according to the Declaration of Helsinki.
Information collected on the LTFU form included demographics, disease status, medication, hospitalization, vaccination history, and post-HCT complications including new malignancies, cardiopulmonary dysfunction, renal compromise, neurological toxicity, avascular necrosis, gastrointestinal complications, cataracts and details regarding GvHD. Data from the LTFU form was merged with data from an institutional database on HCT containing information on conditioning for HCT and GvHD prophylaxis/treatment.
Using this combined data set, we identified cases of renal insufficiency diagnosed by a health care provider. Medical records were reviewed again in order to confirm the presence of CKD using the definition detailed below. CKD was defined as a sustained decrease in glomerular filtration rate (GFR) to levels less than 60 mL/min/1.73 m2 for a period of three months or longer.24 Direct measurement of GFR is complex, expensive, and difficult to do in routine clinical practice, and therefore was not logistically possible in this retrospective study. Thus, the endogenous filtration marker creatinine was used to assess GFR. We used the Modification of Diet in Renal Disease (MDRD) Study equation endorsed by the National Kidney Foundation24 to calculate GFR based on serum creatinine value. The equation is shown below, where Pcr = serum creatinine value in mg/dl:
GFR = 186 × (Pcr) -1.154 × (age) -0.203 (× 0.742 if female) (× 1.210 if African American)25
Using this definition, cumulative incidence of delayed CKD after HCT was calculated taking into consideration the competing risk from death, as described by Gray.26 The time at risk was computed from one year post-HCT to the date of onset of CKD, the date of last contact, or the date of death, whichever came first. Cox proportional hazards regression techniques were used to calculate relative risk (RR) estimates and their 95% confidence intervals (CIs).27 Univariate analyses for all pertinent variables were first performed to estimate relative risk individually. Stepwise regression was employed to select important variables from those that approached statistical significance in the univariate analysis, and a p value of less than 0.10 was used as the selection criterion.
Variables examined in the regression model included age at transplantation, sex, primary diagnosis, type of transplantation (autologous, allogeneic [related and unrelated]), chemotherapeutic agents and radiation used as part of conditioning, presence or history of chronic GvHD, and exposure to immunosuppressive agents used for GvHD prophylaxis and/or treatment. Age at HCT was analyzed both as a continuous variable and as a categorical variable (less than or equal to 45 years vs. more than 45 years of age). Six major drugs were used for GvHD prophylaxis/treatment in the study cohort: methotrexate, systemic steroids (Prednisone), cyclosporine, tacrolimus (FK506), thalidomide, and mycophenolate Mofetil (MMF). Based on prior knowledge of the nephrotoxic potential of these immunosuppressive agents,28 we combined these agents into the following composite variables: use of methotrexate alone for prophylaxis and no agents for GvHD treatment; exposure to cyclosporine with or without other agents listed above, except Tacrolimus; and exposure to cyclosporine and tacrolimus, along with the other agents listed above. All tests of statistical significance were 2-sided, and p-value less or equal to 0.05 was considered statistically significant. Data were analyzed using SAS version 9.1 (SAS Institute, Cary, NC).
RESULTS
Table 1 describes the characteristics of the patient population. Of the 1,190 patients in the cohort, 700 (59%) were males. The median age at transplantation was 35.0 years (range, 18.1 to 68.6 years). The follow-up period for this study extended to May 31, 2005. As of May 2005, 67% of this cohort was alive at last contact, and the median length of follow-up for these patients was 7.1 years from HCT (range, 1 to 24.3 years). For those alive and without CKD, the median time from date of last contact to 5/31/2005 was 3.3 years (range 0.02-17 years). Overall, 75% of the cohort had been followed up within 5 years from 5/31/2005.
Table 1.
Characteristics of the Patient Population
| Characteristic | Total (n=1,190) | Patients with CKD (n=60) | ||
|---|---|---|---|---|
| No. of patients | % | No. of patients | % | |
| Year of HCT | ||||
| 1976-1990 | 347 | 29 | 10 | 17 |
| 1991-1997 | 843 | 71 | 50 | 83 |
| Age at HCT | ||||
| ≤ 45 years | 916 | 77 | 36 | 60 |
| > 45 years | 274 | 23 | 24 | 40 |
| Sex | ||||
| Male | 700 | 59 | 31 | 52 |
| Female | 490 | 41 | 29 | 48 |
| Race | ||||
| Non-Hispanic White | 766 | 64 | 38 | 63 |
| Black | 28 | 2 | 4 | 7 |
| Hispanic White | 296 | 25 | 12 | 20 |
| Asian | 74 | 6 | 6 | 10 |
| Others | 26 | 2 | 0 | 0 |
| Primary diagnosis | ||||
| Non-Hodgkin’s lymphoma | 284 | 24 | 15 | 25 |
| Hodgkin’s disease | 161 | 14 | 3 | 5 |
| Acute myeloid leukemia | 294 | 25 | 16 | 27 |
| Chronic myeloid leukemia | 213 | 18 | 14 | 23 |
| Acute lymphoblastic leukemia | 131 | 11 | 3 | 5 |
| Multiple myeloma | 59 | 5 | 8 | 13 |
| Aplastic anemia | 38 | 3 | 0 | 0 |
| Other | 10 | 1 | 1 | 2 |
| Type of HCT | ||||
| Allogeneic | 628 | 53 | 37 | 62 |
| Related | 561 | 47 | 31 | 52 |
| Unrelated | 67 | 6 | 6 | 10 |
| Autologous | 562 | 47 | 23 | 38 |
| Agents used for conditioning† | ||||
| Cyclophosphamide | 879 | 74 | 42 | 70 |
| Etoposide | 856 | 72 | 37 | 62 |
| Total body irradiation | 899 | 76 | 46 | 77 |
| BCNU | 138 | 12 | 4 | 7 |
| Busulfan | 113 | 10 | 7 | 12 |
| Melphalan | 37 | 3 | 3 | 5 |
| GvHD prophylaxis/ treatment§ | ||||
| No agents/ Methotrexate alone | 637 | 54 | 27 | 45 |
| Cyclosporine without Tacrolimus ζ | 500 | 42 | 27 | 45 |
| Cyclosporine and Tacrolimus ζ | 48 | 4 | 6 | 10 |
| Status at last contact | ||||
| Alive | 789 | 66 | 40 | 67 |
| Dead | 401 | 34 | 20 | 33 |
| Presence of chronic graft vs. host disease* | ||||
| No | 794 | 67 | 34 | 57 |
| Yes | 386 | 33 | 26 | 43 |
4 subjects with missing chemotherapeutic agent information
5 subjects with missing GvHD drug information
10 subjects with unknown chronic GVHD status
Cyclosporine without tacrolimus denotes exposure to cyclosporine with or without Prednisone, Thalidomide, Methotrexate, Mycophenolate Mofetil, but with no exposure to Tacrolimus; Cyclosporine with Tacrolimus, denotes exposure to Cyclosporine and Tacrolimus, with or without Prednisone, Thalidomide, Methotrexate, Mycophenolate Mofetil (MMF), (note: 2 patients in this category received Tacrolimus, without Cyclosporine).
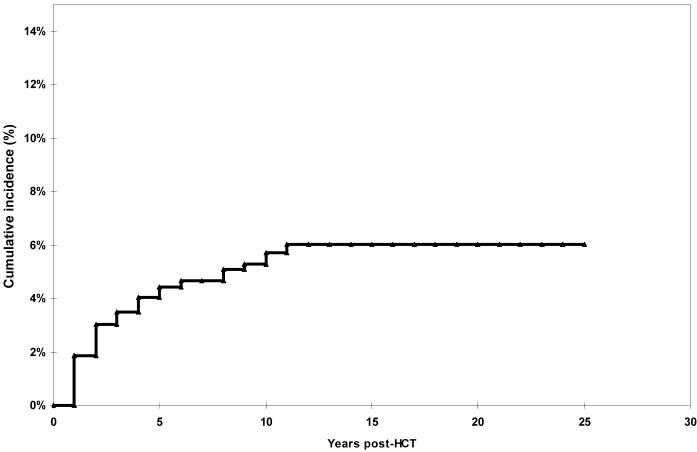
Allogeneic HCT from HLA-matched or partially matched family member donors was performed in 561 patients (47%); 67 (6%) received grafts from unrelated donors matched for HLA-phenotype, and 562 (47%) patients underwent autologous HCT. The major indications for transplant were non-Hodgkin lymphoma (NHL: 24%), Hodgkin disease (HD: 14%), Acute Myeloid Leukemia (AML: 25%), Chronic Myeloid Leukemia (CML: 18%), Acute Lymphoblastic Leukemia (ALL: 11%), Multiple Myeloma (MM: 5%) and severe aplastic anemia (SAA: 3%). The main chemotherapeutic agents used for conditioning were cyclophosphamide (74%), etoposide (72%), TBI (76%), carmustine (12%), busulfan (10%), and melphalan (3%). Multiple drugs were used for GvHD prophylaxis or treatment, including cyclosporine A, methotrexate (prophylaxis), systemic steroids, thalidomide, tacrolimus, and mycophenolate mofetil (primarily treatment). Sixty patients (5% of the entire cohort) developed CKD. These included 31 patients (5.5%) with HCT from related family members; six (9.0%) from unrelated donors, and 23 (4.1%) autologous HCT survivors. As shown in Figure 1, the cumulative incidence of CKD was 4.4% [95% confidence interval (CI), 3.2% to 5.6%] at five years post-HCT and 5.7% (95% CI, 4.2% to 7.2%) at 10 years. Twenty-two events (37%) occurred within two years post-HCT. The large majority of events occurred within the first 10 years, with a plateau after that. Compared to autologous HCT recipients (cumulative incidence 3.8% at five years post transplant), the cumulative incidence was comparable for matched sibling HCT recipients (4.5% at five years, p>0.5), and was borderline significantly higher for patients who had undergone a matched unrelated donor HCT (10.0%) (p=0.09 compared to autologous HCT recipients). Patients with MM can be at an increased risk of CKD due to infiltration of the kidneys with primary disease. In order to examine the risk of CKD due to transplant-related exposures, we repeated the above analyses after excluding patients with MM. The cumulative incidence of delayed CKD by donor source was as follows: autologous HCT recipients: 3.5% at 5 years post-HCT; matched sibling HCT recipients: 4.2% at 5 years (p=0.54 compared to autologous HCT recipients); matched unrelated donor HCT recipients: 10.0% at 5 years (p=0.05 compared to autologous HCT recipients).
Figure 1.
Cumulative Incidence of Chronic Kidney Disease in 1+ year Survivors of HCT
Table 2 presents the results of the univariate analyses of the associations between delayed CKD and demographic factors, chemotherapeutic agents used for conditioning, and drugs used for prophylaxis and/or treatment of GvHD. Stepwise regression (Table 3) revealed older age at HCT (RR per increase of five years, 1.33; 95% CI, 1.17 to 1.51), and prophylaxis/treatment of GvHD using cyclosporine without tacrolimus (RR=1.90; 95% CI 1.07 to 3.41), or with tacrolimus (RR=4.59; 95% CI, 1.84 to 11.46), to be significantly associated with increased risk of CKD. Primary diagnosis of MM (RR=2.51, 95% CI, 1.12 to 5.60) was also associated with an increased risk of CKD. Of note, exposure to TBI did not increase the risk of CKD (RR=0.92; 95% CI, 0.51 to 1.68).
Table 2.
Univariate Analysis of Risk Factors associated with Chronic Kidney Disease after HCT
| Risk Factors | Entire Cohort (n=1190) | |
|---|---|---|
| Number with/without CKD | RR (95% CI) | |
| Year of HCT | ||
| 1976-1990 | 10/337 | 1.00 |
| 1991-1997 | 50/793 | 2.83 (1.39-5.78) |
| Age at HCT | ||
| ≤ 45 | 36/880 | 1.00 |
| > 45 | 24/250 | 2.67(1.59-4.50) |
| Sex | ||
| Male | 31/669 | 1.00 |
| Female | 29/461 | 1.30 (0.78-2.16) |
| Race | ||
| Non-Hispanic white | 38/728 | 1.00 |
| Hispanic | 22/402 | 0.99(0.59-1.67) |
| Primary diagnosis | ||
| Other than myeloma | 52/1079 | 1.00 |
| Multiple Myeloma | 8/51 | 3.68 (1.74-7.79) |
| Cyclophosphamide | ||
| No | 18/289 | 1.00 |
| Yes | 42/837 | 0.86 (0.50-1.50) |
| Etoposide | ||
| No | 23/307 | 1.00 |
| Yes | 37/819 | 0.65 (0.39-1.09) |
| Total Body Irradiation | ||
| No | 14/273 | 1.00 |
| Yes | 46/853 | 0.92 (0.51-1.68) |
| Carmustine | ||
| No | 56/992 | 1.00 |
| Yes | 4/134 | 0.61 (0.22-1.68) |
| Busulfan | ||
| No | 53/1020 | 1.00 |
| Yes | 7/106 | 1.44 (0.66-3.18) |
| Melphalan | ||
| No | 57/1092 | 1.00 |
| Yes | 3/34 | 2.34 (0.73-7.51) |
| Drugs used for GvHD prophylaxis/ treatment | ||
| Cyclosporine, no tacrolimusζ | 27/473 | 1.22 (0.72-2.08) |
| Cyclosporine and tacrolimusζ | 6/42 | 2.77 (1.14-6.70) |
| Source of hematopoietic cells | ||
| Autologous | 23/539 | 1.00 |
| Allogeneic, related | 31/530 | 1.16 (0.72-2.09) |
| Matched, unrelated | 6/61 | 2.70 (1.21-6.01) |
| Chronic GvHD | ||
| No | 34/760 | 1.00 |
| Yes* | 26/360 | 1.57 (0.94-2.62) |
| Limited | 11/171 | 1.30 (0.66-2.56) |
| Extensive | 13/167 | 1.87 (0.99-3.54) |
| Fungal infections | ||
| No | 53/1014 | 1.00 |
| Yes | 7/116 | 1.30 (0.59-2.86) |
10 subjects with unknown chronic GvHD status were excluded
Cyclosporine without Tacrolimus denotes exposure to Cyclosporine with or without systemic steroids, Thalidomide, Methotrexate, Mycophenolate Mofetil, but with no exposure to Tacrolimus; Cyclosporine with Tacrolimus, denotes exposure to Cyclosporine and Tacrolimus, with or without systemic steroids, Thalidomide, Methotrexate, Mycophenolate Mofetil (note: 2 patients in this category received Tacrolimus, without Cyclosporine).
Table 3.
Multivariate analysis of risk factors associated with chronic kidney disease after HCT
| Risk Factors | Overall |
|---|---|
| Relative risk (95% Confidence interval) | |
| Age at HCT | |
| Increments of 5 years | 1.33 (1.17-1.51) |
| Drug combinations for prophylaxis/treatment of GvHD | |
| None/methotrexate alone | 1.00 |
| Cyclosporine without tacrolimusrζ | 1.90 (1.07-3.41) |
| Cyclosporine with tacrolimusζ | 4.59 (1.84-11.46) |
| Primary diagnosis | |
| Primary diagnosis other than myeloma | 1.00 |
| Multiple myeloma | 2.51 (1.12-5.60) |
Cyclosporine without Tacrolimus denotes exposure to Cyclosporine with or without Prednisone, Thalidomide, Methotrexate, Mycophenolate Mofetil (MMF), but with no exposure to Tacrolimus; Cyclosporine with Tacrolimus, denotes exposure to Cyclosporine and Tacrolimus, with or without Prednisone, Thalidomide, Methotrexate, Mycophenolate Mofetil (MMF) (note: 2 patients in this category received Tacrolimus, without Cyclosporine).
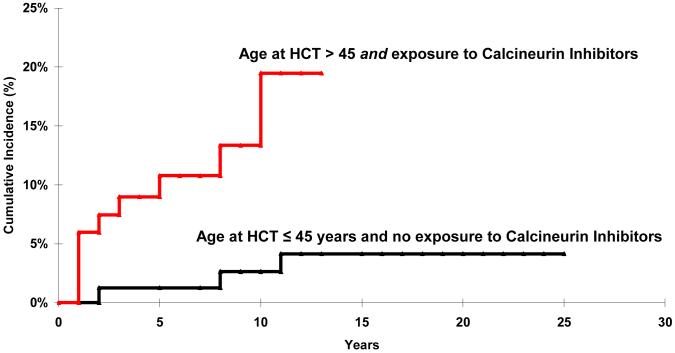
Restricting the analysis to allogeneic HCT recipients, the cumulative incidence of CKD among those who were younger than 45 years of age at HCT and not exposed to calcineurin inhibitors for GvHD prophylaxis/treatment was substantially lower than the cumulative incidence of delayed CKD for those who were older than 45 years of age at HCT and had been exposed to calcineurin inhibitors (2.7% vs. 19.5% at 10 years post-HCT, p<0.01) (Figure 2).
Figure 2.
Cumulative Incidence of Chronic Kidney Disease by age at HCT and by exposure to calcineurin inhibitors.
Comparison with General Population Published Results
According to National Health Interview data published in 2004, 29 the prevalence of CKD (defined by asking “Have you been told in the past 12 months by a doctor or other health care professional that you have weak or failing kidneys [excluding kidney stones, bladder infections, or incontinence]?”), was lower in the general population (0.9% at ages between 18-44; 1.8% at ages between 45-64; 3.4% at ages between 65-74) than in our HCT survivors (the comparable figures were 3.5%, 9.5% and 15.0% respectively, p<0.01).
Outcome after CKD
A detailed review of the 60 patients with CKD was conducted in terms of the need for dialysis. Four patients had gaps in data in the medical records that precluded a detailed evaluation of this outcome. Of the 56 patients that could be evaluated in detail, a total of 4 needed dialysis. Twenty (33.3%) of the 60 patients with CKD have died. The median survival after CKD was 121 months (range, 0 to 159 months). Cause of death included primary disease (9), secondary malignancy (3), cGvHD (1), organ failure (1 liver and 1 respiratory), restrictive lung disease (2), infection (2) and unknown cause (1).
DISCUSSION
Renal dysfunction has been recognized as a complication of HCT for the past 15 years, however, the initial studies were in the form of case reports and case series that were unable to assess magnitude of risk or identify risk factors.3, 6, 8, 30-35 The analytical studies 4, 5, 7, 9-11, 13, 17, 18, 36-43 have been limited by small sample size and a relatively short length of follow-up after HCT44, thus limiting their ability to describe the magnitude of risk with precision and to assess the contribution of various nephrotoxic exposures as well as host and demographic factors in the development of delayed CKD. The current study overcomes these limitations by evaluating the risk of delayed CKD in a large cohort of HCT survivors followed for a median of 7 years, and describes subpopulations at increased risk.
Among the 1190 adult patients who received HCT for hematologic malignancies or severe aplastic anemia at the City of Hope National Medical Center between 1976 and 1997 and survived one year post-HCT, the estimated cumulative incidence of CKD was 4.4% at 5 years, and increased to 5.7% at 10 years after transplantation. Autologous and allogeneic matched sibling HCT recipients were at a comparable risk of developing CKD, with a 5-year cumulative incidence of 3.8% and 4.5%, respectively; unrelated donor HCT recipients, on the other hand, were at a higher risk of developing delayed CKD (10.0%) when compared to autologous HCT recipients. The large majority of events occurred within the first 10 years after HCT, with a plateau thereafter. When compared to published age-matched general population rates, the prevalence of CKD was significantly higher in HCT survivors.
It has been reported that among long-term HCT survivors, about 5-20% will develop CKD.45 The variability in the magnitude of risk of CKD may be partially attributed to the fact that no standard definition for chronic renal dysfunction has been utilized in the previous reports.22 Accurate estimation of kidney function is central to the detection, evaluation, and treatment of CKD, and GFR is widely accepted as the best overall measure of kidney function.46 The National Kidney Foundation Kidney Disease Outcomes Quality Initiative defines CKD as a GFR of less than 60 ml/min/1.73 m2 for three or more months, with or without kidney damage.24 Our study used the Modification of Diet in Renal Disease (MDRD) Study equation endorsed by the National Kidney Foundation24 to calculate GFR based on serum creatinine while taking into consideration of age, sex and race. The MDRD equation has now been recommended for routine clinical use by the National Kidney Foundation, American Society of Nephrology, and the National Kidney Disease Education Program of the National Institute of Health.
Renal dysfunction after HCT can be caused by a number of nephrotoxic agents used in the early phases of disease (chemotherapy prior to transplantation), during conditioning (high dose chemotherapy and TBI), and subsequently in the early post-transplantation period (sepsis and its treatment with antifungal agents, and antibiotics; immunosuppressive agents used to prevent and treat GVHD; and the development of chronic GvHD itself). Previous studies have reported presence or history of GvHD, and exposure to cyclosporine A, tacrolimus, ifosfamide, and TBI to be associated with an increased risk of CKD.3-21 The results of the current multivariate analyses indicated that older age at HCT increases the risk of delayed CKD, and are consistent with two recent studies.4, 37 This could possibly be explained by a higher prevalence of co-morbid conditions that increase the vulnerability of the kidney, or by the fact that renal function decreases with age.24 The current study also finds patients with MM treated with HCT to be at a 2.5-fold increased risk of developing CKD. Renal impairment is a common complication of MM, with a multifactorial pathogenesis.47 Nephrotoxic manifestations of monoclonal immunoglobulin overexpression include the ‘myeloma kidney’, light chain deposition disease, amyloidosis, plasma cell infiltration and glomerulonephritis. Other factors, such as hypercalcemia, hyperuricemia, infection, hyperviscosity and the use of nephrotoxic drugs can precipitate or exacerbate acute and chronic renal failure in patients with MM.
The association between CKD and TBI has been recognized for over a decade22 and has been confirmed by a study that showed a direct dose-response relation, where patients conditioned with 13.5, 12 or 10 Gy, had an estimated probability of CKD at 18 months post transplant of 45%, 26% and 5%, respectively.21 On the other hand, other studies have failed to show an association between TBI and CKD.39, 42 44 The large majority of the our cohort treated with TBI, received 1320 cGy of fractionated TBI for allogeneic HCT and 1200 cGy for autologous HCT. It should also be noted that radiation-related nephropathy has been reported to occur relatively early after HCT - usually between 8-12 months post-HCT 48 - a timeframe we did not investigate in this study. Therefore, the delayed CKD developing after one year of transplant in our cohort probably represented non-radiation related insult to the kidney.
Antifungal agents and aminoglycoside antibiotics are known nephrotoxins but they are tubular rather than glomerular toxins. We did not demonstrate any association between fungal infections (used as a surrogate for exposure to potentially nephrotoxic agents) and CKD. Again, use of antifungals and aminoglycosides decreases after the first year post-HCT, perhaps in part explaining the lack of association in the current study.
Nephrotoxicity has been one of the most serious complications of calcineurin inhibitors.49 Cyclosporine and tacrolimus have been reported to be associated with renal impairment in HCT patients.12-16, 19, 50, 51 Cyclosporine causes glomerular thrombosis and tubular injury in the early months after HCT20 that is reversible upon dose modification or withdrawal of the drug. A longer term syndrome of cyclosporine toxicity involves both arteriolar and tubular lesions, accompanied by interstitial fibrosis.52, 53 It is known that the use of tacrolimus can potentially cause chronic nephrotoxicity as it causes structural lesions in the kidney, and has also been previously reported as a risk factor for kidney disease post-HCT.54 In the current study, exposure to calcineurin inhibitors (cyclosporine and tacrolimus) was associated with a significantly increased risk of delayed CKD after adjustment for age at transplant and primary diagnosis. Furthermore, the effect appeared to be additive, as demonstrated by the increase in the magnitude of risk by the addition of tacrolimus to a cyclosporine exposed setting. We were limited in our ability to collect details regarding the dose or duration of exposure to immunosuppressive agents used for prophylaxis/treatment of GvHD, and could not therefore explore a detailed dose-response association.
Chronic GvHD has been reported to play a role in the development of CKD after HCT.21 We did not demonstrate an independent association between chronic GvHD and CKD, probably because the strong correlation between the use of nephrotoxic drugs to prevent and/ or treat chronic GvHD and the presence of chronic GvHD would make it impossible to identify the role of chronic GvHD independent of the use of these agents in the development of CKD.
Using rigorous standardized definitions for identifying patients with chronic kidney disease, this study describes the magnitude of risk of CKD in a large cohort of long-term survivors of autologous and allogeneic HCT. It demonstrates that the risk of CKD is elevated for the first ten years after HCT, and plateaus after that. Furthermore, this study identifies high risk groups in this population, in particular, older patients; patients with a diagnosis of MM; and those exposed to calcineurin inhibitors. Findings from this study could guide clinicians in monitoring high-risk subpopulations, as well as help establish guidelines for appropriate follow-up for these survivors.
Acknowledgments
Supported in part by NIH R01 CA078938 (S.B.), P01 CA30206 (S.J.F.), and Lymphoma/Leukemia Society Scholar Award for Clinical Research 2191-02 (S.B.)
References
- 1.Pasquini M. Part I - CIBMTR Summary Slides, 2005. CIBMTR newsletter. 2006;12:5–8. [Google Scholar]
- 2.McClellan WM. Epidemiology and risk factors for chronic kidney disease. Med. Clin. North Am. 2005;89:419–445. doi: 10.1016/j.mcna.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 3.Chappell ME, Keeling DM, Prentice HG, Sweny P. Haemolytic uraemic syndrome after bone marrow transplantation: an adverse effect of total body irradiation? Bone Marrow Transplant. 1988;3:339–347. [PubMed] [Google Scholar]
- 4.Delgado J, Cooper N, Thomson K, et al. The importance of age, fludarabine, and total body irradiation in the incidence and severity of chronic renal failure after allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2006;12:75–83. doi: 10.1016/j.bbmt.2005.08.036. [DOI] [PubMed] [Google Scholar]
- 5.Frisk P, Bratteby LE, Carlson K, Lonnerholm G. Renal function after autologous bone marrow transplantation in children: a long-term prospective study. Bone Marrow Transplant. 2002;29:129–136. doi: 10.1038/sj.bmt.1703312. [DOI] [PubMed] [Google Scholar]
- 6.Lawton CA, Cohen EP, Barber-Derus SW, et al. Late renal dysfunction in adult survivors of bone marrow transplantation. Cancer. 1991;67:2795–2800. doi: 10.1002/1097-0142(19910601)67:11<2795::aid-cncr2820671114>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 7.Leblond V, Sutton L, Jacquiaud C, et al. Evaluation of renal function in 60 long-term survivors of bone marrow transplantation. J. Am. Soc. Nephrol. 1995;6:1661–1665. doi: 10.1681/ASN.V661661. [DOI] [PubMed] [Google Scholar]
- 8.Tarbell NJ, Guinan EC, Chin L, Mauch P, Weinstein HJ. Renal insufficiency after total body irradiation for pediatric bone marrow transplantation. Radiother. Oncol. 1990;18(Suppl 1):139–142. doi: 10.1016/0167-8140(90)90195-3. [DOI] [PubMed] [Google Scholar]
- 9.Van Why SK, Friedman AL, Wei LJ, Hong R. Renal insufficiency after bone marrow transplantation in children. Bone Marrow Transplant. 1991;7:383–388. [PubMed] [Google Scholar]
- 10.Gubbins PO, Penzak SR, Polston S, McConnell SA, Anaissie E. Characterizing and predicting amphotericin B-associated nephrotoxicity in bone marrow or peripheral blood stem cell transplant recipients. Pharmacotherapy. 2002;22:961–971. doi: 10.1592/phco.22.12.961.33607. [DOI] [PubMed] [Google Scholar]
- 11.Miralbell R, Sancho G, Bieri S, et al. Renal insufficiency in patients with hematologic malignancies undergoing total body irradiation and bone marrow transplantation: a prospective assessment. Int. J. Radiat. Oncol. Biol. Phys. 2004;58:809–816. doi: 10.1016/j.ijrobp.2003.06.001. [DOI] [PubMed] [Google Scholar]
- 12.Bacigalupo A, Di Giorgio F, Frassoni F, et al. Cyclosporin A serum and blood levels in marrow graft recipients: correlation with administered dose, serum creatinine and graft-versus-host disease. Acta Haematologica. 1984;72:155–162. doi: 10.1159/000206381. [DOI] [PubMed] [Google Scholar]
- 13.Caliskan Y, Besisik SK, Sargin D, Ecder T. Early renal injury after myeloablative allogeneic and autologous hematopoietic cell transplantation. Bone Marrow Transplant. 2006;38:141–147. doi: 10.1038/sj.bmt.1705412. [DOI] [PubMed] [Google Scholar]
- 14.Devine SM, Geller RB, Lin LB, et al. The outcome of unrelated donor bone marrow transplantation in patients with hematologic malignancies using tacrolimus (FK506) and low dose methotrexate for graft-versus-host disease prophylaxis. Biol Blood Marrow Transplant. 1997;3:25–33. [PubMed] [Google Scholar]
- 15.Dieterle A, Gratwohl A, Nizze H, et al. Chronic cyclosporine-associated nephrotoxicity in bone marrow transplant patients. Transplantation. 1990;49:1093–1100. doi: 10.1097/00007890-199006000-00013. [DOI] [PubMed] [Google Scholar]
- 16.Furlong T, Storb R, Anasetti C, et al. Clinical outcome after conversion to FK 506 (tacrolimus) therapy for acute graft-versus-host disease resistant to cyclosporine or for cyclosporine-associated toxicities. Bone Marrow Transplant. 2000;26:985–991. doi: 10.1038/sj.bmt.1702639. [DOI] [PubMed] [Google Scholar]
- 17.Hows JM, Chipping PM, Fairhead S, Smith J, Baughan A, Gordon-Smith EC. Nephrotoxicity in bone marrow transplant recipients treated with cyclosporin A. Br. J. Haematol. 1983;54:69–78. doi: 10.1111/j.1365-2141.1983.tb02068.x. [DOI] [PubMed] [Google Scholar]
- 18.Irle C, Deeg HJ, Buckner CD, et al. Marrow transplantation for leukemia following fractionated total body irradiation. A comparative trial of methotrexate and cyclosporine. Leuk. Res. 1985;9:1255–1261. doi: 10.1016/0145-2126(85)90153-5. [DOI] [PubMed] [Google Scholar]
- 19.Nizze H, Mihatsch MJ, Zollinger HU, et al. Cyclosporine-associated nephropathy in patients with heart and bone marrow transplants. Clinical Nephrology. 1988;30:248–260. [PubMed] [Google Scholar]
- 20.Shulman H, Striker G, Deeg HJ, Kennedy M, Storb R, Thomas ED. Nephrotoxicity of cyclosporin A after allogeneic marrow transplantation: glomerular thromboses and tubular injury. N. Engl. J. Med. 1981;305:1392–1395. doi: 10.1056/NEJM198112033052306. [DOI] [PubMed] [Google Scholar]
- 21.Miralbell R, Bieri S, Mermillod B, et al. Renal toxicity after allogeneic bone marrow transplantation: the combined effects of total-body irradiation and graft-versus-host disease. J. Clin. Oncol. 1996;14:579–585. doi: 10.1200/JCO.1996.14.2.579. [DOI] [PubMed] [Google Scholar]
- 22.Kal HB, van Kempen-Harteveld ML. Renal dysfunction after total body irradiation: dose-effect relationship. Int. J. Radiat. Oncol. Biol. Phys. 2006;65:1228–1232. doi: 10.1016/j.ijrobp.2006.02.021. [DOI] [PubMed] [Google Scholar]
- 23.Kist-van Holthe JE, Goedvolk CA, Brand R, et al. Prospective study of renal insufficiency after bone marrow transplantation. Pediatr. Nephrol. 2002;17:1032–1037. doi: 10.1007/s00467-002-0989-9. [DOI] [PubMed] [Google Scholar]
- 24.National Kidney Foundation K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am. J. Kidney Dis. 2002;39:S1–266. [PubMed] [Google Scholar]
- 25.Anonymous Effects of diet and antihypertensive therapy on creatinine clearance and serum creatinine concentration in the Modification of Diet in Renal Disease Study. J. Am. Soc. Nephrol. 1996;7:556–566. doi: 10.1681/ASN.V74556. [DOI] [PubMed] [Google Scholar]
- 26.Gray RJ. A class of k-sample tests for comparing the cumulative incidence of a competing risk. Ann Stat. 1988;16:1141–1154. [Google Scholar]
- 27.Cox D. Regression models and life tables. J. Roy. Stat. Soc. 1972;B:187–220. [Google Scholar]
- 28.Olyaei AJ, de Mattos AM, Bennett WM. Nephrotoxicity of immunosuppressive drugs: new insight and preventive strategies. Current Opinion in Critical Care. 2001;7:384–389. doi: 10.1097/00075198-200112000-00003. [DOI] [PubMed] [Google Scholar]
- 29.Lethbridge-Çejku M, Rose D, Vickerie J. Summary health statistics for U.S. Adults: National Health Interview Survey, 2004.: Vital Health Stat. 2006;10(228) [PubMed] [Google Scholar]
- 30.Antignac C, Gubler MC, Leverger G, Broyer M, Habib R. Delayed renal failure with extensive mesangiolysis following bone marrow transplantation. Kidney Int. 1989;35:1336–1344. doi: 10.1038/ki.1989.132. [DOI] [PubMed] [Google Scholar]
- 31.Atkinson K, Biggs JC, Hayes J, et al. Cyclosporin A associated nephrotoxicity in the first 100 days after allogeneic bone marrow transplantation: three distinct syndromes. Br. J. Haematol. 1983;54:59–67. doi: 10.1111/j.1365-2141.1983.tb02067.x. [DOI] [PubMed] [Google Scholar]
- 32.Cruz DN, Perazella MA, Mahnensmith RL. Bone marrow transplant nephropathy: a case report and review of the literature. J. Am. Soc. Nephrol. 1997;8:166–173. doi: 10.1681/ASN.V81166. [DOI] [PubMed] [Google Scholar]
- 33.Gomez-Garcia P, Herrera-Arroyo C, Torres-Gomez A, et al. Renal involvement in chronic graft-versus-host disease: a report of two cases. Bone Marrow Transplant. 1988;3:357–362. [PubMed] [Google Scholar]
- 34.Guinan EC, Tarbell NJ, Niemeyer CM, Sallan SE, Weinstein HJ. Intravascular hemolysis and renal insufficiency after bone marrow transplantation. Blood. 1988;72:451–455. [PubMed] [Google Scholar]
- 35.Juckett M, Perry EH, Daniels BS, Weisdorf DJ. Hemolytic uremic syndrome following bone marrow transplantation. Bone Marrow Transplant. 1991;7:405–409. [PubMed] [Google Scholar]
- 36.Glynne P, Powles R, Steele J, et al. Renal dysfunction following autologous bone marrow transplantation in adult patients with acute leukemia. Acta Oncologica. 1996;35:709–712. doi: 10.3109/02841869609084003. [DOI] [PubMed] [Google Scholar]
- 37.Haight AE, Kaste SC, Goloubeva OG, Xiong XP, Bowman LC. Nephrotoxicity of iopamidol in pediatric, adolescent, and young adult patients who have undergone allogeneic bone marrow transplantation. Radiology. 2003;226:399–404. doi: 10.1148/radiol.2262011471. [DOI] [PubMed] [Google Scholar]
- 38.Kagawa Y, Sawada J-I, Yamada S, et al. Relationship between development of nephrotoxicity and blood concentration of cyclosporine A in bone-marrow transplanted recipients who received the continuous intravenous infusion. Biol. Pharm. Bull. 2003;26:1115–1119. doi: 10.1248/bpb.26.1115. [DOI] [PubMed] [Google Scholar]
- 39.Kist-van Holthe JE, Bresters D, Ahmed-Ousenkova YM, et al. Long-term renal function after hemopoietic stem cell transplantation in children. Bone Marrow Transplant. 2005;36:605–610. doi: 10.1038/sj.bmt.1705110. [DOI] [PubMed] [Google Scholar]
- 40.Lawton CA, Cohen EP, Murray KJ, et al. Long-term results of selective renal shielding in patients undergoing total body irradiation in preparation for bone marrow transplantation. Bone Marrow Transplant. 1997;20:1069–1074. doi: 10.1038/sj.bmt.1701022. [DOI] [PubMed] [Google Scholar]
- 41.Parikh CR, McSweeney PA, Korular D, et al. Renal dysfunction in allogeneic hematopoietic cell transplantation. Kidney Int. 2002;62:566–573. doi: 10.1046/j.1523-1755.2002.00455.x. [DOI] [PubMed] [Google Scholar]
- 42.Patzer L, Ringelmann F, Kentouche K, et al. Renal function in long-term survivors of stem cell transplantation in childhood. A prospective trial. Bone Marrow Transplant. 2001;27:319–327. doi: 10.1038/sj.bmt.1702763. [DOI] [PubMed] [Google Scholar]
- 43.Kersting S, Hene RJ, Koomans HA, Verdonck LF. Chronic kidney disease after myeloablative allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2007;13:1169–1175. doi: 10.1016/j.bbmt.2007.06.008. [DOI] [PubMed] [Google Scholar]
- 44.Hingorani S, Guthrie KA, Schoch G, Weiss NS, McDonald GB. Chronic kidney disease in long-term survivors of hematopoietic cell transplant. Bone Marrow Transplant. 2007;39:223–229. doi: 10.1038/sj.bmt.1705573. [DOI] [PubMed] [Google Scholar]
- 45.Cohen EP. Radiation nephropathy after bone marrow transplantation. Kidney Int. 2000;58:903–918. doi: 10.1046/j.1523-1755.2000.00241.x. [DOI] [PubMed] [Google Scholar]
- 46.Stevens LA, Levey AS. Measurement of kidney function. Med. Clin. North Am. 2005;89:457–473. doi: 10.1016/j.mcna.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 47.Clark AD, Shetty A, Soutar R. Renal failure and multiple myeloma: pathogenesis and treatment of renal failure and management of underlying myeloma. Blood Rev. 1999;13:79–990. doi: 10.1016/s0268-960x(99)90014-0. [DOI] [PubMed] [Google Scholar]
- 48.Cohen EP. Renal failure after bone-marrow transplantation. Lancet. 2001;357:6–7. doi: 10.1016/S0140-6736(00)03561-3. [DOI] [PubMed] [Google Scholar]
- 49.Olyaei AJ, de Mattos AM, Bennett WM. Immunosuppressant-induced nephropathy: pathophysiology, incidence and management. Drug Saf. 1999;21:471–488. doi: 10.2165/00002018-199921060-00004. [DOI] [PubMed] [Google Scholar]
- 50.Deeg HJ, Storb R, Thomas ED, et al. Cyclosporine as prophylaxis for graft-versus-host disease: a randomized study in patients undergoing marrow transplantation for acute nonlymphoblastic leukemia. Blood. 1985;65:1325–1334. [PubMed] [Google Scholar]
- 51.Miach PJ. Cyclosporin A in organ transplantation. Med. J. Aust. 1986;145:146–150. doi: 10.5694/j.1326-5377.1986.tb113775.x. [DOI] [PubMed] [Google Scholar]
- 52.Myers BD, Sibley R, Newton L, et al. The long-term course of cyclosporine-associated chronic nephropathy. Kidney Int. 1988;33:590–600. doi: 10.1038/ki.1988.38. [DOI] [PubMed] [Google Scholar]
- 53.Palestine AG, Austin HA, 3rd, Balow JE, et al. Renal histopathologic alterations in patients treated with cyclosporine for uveitis. N. Engl. J. Med. 1986;314:1293–1298. doi: 10.1056/NEJM198605153142005. [DOI] [PubMed] [Google Scholar]
- 54.Andoh TF, Burdmann EA, Bennett WM. Nephrotoxicity of immunosuppressive drugs: experimental and clinical observations. Semin. Nephrol. 1997;17:34–45. [PubMed] [Google Scholar]