Abstract
This report describes the safety observations following administration of a polyvalent DNA prime-protein boost HIV-1 vaccine formulated with adjuvant QS21. Local injection site reactionswere the most common (65% of subjects), and included type IV delayed-type hypersensitivity (DTH) reactions at prior DNA inoculation sites in 12 of 28 (43%) subjects following protein vaccination. Systemic reactions revealed two cases of vasculitis temporally related to inoculation with recombinant Env protein + QS21 adjuvant. Questions remain regarding the cause of the vasculitis, but the unique DTH observation may have contributed to the high level of immune responses previously reported for this vaccine.
Keywords: HIV, vaccine, adverse event, vasculitis
Over the past two decades, various HIV vaccines have been tested for safety and several for efficacy in humans. However, the majority have not reproduced, in humans, the high immunogenicity seen in preclinical testing. Recently, we reported that a novel multi-gene, polyvalent DNA prime-recombinant protein boost HIV vaccine formulation, DP6-001, elicited strong and balanced humoral and cell-mediated immunity in healthy, HIV-1-negative adult subjects [1]. Preclinical testing of DP6-001 demonstrated no significant adverse reactions in either rabbits or non-human primates despite the induction of robust immunity [2, 3]. Previously tested HIV DNA vaccines have demonstrated excellent human safety profiles [4–9]. Recombinant protein-based HIV vaccines formulated with QS21 adjuvant have reported local reactions, but have shown limited systemic adverse events in humans [10–15]. The current report summarizes the phase 1 clinical safety and tolerability data, highlighting local and systemic reactions not previously associated with HIV Env-based vaccines, including delayed-type hypersensitivity reactions occurring at previous vaccination sites, cutaneous leukocytoclastic vasculitis (LCV) and Henoch-Schonlein Purpura (HSP).
The clinical trial was an open-label, 3-arm design requiring each subject to receive two vaccine components; three sequential inoculations using a DNA plasmid vaccine administered either intradermally (ID) or intramuscularly (IM) followed by two sequential inoculations using a protein vaccine as shown in Table 1. Healthy HIV-negative adults (aged 18–50) were enrolled under informed consent as previously described [1]. This study involved 2 dose levels of DNA vaccines (1.2mg (Groups A and B); 7.2mg (Group C)) expressing five gp120 Env antigens (HIV-1 clades A, B, C and E) and one Gag antigen (HIV-1 clade C), and a single dose level of the same five recombinant expressed gp120 protein vaccines, produced and purified from CHO cells, and mixed with QS21 adjuvant at the time of injection.
Table 1.
Study Design and Subject Demographics
| Demographics | Study Design | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| DNA componenta Days 0, 28, 64 | Protein componentb Days 140, 196 | ||||||||
| Group(N) | Mean age in yrs | Gender (N) M/F | Race (N)Caucasian/Asian | Total dose(mg) | No. of sitesc | Routed | Total dose(mg) | No. of sites | Routed |
| A (12) | 35.8 | 8 / 4 | 12 / 0 | 1.2 | 4 | ID | 0.375 | 1 | IM |
| B (12) | 30.8 | 9 / 3 | 11 / 1 | 1.2 | 2 | IM | 0.375 | 1 | IM |
| C (10) | 32.0 | 8 / 2 | 9 / 1 | 7.2 | 2 | IM | 0.375 | 1 | IM |
For all groups, the DNA component consisted of equal amounts of 6 plasmids (5 Env and 1 Gag) at a final concentration of 3 mg/ml.
For all groups, the protein component consists of equal amounts of 5 Env proteins formulated in QS21.
Total dose divided equally between the number of sites
ID = intradermal; IM = intramuscular
Vaccinations were halted following a case of LCV in a Group C subject after the first protein boost, affecting the number of vaccinations administered and enrollment of subjects into Group C. In this trial, 33 of 34 subjects received all three DNA vaccinations; one subject (Group C) received only one DNA inoculation. Twenty-nine subjects (12 of 12, 11 of 12, and 6 of 10 in Groups A-C, respectively) received at least one protein boost. Among them, twenty-two subjects (11 in Group A and 11 in Group B) completed two protein boost inoculations (Table 2).
Table 2.
Clinical adverse events related to the vaccine1 within 14 days of vaccination
| Vaccine | Event | Number of subjects | P-value* | ||
|---|---|---|---|---|---|
| Group A | Group B | Group C2 | |||
| DNA #1 | N | 12 | 12 | 10 | |
| Fever (>37.7°C) | 4 | 3 | 1 | N.S. | |
| Myalgia | 0 | 1 | 1 | N.S. | |
| Erythema | 7 | 1 | 1 | 0.010 | |
| Pain-injection site | 4 | 2 | 7 | 0.047 | |
| Arthralgia | 1 | 0 | 1 | N.S. | |
| Fatigue | 2 | 2 | 0 | N.S. | |
| Headache | 1 | 3 | 3 | N.S. | |
| DNA #2 | N | 12 | 12 | 10 | |
| Fever (>37.7°C) | 1 | 5 | 0 | 0.032 | |
| Myalgia | 0 | 1 | 0 | N.S. | |
| Erythema | 7 | 1 | 0 | 0.002 | |
| Pain-injection site | 1 | 4 | 4 | N.S. | |
| Arthralgia | 0 | 0 | 0 | N.S. | |
| Fatigue | 0 | 1 | 0 | N.S. | |
| Headache | 1 | 3 | 2 | N.S. | |
| DNA #3 | N | 12 | 12 | 9 | |
| Fever (>37.7°C) | 2 | 1 | 0 | N.S. | |
| Myalgia | 1 | 0 | 0 | N.S. | |
| Erythema | 7 | 1 | 0 | 0.003 | |
| Pain-injection site | 0 | 2 | 5 | 0.009 | |
| Arthralgia | 0 | 0 | 0 | N.S. | |
| Fatigue | 0 | 1 | 0 | N.S. | |
| Headache | 1 | 1 | 2 | N.S. | |
| Protein #1 | N | 12 | 11 | 6 | |
| Fever (>37.7°C) | 1 | 4 | 2 | N.S. | |
| Myalgia | 0 | 4 | 5 | 0.001 | |
| Erythema | 5 | 5 | 1 | N.S. | |
| Pain-injection site | 10 | 9 | 5 | N.S. | |
| Arthralgia | 0 | 2 | 0 | N.S. | |
| Fatigue | 0 | 1 | 0 | N.S. | |
| Headache | 1 | 3 | 6 | <0.0001 | |
| Protein #2 | N | 11 | 11 | NA | |
| Fever (>37.7°C) | 3 | 4 | NA | N.S. | |
| Myalgia | 3 | 7 | NA | 0.198 | |
| Erythema | 4 | 6 | NA | N.S. | |
| Pain-injection site | 10 | 11 | NA | N.S. | |
| Arthralgia | 1 | 1 | NA | N.S. | |
| Fatigue | 3 | 0 | NA | N.S. | |
| Headache | 1 | 2 | NA | N.S. | |
Relatedness categories were noted as: not related, doubtful, possibly, probably and definitely, with the latter three summarized in the Table.
Group C: 1 subject received a single DNA injection; 9 three DNA, and 6 three DNA plus one protein vaccination.
Difference between Groups A and B. Fisher exact test; p<0.050 significant, values listed only for p <0.200. NA: not applicable. N.S: difference was not significant.
Adverse Events (AEs) were grouped into either local injection site reactions (ISR) including pain, erythema, swelling and pruritis or systemic reactions including fever, myalgias, joint pain, anorexia, headache, nausea, malaise, and generalized skin rash. Clinical and laboratory adverse events were assessed for relatedness to the vaccine and were graded as mild to severe (1 – 4), according to the NIH’s Table for Grading the Severity of Adult and Pediatric Adverse Events, Version 1.0, Dec 2004 (http://rcc.techres.com/DAIDS%20RCC%20FORMS/ToxicityTables_DAIDS_AE_GradingTable_FinalDec2004.pdf).
The AEs occurring in two or more subjects within 14 days of the indicated vaccination are tabulated in Table 2. Local skin reactions comprised the majority of AE regardless of treatment group. After each DNA immunization, Group A (ID) had significantly more skin reactions than Groups B and C (IM), with no difference in severity (data not shown). Systemic reactions myalgia (p=0.001), headaches (p < 0.001) and fever, although not significant, were more common after protein inoculation in Groups B and C, compared to A, and severity tended to increase with DNA dose (data not shown). Grade 3 and 4 AEs, including two laboratory AEs (elevated gamma-glutamyl transferase [γGT] and hypoglycemia), vasculitis, and fever were observed separately in four subjects.
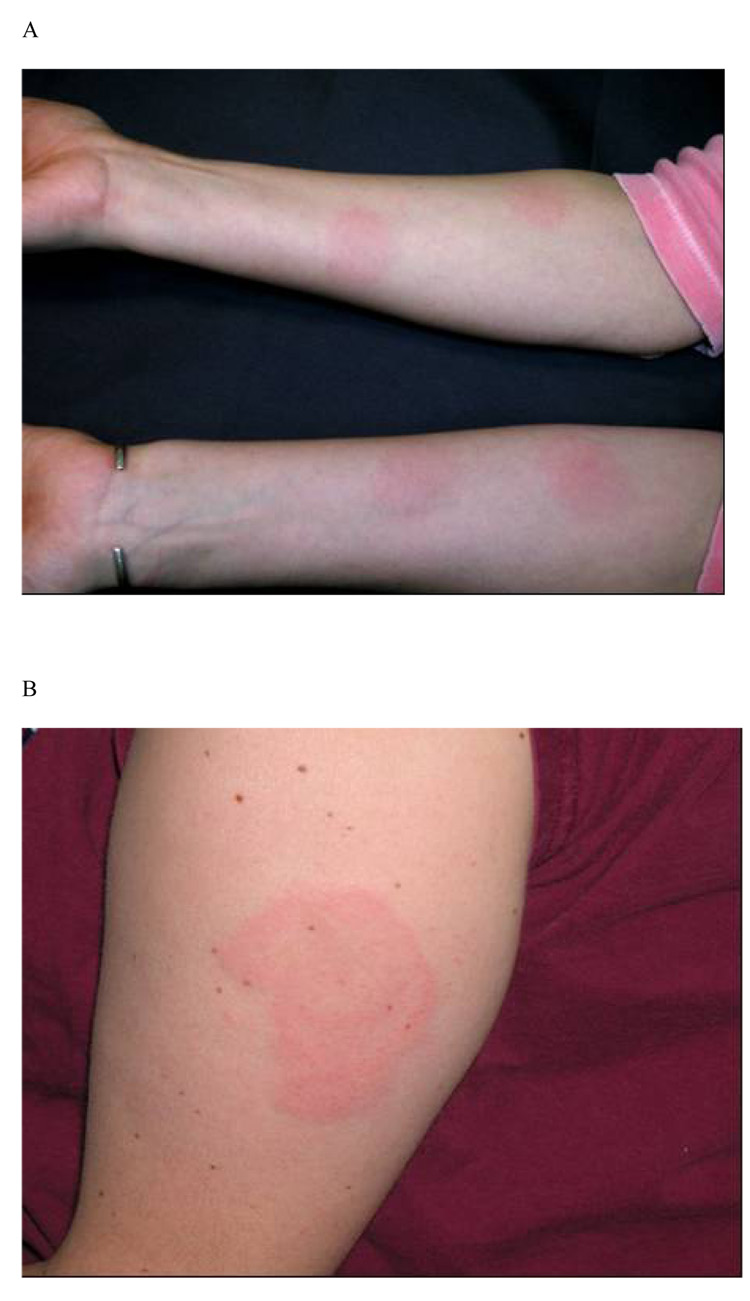
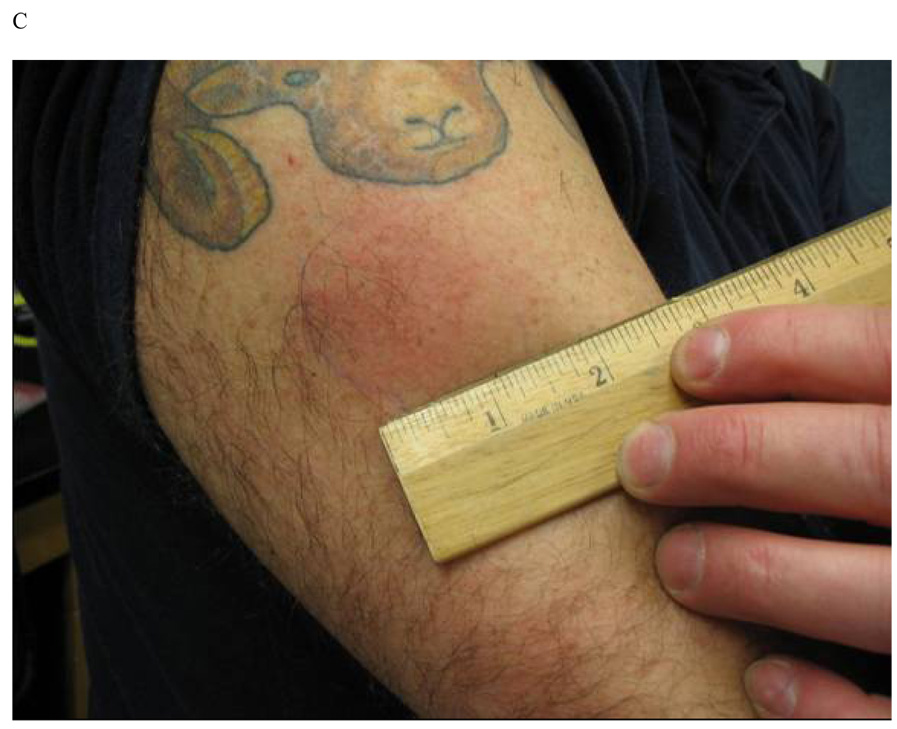
There were a total of 87 skin reactions (one unrelated to a vaccination site) in the 34 subjects. Based on the appearance and location, three clinically distinct types of skin reactions were encountered and are shown in Figure 1 and Figure 2. Twenty-two of the 34 subjects (65%) in the study developed localized skin erythema within hours after either DNA or protein vaccines. Twenty DTH-like reactions were observed in 13 (39%) of 33 subjects who received at least two vaccine doses. These reactions typically appeared 36–72 hours after injection of either the DNA (8 reactions) or the protein vaccine (12 reactions). Fig. 1A depicts reactions at prior forearm ID sites of DNA inoculation in a Group A subject following protein inoculation in the deltoid. Fig. 1B is a reaction following the 1st protein boost that also developed in subjects at the contralateral deltoid site, indicating reaction to previous IM DNA administration (Groups B and C). The first four subjects with this type of skin reaction were confirmed by a dermatology consultation as type IV hypersensitivity reactions. The occurrence of DTH-like reactions was more common in Group A (ID) (13 events in 8 of 12 subjects) compared to Group B (IM) (1 event among 12 subjects) (p=0.004), but was not different from Group C (high dose IM) (6 events in 4 of 9 subjects).
Figure 1.
Representative photographs of skin reactions observed after one protein vaccination in subjects primed with DNA vaccines. (A) Type IV hypersensitivity skin reaction (DTH-like) observed at prior sites of intradermal DNA inoculation in a Group A subject; (B) DTH reaction at the contra-lateral intramuscular deltoid (DNA) site in a Group B subject; and (C) Env-QS21 hypersensitivity reaction observed in < 24hr at the site of the intramuscular deltoid inoculation in a Group B subject.
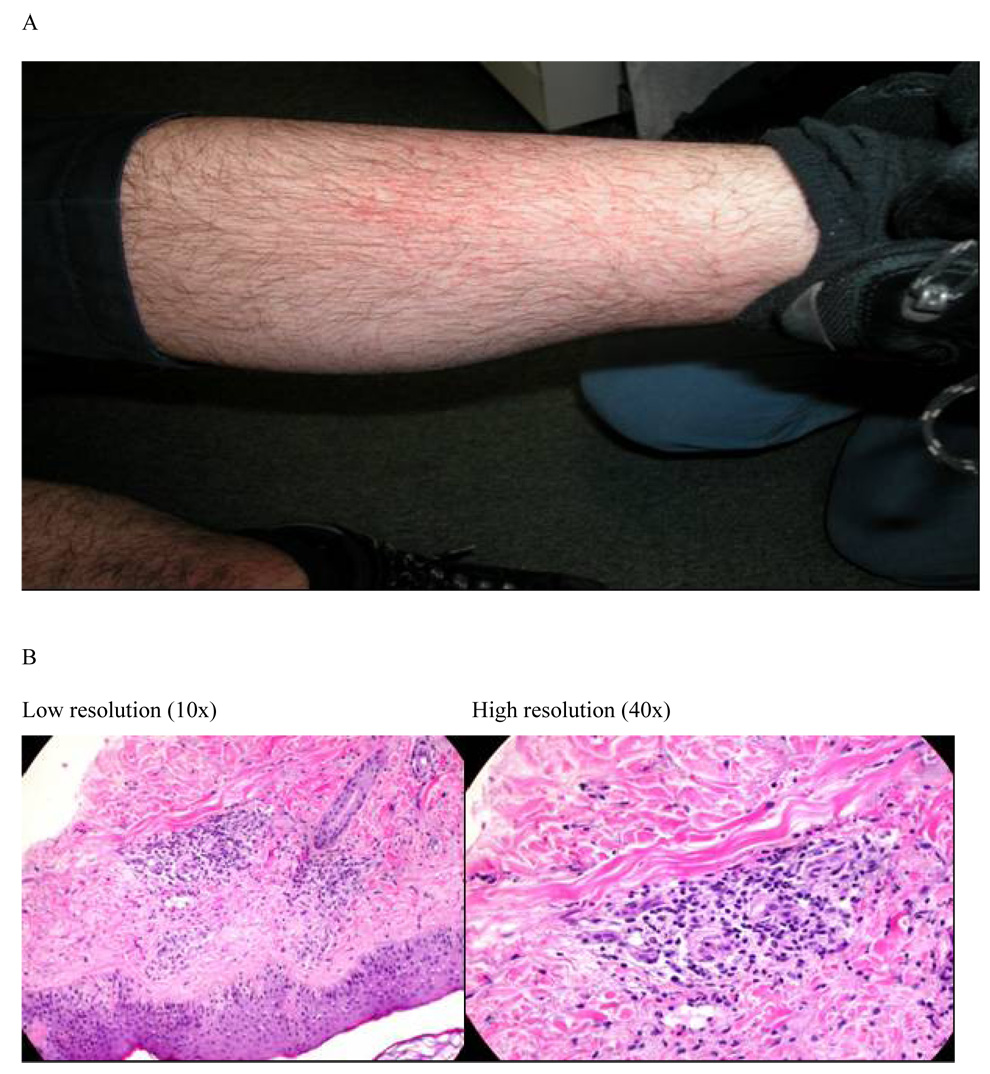
Figure 2.
Lower extremity skin lesions in photograph (A) and light microscopy of the skin biopsy (B) for leukocytoclastic vasculitis in a Group C subject
The second type of skin reaction (Fig. 1C) was probably related to QS21 based on prior reports of similar reactions to Env proteins formulated with QS21 [10, 15]. This pattern of erythema typically developed ≤24 hours after inoculation and was associated with greater induration, distinguishing it from the reactions depicted in Figures 1A and 1B. However, a hypersensitivity reaction to the HIV antigens cannot be definitively excluded as a cause.
The third type of skin reaction occurred in two subjects, both developing vasculitis following inoculation of the protein vaccine component. The Group C subject had grade 1 injection site skin reactions following the DNA priming regimen consistent with DTH and after the first protein inoculation complained of flu-like symptoms and subsequently developed a bilateral rash extending proximally from the ankle to the mid calf (Figure 2A). A skin biopsy confirmed the diagnosis of leukocytoclastic vasculitis (Figure 2B). The rash had near complete resolution at the Day 7 safety visit following topical corticosteroid treatment. All symptoms resolved in 30 days and the 1-year closeout visit was normal. The second subject (Group B) had grade 2 injection site skin reactions (DTH) after DNA immunizations. Following the first protein boost, the subject presented with RUQ abdominal pain, elevated liver enzymes (Grade-4 Laboratory AE; γGT), elevated ESR, and transient trace hematuria initially attributed to a new prescription for bupropion. No further vaccination was given to this subject. At the 1-year closeout visit, the subject reported developing multiple flat purple-red lesions on the lower extremities about 8 weeks after receiving the protein vaccination. Physical examination at the closeout visit revealed new multiple bilateral lower extremity lesions consistent with resolved purpura. The subject related a prior diagnosis of Henoch-Schonlein Purpura as a young adult that was not reported during the screening visit. The biochemical and clinical signs of hepatitis and purpura following the 1st protein vaccination are consistent with reactivation of HSP. A panel of tests for autoimmune responses conducted for the cases of vasculitis and four individuals with DTH were negative, with only grade-1 elevations of CH50 in the LCV case and ESR in the case of HSP.
These results raise questions regarding the reactogenicity of our DNA prime-protein boost vaccination approach when a potent immune adjuvant, such as QS21, is included with the protein components. One major difference between the current trial and previous QS21-adjuvanted recombinant Env protein-based HIV vaccine trials [10, 15] is that the current trial included a series of three DNA priming inoculations. The high titer and quick rise of antibody responses after only one protein boost [1] indicated a strong induction of immune priming by the DNA vaccine. The different sites used for the sequential DNA priming, as well as two sequential protein boost inoculations, enabled the clinical separation of DTH-like reactions from local reactions induced by QS21 as reported in a previous HIV Env-QS21 vaccine study [10]. DTH reactions have been reported in other vaccine studies involving prophylactic vaccines against HIV [16], syphilis [17] and malaria [18], and a therapeutic vaccine for HIV [19]. A previous detailed report of exaggerated skin reactions following re-administration of gp160 in HIV+ subjects was attributed to contaminating insect cell proteins [20], and is not similar to our observations of DTH or vasculitis. Our rabbit toxicology study demonstrated gradual clearance of DNA plasmids over several months at skin and muscle sites of DNA inoculations [3], suggesting that some skin reactions observed may be attributable to prolonged presence of Env antigen at the DNA inoculation sites.
Vasculitis has been reported previously to occur following administration of several protein antigen-based vaccines and is described in FDA package insert warnings on a variety of other vaccines [21–23]. These two cases of vasculitis are the first to be reported that were temporarily related to inoculation using HIV-1 Env in combination with the saponin adjuvant QS21 in humans. The etiology of LCV is not well understood but has been shown to be associated with immune complexes, autoantibodies such as antineutrophil cytoplasmic antibody (ANCA), and inflammatory mediators particularly those associated with endothelial adhesion molecules [23, 24]. In the general population, hypersensitivity vasculitis occurs in 10–30 cases per million/year and 14 cases of Henoch-Schonlein purpura per million/year have been reported [23] with up to one-half being idiopathic in nature. In our cases, no evidence of infection (including CMV, EBV, and HBV), inflammatory bowel disease, malignancy, drugs or changes in diet could account for the two cases of vasculitis. The temporal relationship to vaccination suggests mechanisms for the vasculitis in our study could include a strong cytokine production or enhanced reactions to Env antigen resulting from the adjuvant used, and/or another unknown immune activation mechanism resulting in an amplified reaction to the protein-adjuvant mix when the host is DNA primed. In support of a role for an adjuvant in the systemic events observed, reactogenic events associated with several well known adjuvants, including QS21 have been reported [15, 21, 22, 25–29].
The design of the phase I study does not allow firm conclusions to be drawn regarding the etiology of these unusual local and systemic reactions. Given their occurrence in association with the high antibody and cellular immune responses to HIV Env induced by this DNA prime-protein boost vaccine, along with the recognition that HIV infected individuals display a varied autoimmune antibody repertoire to self-antigens [30–35], a potential relationship to HIV Env warrants consideration in the design and analysis of this and other vaccine trials.
Acknowledgements
We are grateful to the volunteers in this study for their dedication, passion and willingness to participate in the critical role of testing an experimental HIV vaccine. We express thanks the members of our scientific advisory board, DAIDS-NIH and in particular Drs. Stuart Shapiro, Alan Fix, Dale Lawrence of DIADS-NIH, and Francis Ennis, UMMS for their insightful comments, discussions and scientific expertise in bringing this vaccine from the concept stage to human clinical trials.
This study was supported by the contract NIH, NIAID, N01-AI-05394.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Wang S, Kennedy JS, West K, Montefiori DC, Coley S, Lawrence J, et al. Cross-subtype antibody and cellular immune responses induced by a polyvalent DNA prime-protein boost HIV-1 vaccine in healthy human volunteers. Vaccine. 2008 Feb 20;26(8):1098–1110. doi: 10.1016/j.vaccine.2007.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pal R, Kalyanaraman VS, Nair BC, Whitney S, Keen T, Hocker L, et al. Immunization of rhesus macaques with a polyvalent DNA prime/protein boost human immunodeficiency virus type 1 vaccine elicits protective antibody response against simian human immunodeficiency virus of R5 phenotype. Virology. 2006 May 10;348(2):341–353. doi: 10.1016/j.virol.2005.12.029. [DOI] [PubMed] [Google Scholar]
- 3.Pal R, Yu Q, Wang S, Kalyanaraman VS, Nair BC, Hudacik L, et al. Definitive toxicology and biodistribution study of a polyvalent DNA prime/protein boost human immunodeficiency virus type 1 (HIV-1) vaccine in rabbits. Vaccine. 2006 Feb 20;24(8):1225–1234. doi: 10.1016/j.vaccine.2005.07.112. [DOI] [PubMed] [Google Scholar]
- 4.Robinson HL. New hope for an AIDS vaccine. Nat Rev Immunol. 2002 Apr 2;4:239–250. doi: 10.1038/nri776. [DOI] [PubMed] [Google Scholar]
- 5.Boyer JD, Cohen AD, Vogt S, Schumann K, Nath B, Ahn L, et al. Vaccination of seronegative volunteers with a human immunodeficiency virus type 1 env/rev DNA vaccine induces antigen-specific proliferation and lymphocyte production of beta-chemokines. J Infect Dis. 2000 Feb;181(2):476–483. doi: 10.1086/315229. [DOI] [PubMed] [Google Scholar]
- 6.MacGregor RR, Boyer JD, Ciccarelli RB, Ginsberg RS, Weiner DB. Safety and immune responses to a DNA-based human immunodeficiency virus (HIV) type I env/rev vaccine in HIV-infected recipients: follow-up data. J Infect Dis. 2000 Jan;181(1):406. doi: 10.1086/315199. [DOI] [PubMed] [Google Scholar]
- 7.Tavel JA, Martin JE, Kelly GG, Enama ME, Shen JM, Gomez PL, et al. Safety and immunogenicity of a Gag-Pol candidate HIV-1 DNA vaccine administered by a needle-free device in HIV-1-seronegative subjects. J Acquir Immune Defic Syndr. 2007 Apr 15;44(5):601–605. doi: 10.1097/QAI.0b013e3180417cb6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harari A, Bart PA, Stohr W, Tapia G, Garcia M, Medjitna-Rais E, et al. An HIV-1 clade C DNA prime, NYVAC boost vaccine regimen induces reliable, polyfunctional, and long-lasting T cell responses. The Journal of experimental medicine. 2008 Jan 21;205(1):63–77. doi: 10.1084/jem.20071331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Graham BS, Koup RA, Roederer M, Bailer RT, Enama ME, Moodie Z, et al. Phase 1 safety and immunogenicity evaluation of a multiclade HIV-1 DNA candidate vaccine. The Journal of infectious diseases. 2006 Dec 15;194(12):1650–1660. doi: 10.1086/509259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Evans TG, McElrath MJ, Matthews T, Montefiori D, Weinhold K, Wolff M, et al. QS-21 promotes an adjuvant effect allowing for reduced antigen dose during HIV-1 envelope subunit immunization in humans. Vaccine. 2001 Feb 28;19(15–16):2080–2091. doi: 10.1016/s0264-410x(00)00415-1. [DOI] [PubMed] [Google Scholar]
- 11.Gahery-Segard H, Pialoux G, Figueiredo S, Igea C, Surenaud M, Gaston J, et al. Long-term specific immune responses induced in humans by a human immunodeficiency virus type 1 lipopeptide vaccine: characterization of CD8+-T-cell epitopes recognized. J Virol. 2003 Oct;77(20):11220–11231. doi: 10.1128/JVI.77.20.11220-11231.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pialoux G, Gahery-Segard H, Sermet S, Poncelet H, Fournier S, Gerard L, et al. Lipopeptides induce cell-mediated anti-HIV immune responses in seronegative volunteers. Aids. 2001 Jul 6;15(10):1239–1249. doi: 10.1097/00002030-200107060-00005. [DOI] [PubMed] [Google Scholar]
- 13.McCormack S, Tilzey A, Carmichael A, Gotch F, Kepple J, Newberry A, et al. A phase I trial in HIV negative healthy volunteers evaluating the effect of potent adjuvants on immunogenicity of a recombinant gp120W61D derived from dual tropic R5X4 HIV-1ACH320. Vaccine. 2000 Jan 18;18(13):1166–1177. doi: 10.1016/s0264-410x(99)00388-6. [DOI] [PubMed] [Google Scholar]
- 14.Jones GJ, von Hoegen P, Weber J, Rees AD. Immunization with human immunodeficiency virus type 1 rgp120W61D in QS21/MPL adjuvant primes T cell proliferation and C-C chemokine production to multiple epitopes within variable and conserved domains of gp120W61D. J Infect Dis. 1999 Mar;179(3):558–566. doi: 10.1086/314626. [DOI] [PubMed] [Google Scholar]
- 15.Keefer MC, Wolff M, Gorse GJ, Graham BS, Corey L, Clements-Mann ML, et al. Safety profile of phase I and II preventive HIV type 1 envelope vaccination: experience of the NIAID AIDS Vaccine Evaluation Group. AIDS Res Hum Retroviruses. 1997 Sep 20;13(14):1163–1177. doi: 10.1089/aid.1997.13.1163. [DOI] [PubMed] [Google Scholar]
- 16.Girard MP, Osmanov SK, Kieny MP. A review of vaccine research and development: the human immunodeficiency virus (HIV) Vaccine. 2006 May 8;24(19):4062–4081. doi: 10.1016/j.vaccine.2006.02.031. [DOI] [PubMed] [Google Scholar]
- 17.Sell S, Hsu PL. Delayed hypersensitivity, immune deviation, antigen processing and T-cell subset selection in syphilis pathogenesis and vaccine design. Immunol Today. 1993 Dec;14(12):576–582. doi: 10.1016/0167-5699(93)90196-R. [DOI] [PubMed] [Google Scholar]
- 18.Kublin JG, Lowitt MH, Hamilton RG, Oliveira GA, Nardin EH, Nussenzweig RS, et al. Delayed-type hypersensitivity in volunteers immunized with a synthetic multi-antigen peptide vaccine (PfCS-MAP1NYU) against Plasmodium falciparum sporozoites. Vaccine. 2002 Mar 15;20(13–14):1853–1861. doi: 10.1016/s0264-410x(02)00037-3. [DOI] [PubMed] [Google Scholar]
- 19.Levine AM, Groshen S, Allen J, Munson KM, Carlo DJ, Daigle AE, et al. Initial studies on active immunization of HIV-infected subjects using a gp120-depleted HIV-1 Immunogen: long-term follow-up. J Acquir Immune Defic Syndr Hum Retrovirol. 1996 Apr 1;11(4):351–364. doi: 10.1097/00042560-199604010-00005. [DOI] [PubMed] [Google Scholar]
- 20.Smith KJ, Skelton HG, Ruiz N, Samlaska K, Wagner KF. Cutaneous reactions at the site of post-infection gp160 vaccination therapy in HIV-1+ patients. Military Medical Consortium for the Advancement of Military Medicine. International journal of dermatology. 1998 Apr;37(4):293–298. doi: 10.1046/j.1365-4362.1998.00272.x. [DOI] [PubMed] [Google Scholar]
- 21.Nikkels AF, Nikkels-Tassoudji N, Pierard GE. Cutaneous adverse reactions following anti-infective vaccinations. Am J Clin Dermatol. 2005;6(2):79–87. doi: 10.2165/00128071-200506020-00002. [DOI] [PubMed] [Google Scholar]
- 22.Chave T, Neal C, Camp R. Henoch-Schonlein purpura following hepatitis B vaccination. J Dermatolog Treat. 2003 Sep;14(3):179–181. doi: 10.1080/09546630310004199. [DOI] [PubMed] [Google Scholar]
- 23.Blanco R, Martinez-Taboada VM, Rodriguez-Valverde V, Garcia-Fuentes M. Cutaneous vasculitis in children and adults. Associated diseases and etiologic factors in 303 patients. Medicine (Baltimore) 1998 Nov;77(6):403–418. doi: 10.1097/00005792-199811000-00007. [DOI] [PubMed] [Google Scholar]
- 24.Carlson JA, Chen KR. Cutaneous vasculitis update: small vessel neutrophilic vasculitis syndromes. The American Journal of dermatopathology. 2006 Dec;28(6):486–506. doi: 10.1097/01.dad.0000246646.45651.a2. [DOI] [PubMed] [Google Scholar]
- 25.Orgogozo JM, Gilman S, Dartigues JF, Laurent B, Puel M, Kirby LC, et al. Subacute meningoencephalitis in a subset of patients with AD after Abeta42 immunization. Neurology. 2003 Jul 8;61(1):46–54. doi: 10.1212/01.wnl.0000073623.84147.a8. [DOI] [PubMed] [Google Scholar]
- 26.Stewart TJ, Drane D, Malliaros J, Elmer H, Malcolm KM, Cox JC, et al. ISCOMATRIX adjuvant: an adjuvant suitable for use in anticancer vaccines. Vaccine. 2004 Sep 9;22(27–28):3738–3743. doi: 10.1016/j.vaccine.2004.03.026. [DOI] [PubMed] [Google Scholar]
- 27.Livingston PO, Adluri S, Helling F, Yao TJ, Kensil CR, Newman MJ, et al. Phase 1 trial of immunological adjuvant QS-21 with a GM2 ganglioside-keyhole limpet haemocyanin conjugate vaccine in patients with malignant melanoma. Vaccine. 1994 Nov;12(14):1275–1280. doi: 10.1016/s0264-410x(94)80052-2. [DOI] [PubMed] [Google Scholar]
- 28.Vandepapeliere P, Rehermann B, Koutsoukos M, Moris P, Garcon N, Wettendorff M, et al. Potent enhancement of cellular and humoral immune responses against recombinant hepatitis B antigens using AS02A adjuvant in healthy adults. Vaccine. 2005 Apr 8;23(20):2591–2601. doi: 10.1016/j.vaccine.2004.11.034. [DOI] [PubMed] [Google Scholar]
- 29.Cribbs DH, Ghochikyan A, Vasilevko V, Tran M, Petrushina I, Sadzikava N, et al. Adjuvant-dependent modulation of Th1 and Th2 responses to immunization with beta-amyloid. Int Immunol. 2003 Apr;15(4):505–514. doi: 10.1093/intimm/dxg049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Haynes BF, Fleming J, St Clair EW, Katinger H, Stiegler G, Kunert R, et al. Cardiolipin polyspecific autoreactivity in two broadly neutralizing HIV-1 antibodies. Science. 2005 Jun 24;308(5730):1906–1908. doi: 10.1126/science.1111781. [DOI] [PubMed] [Google Scholar]
- 31.Trkola A, Kuster H, Rusert P, Joos B, Fischer M, Leemann C, et al. Delay of HIV-1 rebound after cessation of antiretroviral therapy through passive transfer of human neutralizing antibodies. Nat Med. 2005 Jun;11(6):615–622. doi: 10.1038/nm1244. [DOI] [PubMed] [Google Scholar]
- 32.Ditzel HJ, Barbas SM, Barbas CF, 3rd, Burton DR. The nature of the autoimmune antibody repertoire in human immunodeficiency virus type 1 infection. Proc Natl Acad Sci U S A. 1994 Apr 26;91(9):3710–3714. doi: 10.1073/pnas.91.9.3710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Walsh CM, Nardi MA, Karpatkin S. On the mechanism of thrombocytopenic purpura in sexually active homosexual men. N Engl J Med. 1984 Sep 6;311(10):635–639. doi: 10.1056/NEJM198409063111004. [DOI] [PubMed] [Google Scholar]
- 34.Karpatkin S, Nardi M, Lennette ET, Byrne B, Poiesz B. Anti-human immunodeficiency virus type 1 antibody complexes on platelets of seropositive thrombocytopenic homosexuals and narcotic addicts. Proc Natl Acad Sci U S A. 1988 Dec;85(24):9763–9767. doi: 10.1073/pnas.85.24.9763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stricker RB, Abrams DI, Corash L, Shuman MA. Target platelet antigen in homosexual men with immune thrombocytopenia. N Engl J Med. 1985 Nov 28;313(22):1375–1380. doi: 10.1056/NEJM198511283132202. [DOI] [PubMed] [Google Scholar]