Abstract
Oncogenic transformation of cells alters their morphology, cytoskeletal organization, and adhesive interactions. When the mammary epithelial cell line MCF10A is transformed by activated H-Ras, the cells display a mesenchymal/fibroblastic morphology with decreased cell–cell junctions but increased focal adhesions and stress fibers. We have investigated whether the transformed phenotype is due to Rho activation. The Ras-transformed MCF10A cells have elevated levels of myosin light chain phosphorylation and are more contractile than their normal counterparts, consistent with the activation of Rho. Furthermore, inhibitors of contractility restore a more normal epithelial phenotype to the Ras-transformed MCF10A cells. However, inhibiting Rho by microinjection of C3 exotransferase or dominant negative RhoA only partially restores the normal phenotype, in that it fails to restore normal junctional organization. This result prompted us to examine the effect that inhibiting Rho would have on the junctions of normal MCF10A cells. We have found that inhibiting Rho by C3 microinjection leads to a disruption of E-cadherin cytoskeletal links in adherens junctions and blocks the assembly of new adherens junctions. The introduction of constitutively active Rho into normal MCF10A cells did not mimic the Ras-transformed phenotype. Thus, these results lead us to conclude that some, but not all, characteristics of Ras-transformed epithelial cells are due to activated Rho. Whereas Rho is needed for the assembly of adherens junctions, high levels of activated Rho in Ras-transformed cells contribute to their altered cytoskeletal organization. However, additional events triggered by Ras must also be required for the disruption of adherens junctions and the full development of the transformed epithelial phenotype.
INTRODUCTION
Oncogenic transformation often results in epithelial cells losing many of their distinctive epithelial characteristics. Transformed epithelia frequently lose their polarized morphology, reveal less organized cell–cell junctions, and become more migratory (Behrens et al., 1989; Birchmeier et al., 1993). In many ways, these cells appear more mesenchymal. One of the commonest oncogenes to be activated in human cancers is Ras (Slamon et al., 1984; Thor et al., 1986; Clark and Der, 1995). This low molecular weight GTP-binding protein has been shown to be involved in multiple signaling pathways, the best characterized being the Raf/mitogen-activated protein kinase (MAPK) cascade (Roberts, 1992; Marshall, 1996). This pathway ultimately affects gene expression. Ras is also involved in regulating the organization of the actin cytoskeleton, with Rho and Rac being downstream effectors (Hall, 1994). In addition, there are several other effectors found to be associated and regulated by Ras, such as phosphatidylinositol 3-kinase and Ral guanine nucleotide dissociation stimulator (Rodriguez-Viciana et al., 1994; Spaargaren and Bischoff, 1994; Marshall, 1996). Studies from several groups have indicated that oncogenic Ras activation of pathways in addition to the Raf/MAPK pathway, such as those involving Rac and Rho, are essential for tumorigenic transformation of both fibroblasts and epithelial cells (Khosravi-Far et al., 1995, 1996; Prendergast et al., 1995; Qiu et al., 1995a,b; Oldham et al., 1996).
As a model to better understand how activated Ras may transform epithelial cells, we have used MCF10A breast epithelial cells transfected with either normal H-Ras or oncogenically activated H-Ras (12V; Basolo et al., 1991). The Ras-transformed MCF10A cells appear more mesenchymal (fibroblastic) in their morphology, in the organization of their cell–cell junctions and their actin cytoskeletons (Kinch et al., 1995). Whereas normal MCF10A cells grow in culture as tightly clustered epithelial colonies, the Ras-transformed MCF10A cells are more loosely arranged. The normal MCF10A cells reveal circumferential bundles of actin filaments, typical of polarized epithelia. In contrast, the Ras-transformed MCF10A cells possess large bundles of actin filaments (stress fibers) that extend across the cells and terminate in focal adhesions (Kinch et al., 1995). This latter type of junction is generally absent from the normal MCF10A cells in culture, except at the periphery of a colony or when the MCF10A cells are growing without contact with their neighbors. The prominent stress fibers and focal adhesions of Ras-transformed MCF10A cells are reminiscent of cells in which the Ras family member Rho has been activated (Ridley and Hall, 1992). Rho stimulates the assembly of focal adhesions and stress fibers by increasing the contractility of cells (Chrzanowska-Wodnicka and Burridge, 1996). Herein we have investigated whether the fibroblastic phenotype of Ras-transformed epithelial cells is due to activation of Rho and, in particular, due to the stimulation of contractility. A portion of this work has been presented (Zhong et al., 1996).
MATERIALS AND METHODS
Cell Culture
MCF10A cells originated from a spontaneous immortalization of mammary tissue from a patient with fibrocystic disease were transfected with the normal human H-Ras protooncogene (N) or with the 12V-mutated form of H-Ras oncogene (T) and maintained in DMEM and Ham’s F-12 medium (1:1, vol/vol) containing 5% horse serum, 20 ng/ml epidermal growth factor, 10 μg/ml insulin, and 0.5 μg/ml hydrocortisone under a 5% CO2, 95% air atmosphere (Soule et al., 1990; Basolo et al., 1991). Subconfluent dishes were treated with various reagents for 1 to 2 h and later were either lysed with lysis buffer (see below) for biochemical assays or trypsinized and plated on coverslips for immunofluorescence study. Contractility was assayed with cells plated on silicone rubber substrates overnight at high density as described previously (Harris et al., 1980) with some modifications (Chrzanowska-Wodnicka and Burridge, 1996). To ensure better adhesiveness of cells to the substrate, dishes covered with silicone rubber substrates were further coated with palladium/gold by using a cold sputter coater for 16 s in >99% argon-filled chamber. The evenly distributed metal surface makes the cells adhere and spread more quickly.
Reagents and Antibodies
KT5926 (Calbiochem, La Jolla, CA; Nakanishi et al., 1990) was used at 60 μM; 1-(5-isoquinolinylsulfonyl)-2-methylpiperazine (Sigma, St. Louis, MO) was used at 150 μM (Chrzanowska-Wodnicka and Burridge, 1996); 2,3-butanedione 2-monoxime (BDM, Sigma) was used at 20 mM (Higuchi and Takemori, 1989; Hermann et al., 1993; Osterman et al., 1993; McKillop et al., 1994; Chrzanowska-Wodnicka and Burridge, 1996); ML-7 (Biomol, Plymouth Meeting, PA) was used at 25 μM (Saitoh et al., 1987). Drugs were either dissolved in dimethyl sulfoxide or culture medium as stock solution stored at −20°C and diluted right before use. Mouse monoclonal antibody specific for E-cadherin was purchased from Transduction Laboratories (Lexington, KY). Monoclonal rat anti-E-cadherin (DECMA) was obtained from Sigma. A monoclonal antibody against vinculin (7F9) was a gift from Dr. A. Belkin (Glukhova et al., 1990). A monoclonal antibody against paxillin was from Dr. J. Glenney (Turner et al., 1990). Fluorescein- or rhodamine-conjugated phalloidin was purchased from Molecular Probes (Eugene, OR). C3 exotransferase was purchased (Upstate Biotechnology, Lake Placid, NY) or made in the laboratory as a glutathione S-transferase (GST) fusion protein (see below; Aktories and Hall, 1989).
Immunofluorescence Microscopy
Cells plated on coverslips were fixed with 3.7% formaldehyde in PBS and permeabilized with 0.5% Triton X-100 as described elsewhere (Kinch et al., 1995). Cells were incubated with the primary antibodies and then rhodamine-conjugated goat anti-mouse or donkey anti-rabbit antibodies (Chemicon International, el Segundo, CA) for 30 min, respectively, before being mounted and viewed on an Axiophot microscope (Carl Zeiss, Thornwood, NY). Fluorescence micrographs were taken on T-Max 400 film (Eastman Kodak, Rochester, NY). To visualize molecules associated with the actin cytoskeleton, cells were permeabilized with 0.5% Triton X-100 first, followed by fixation and incubation with the primary antibodies.
Metabolic Labeling, Immunoprecipitation, SDS-PAGE, Western Blot, and Autoradiography
In some experiments, cells were metabolically labeled with 160 μCi/ml Translabel (a mixture of [35S]methionine and [35S]cysteine; ICN Biomedicals, Irvine, CA) in methionine-free medium containing 1% fetal calf serum, 2 mM glutamine, and all other components required for MCF10A cells. After labeling, cells were washed four times with PBS. Cells were then lysed with RIPA buffer containing 1% deoxycholate acid, 0.5% Triton X-100, 0.1% SDS, 1 mM phenylmethylsulfonyl fluoride, 1 mM benzamidine, 10 μg/ml leupeptin, and 10 μg/ml aprotinin, and the total amounts of de novo-synthesized proteins in different samples were measured by using an LS5000CE scintillation counter (Beckman, Palo Alto, CA). For myosin light chain phosphorylation assay, cells were double-labeled with 35S and 32P as described previously (Chrzanowska-Wodnicka and Burridge, 1996). Myosin light chain was separated by SDS-PAGE on 15% gels. In all cases, protein bands were either viewed by autoradiography or PhosphorImager (Molecular Dynamics, Sunnyvale, CA) and the intensity of light chain phosphorylation was represented as the ratio of 32P in the light chains relative to 35S in the heavy chains. To study the cytoskeletal association of E-cadherin, cells were sequentially extracted first with 0.5% digitonin in a buffer containing 20 mM Tris(hydroxymethyl)aminomethane hydrochloride, 100 mM NaCl, 0.5 mM ethylene glycol-bis(β-aminoethyl ether)-N,N,N′,N′-tetraacetic acid, and 1 mM MgCl2 and then with RIPA buffer. Cell lysates were immunoprecipitated with DECMA and 10% protein A-Sepharose to which had been bound anti-rat IgG, separated on 10% polyacrylamide gels as described (Laemmli, 1970) with bisacrylamide concentration of 0.13%, and probed by monoclonal anti-E-cadherin antibody (Transduction Laboratories).
Microinjection of Fusion Proteins and Plasmids
Recombinant 14V RhoA and C3 (gifts from Dr. A. Hall, University College, London, UK, and Dr. L. Feig, Tufts University, Boston, MA, respectively) were expressed as GST fusion proteins in Escherichia coli. Expression of the fusion proteins was induced by isopropyl β-d-thiogalactopyranoside (Boehringer, Mannheim, Germany) and bacteria were lysed by sonication at 4°C. The fusion proteins were further purified with glutathione-agarose beads (Pharmacia, Piscataway, NJ). For 14V RhoA preparation, protein was released by triple elution with 25 mM reduced glutathione, pH 8.0. For C3, protein was cleaved by thrombin (Sigma) and all the unused thrombin was removed by incubation with 50% p-aminobenzamide beads. Both proteins were dialyzed against microinjection buffer containing 50 mM Tris(hydroxymethyl)aminomethane, pH 7.5, 50 mM NaCl, 5 mM MgCl2, and 0.1 mM dithiothreitol and concentrated to 1–2.5 mg/ml for 14V RhoA and 100 μg/ml for C3 before microinjection. GST protein alone was generated as above to serve as a control. Cells plated on coverslips were microinjected by using the method described by Graessmann et al. (1980). Control injections were performed by using GST alone or bovine serum albumin (BSA) in the same microinjection buffer. Cells were injected for 15 to 30 min and then returned to the incubator for another 30 min to 7 h, as needed for different experiments. Injected cells were visualized by the coinjection of coumarin-conjugated BSA or by staining with an anti-GST polyclonal antibody followed by rhodamine-conjugated donkey anti-rabbit IgG or coinjection of 1 mg/ml propidium iodide (Sigma). Propidium iodide labels DNA in the nuclei of injected cells and was particularly useful when cells were permeabilized before fixation because the label was not lost with permeabilization.
For nuclear injection, plasmids were diluted in an injection buffer containing 5 mM potassium glutamate (Fluka, Buchs, Switzerland) and 130 mM KCl. Cells plated on coverslips were injected with plasmid pGreen Lantern either alone (20 μg/ml, Life Technologies, Gaithersburg, MD) or together with 19N-RhoA plasmid at a final concentration of 30 μg/ml (kindly provided by Dr. Marc Symons, Onyx Pharmaceuticals, Richmond, CA). Twenty-four hours later cells were fixed and stained. Microinjected cells were visualized by the expression of green fluorescent protein in the cytoplasm.
Proliferation and Motility Assays
To measure DNA synthesis, cells plated on coverslips were incubated with 100 μM 5-bromo-2′-deoxyuridine (BrdUrd, Sigma) for 24 h, fixed, permeabilized, and stained with an anti-BrdUrd monoclonal antibody (Sigma). Cell nuclei were visualized by staining with Hoechst dye. For motility assays, MCF10A cells were plated at low density onto 35-mm tissue culture dishes (Costar, Cambridge, MA) and incubated overnight in growth medium. Next day the medium was supplemented with 20 mM N-(2-hydroxyethyl)piperazine-N′-(2-ethanesulfonic acid) and all the following steps were done with the dishes on a microscope stage maintained at 37°C. Cell movement was then recorded with a time-lapse video recorder with a 60-fold time compression. The rates of cell motility were calculated by measuring the displacement of individual cells over a 2-h period.
RESULTS
Ras-transformed MCF10A Cells Reveal Increased Contractility That Is Rho Dependent
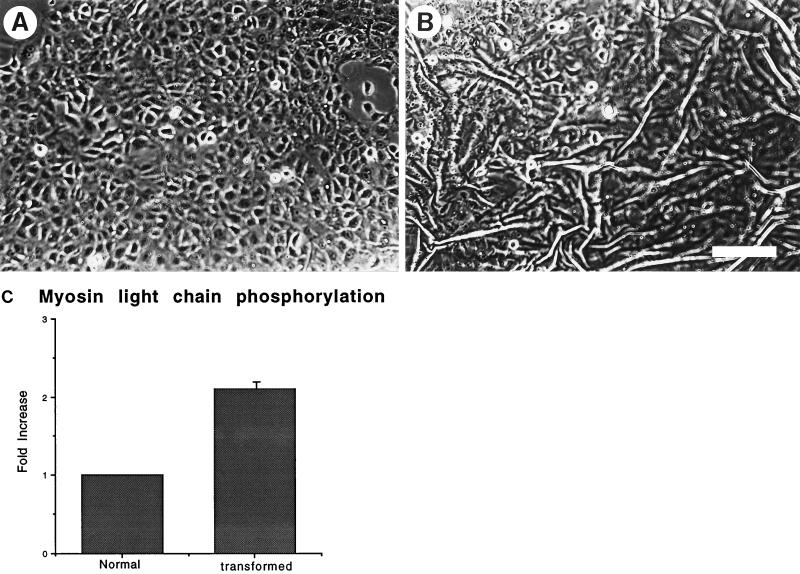
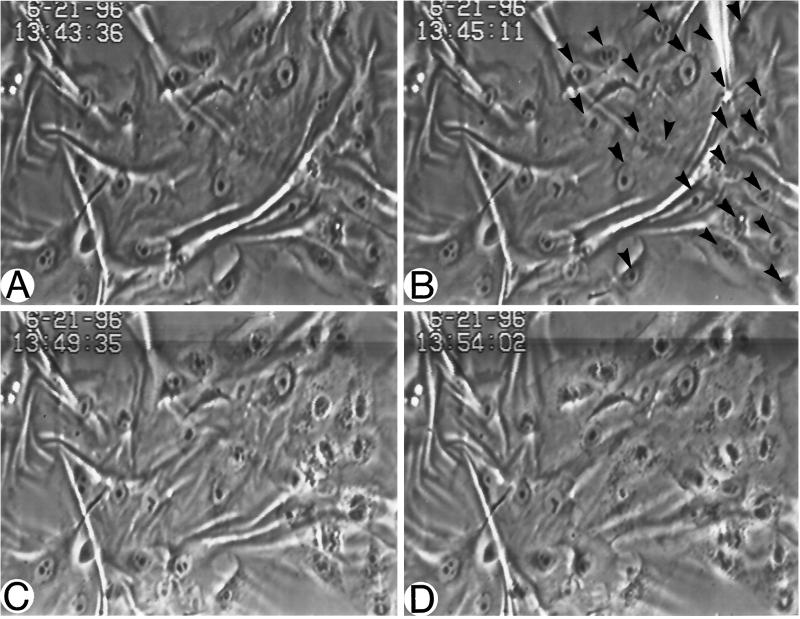
Previous work from this laboratory has demonstrated that RhoA activation is associated with increased contractility (Chrzanowska-Wodnicka and Burridge, 1996). To determine whether the Ras-transformed MCF10A cells revealed increased contractility compared with their normal counterparts, these cells were plated on flexible silicone-rubber substrates. These flexible substrates develop wrinkles in response to tension generated by cells adhering to them. Normal MCF10A cells usually developed few wrinkles (Figure 1A) and those that were developed tended to extend across large areas of confluent cells, indicating that the tension was being generated by islands of epithelia. In contrast, the Ras-transformed MCF10A cells generated many more small wrinkles in the underlying rubber substrates (Figure 1B). These wrinkles could often be seen to be generated by single cells. Because activation of Rho could lead to elevated phosphorylation of the regulatory myosin light chains (Amano et al., 1996; Chrzanowska-Wodnicka and Burridge, 1996; Kimura et al., 1996) and myosin light chain phosphorylation has been shown to be involved in the activation of contractility of smooth muscle and nonmuscle cells (Craig et al., 1983; Kolodney and Elson, 1993; Goeckeler and Wysolmerski, 1995), we examined the state of myosin light chain phosphorylation in the Ras-transformed MCF10A cells compared with the nontransformed parental cells. The Ras-transformed cells revealed elevated myosin light chain phosphorylation (Figure 1C), consistent with increased contractility and activation of Rho. To further determine whether Rho was involved in causing the tension on the underlying substratum, Ras-transformed MCF10A cells were plated on silicone rubber substrates under subconfluent conditions overnight. Cells that were actively wrinkling the rubber (Figure 2A) were microinjected with the C3 exotransferase which ADP-ribosylates and inactivates Rho (Figure 2B, arrowheads). The behavior of the cells was followed by time-lapse videomicroscopy. Within about 5 min of microinjection of the C3 exotransferase, the wrinkles started to be released (Figure 2C) and were greatly decreased in less than 10 min (Figure 2D). Similar cells injected with control proteins or buffer alone did not release the wrinkles (our unpublished observations).
Figure 1.
Ras-transformed MCF10A cells reveal increased contractility. Normal (A) and Ras-transformed (B) MCF10A cells were cultured on flexible silicone rubber substrates and visualized by phase microscopy. The Ras-transformed cells exerted sufficient tension on the underlying rubber to generate wrinkles (B). Bar, 200 μm. (C) The levels of myosin light chain phosphorylation in the normal and Ras-transformed cells were compared.
Figure 2.
Rho activity contributes to the increased contractility of Ras-transformed MCF10A cells. Ras-transformed MCF10A cells were cultured on silicone rubber substrates at high density. A–D are images of successive time points from a video recording of cells microinjected with the Rho-inhibitor C3. Cells before injection are seen in A. B shows the cells at the time of injection. Cells were injected with C3 on the right side of the field and are indicated with arrowheads. Within 4.5 min after injection (C), many of the wrinkles in the rubber have decreased. This decrease in wrinkling is more pronounced at about 9 min after injection (D).
Inhibition of Contractility Restores a More Normal Epithelial Phenotype to the Ras-transformed MCF10A Cells
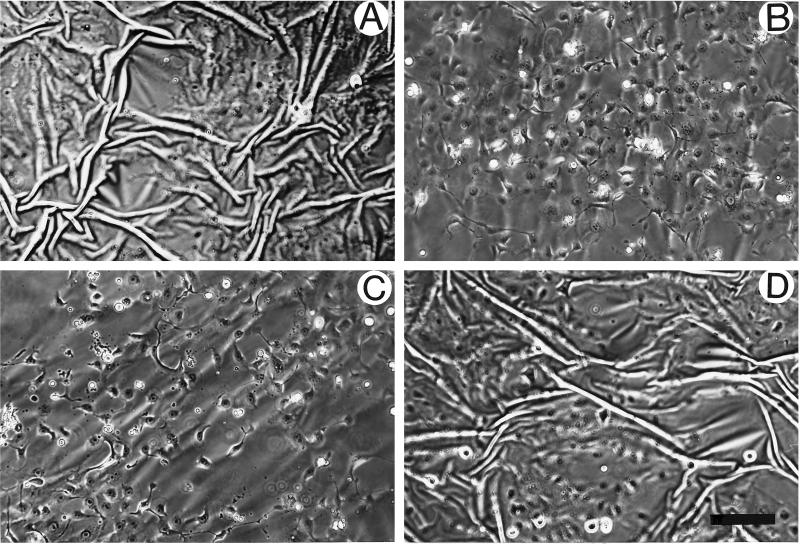
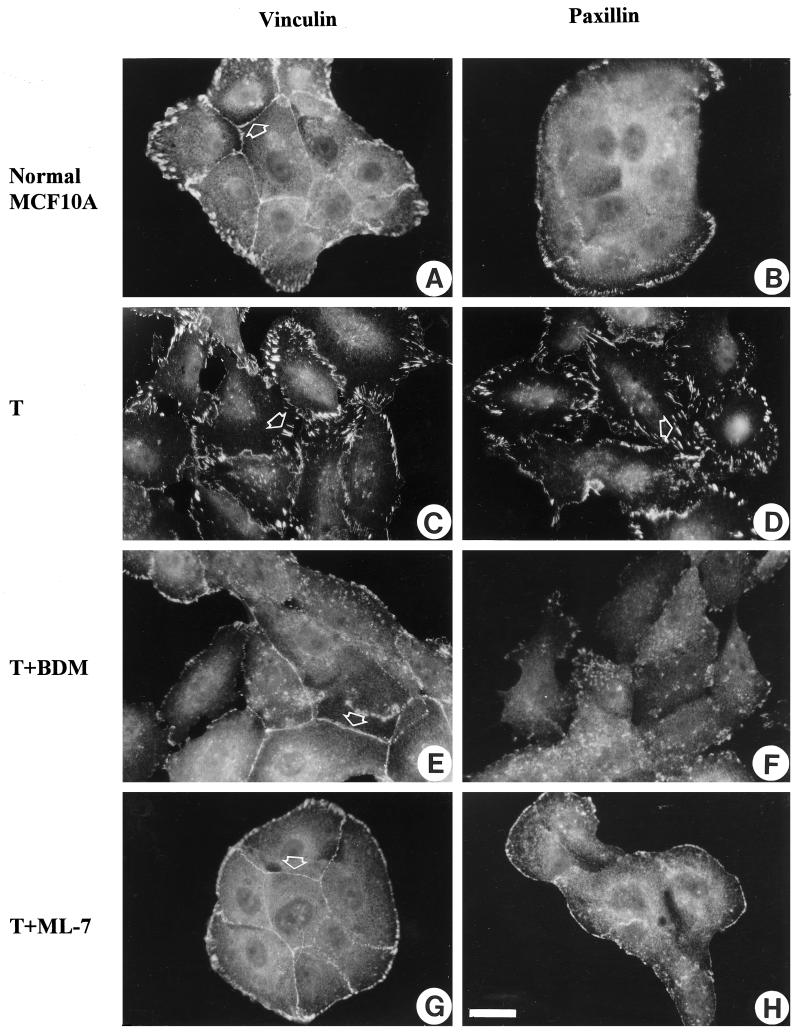
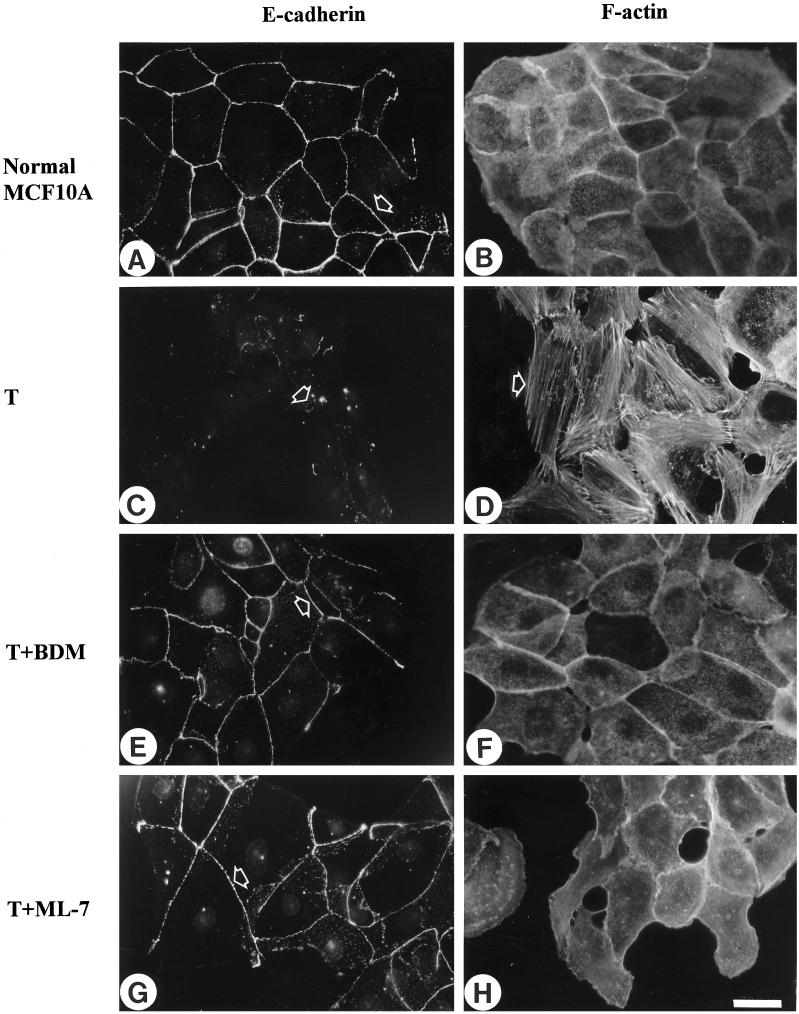
The formation of stress fibers and focal adhesions has been attributed to the development of isometric tension (Burridge, 1981; Burridge and Chrzanowska-Wodnicka, 1996; Chrzanowska-Wodnicka and Burridge, 1996). We wished to determine whether blocking contractility and the development of tension would not only inhibit the formation of stress fibers and focal adhesions in the Ras-transformed epithelial cells but would also restore more normal cell–cell junctions. Several inhibitors of contractility were used in this study, including inhibitors of MLCK (KT5926 and ML-7), an inhibitor of myosin ATPase activity and, therefore, myosin–actin interaction (BDM) and the broad spectrum kinase inhibitor 1-(5-isoquinolinylsulfonyl)-2-methylpiperazine, which has previously been shown to inhibit both fibroblast and epithelial contractility (Volberg et al., 1994; Chrzanowska-Wodnicka and Burridge, 1996). All of these inhibitors were effective in blocking the contractility of the Ras-transformed MCF10A cells in a dose-dependent manner, as judged by the release of wrinkles generated by these cells growing on silicone rubber substrates. This is shown for Ras-transformed MCF10A cells treated with either 20 mM BDM (Figure 3B) or 25 μM ML-7 (Figure 3C). If these inhibitors were removed, wrinkling of the substrate was restored within 1 h, indicating that the effects were reversible (Figure 3D). We also examined the action of these inhibitors on the organization of the actin cytoskeleton and on focal adhesions and adherens junctions (Figures 4 and 5). At concentrations that inhibited the wrinkling of the rubber substrates, the inhibitors had a striking effect on stress fibers, focal adhesions, and adherens junctions. The prominent focal adhesions of the Ras-transformed MCF10A cells were greatly decreased by the inhibitors of contractility as revealed by vinculin (Figure 4, C, E, and G) and paxillin staining (Figure 4, D, F, and H). Few, if any focal adhesions were seen in many cells, particularly within the center of colonies. Cells with free borders revealed some focal adhesions, but these were typically small and confined to the free margin of the cells. Similarly, stress fibers were abolished and the actin became organized into circumferential bundles that resembled the organization of actin in the normal MCF10A cells (Figure 5, B, D, F, and H). Moreover, the inhibitors of contractility resulted in a marked restoration of adherens junctions between adjacent cells. This was revealed by the distribution of both vinculin (Figure 4, A, C, E, and G) and E-cadherin (Figure 5, A, C, E, and G). The borders of adjacent cells became tightly defined, as judged by staining with antibodies against vinculin and E-cadherin, in contrast to the “jagged” or discontinuous staining pattern that was often noted for the untreated Ras-transformed MCF10A cells. By using [35S]methionine-labeled cells and BrdUrd incorporation, BDM and ML-7 showed no significant effects on protein synthesis or cell proliferation, although we found a significant decrease in the motility of single Ras-transformed MCF10A cells after treatment with these contractility inhibitors (our unpublished observations).
Figure 3.
BDM and ML-7 are potent inhibitors of contractility in Ras-transformed MCF10A cells. Ras-transformed MCF10A cells plated on silicone rubber substrates were either untreated (A) or treated with 20 mM BDM (B) or 25 μM ML-7 (C) for 1 h. The effects of both inhibitors were reversible. (D) Cells were exposed to 20 mM BDM for 1 h and then treated with normal medium for another 1 h. Bar, 200 μm.
Figure 4.
Inhibition of contractility abolishes focal adhesions in Ras-transformed MCF10A cells. Normal MCF10A cells (A and B) or MCF10A cells transformed (T) by oncogenic H-Ras (C–H) were untreated (C and D) or treated either with 20 mM BDM (E and F) or 25 μM ML-7 (G and H) for 2 h. Cells were fixed and stained with vinculin (A, C, E, and G) or paxillin (B, D, F, and H). (A, E, and G) Arrowheads indicate vinculin that is in adherens junctions. (C and D) Arrowheads indicate vinculin or paxillin in prominent focal adhesions within confluent regions of the culture. After treatment with contractility inhibitors, the number and size of focal adhesions in Ras-transformed MCF10A cells within confluent areas are diminished. Bar, 20 μm.
Figure 5.
Inhibition of contractility restores more normal cell–cell adherens junctions and cytoskeletal organization to Ras-transformed MCF10A cells. Normal MCF10A cells (A and B) or MCF10A cells transformed (T) by oncogenic H-Ras (C–H) were untreated (C and D) or treated either with 20 mM BDM (E and F) or 25 μM ML-7 (G and H) for 2 h. Cells were stained for E-cadherin after permeabilization (A, C, E, and G) and stained for actin (B, D, F, and H). Arrowheads in A, E, and G mark prominent adherens junctions. In contrast the arrowhead in C indicates a region of cell–cell contact in the untreated Ras-transformed cells exhibiting little E-cadherin staining. The arrowhead in D indicates actin organized in stress fibers, which are absent from the cells in B, F, and H. Bar, 20 μm.
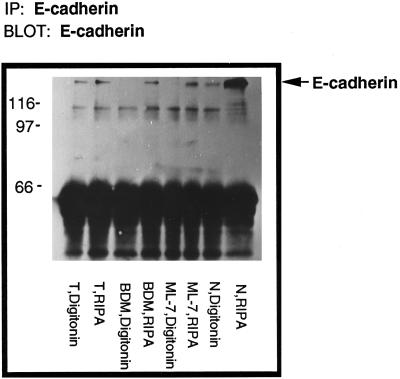
Because E-cadherin binds to the actin cytoskeleton via β-catenin and α-catenin in stabilized cell–cell adherens junctions and becomes detergent insoluble (Hinck et al., 1994; Ewing et al., 1995; Takeichi, 1995), the effect of contractility inhibitors on the detergent solubility of E-cadherin was also examined. Compared with their normal counterparts, the Ras-transformed MCF10A cells revealed that a significant fraction of E-cadherin was soluble after permeabilization with buffers containing nonionic detergents (Kinch et al., 1995; Figure 6, T digitonin). Interestingly, when the Ras-transformed MCF10A cells were treated with inhibitors of contractility, almost all the E-cadherin was driven back into the detergent-insoluble cytoskeleton-associated pool, suggesting that cell–cell adherens junctions were restored to a more normal state (Figure 6).
Figure 6.
BDM and ML-7 drive E-cadherin into the detergent-insoluble fraction of Ras-transformed MCF10A cells. Monolayers of normal MCF10A (N), Ras-transformed MCF10A (T), Ras-transformed cells treated with either 20 mM BDM (BDM) or 25 μM ML-7 (ML-7) for 2 h were sequentially extracted with 0.5% digitonin and modified RIPA buffer. Total amount of protein in each sample was equalized before immunoprecipitation. All the fractions were immunoprecipitated with anti-E-cadherin antibody and immunoblotted for E-cadherin.
Inhibition of Rho Activity Partially Restores a More Normal Epithelial Phenotype
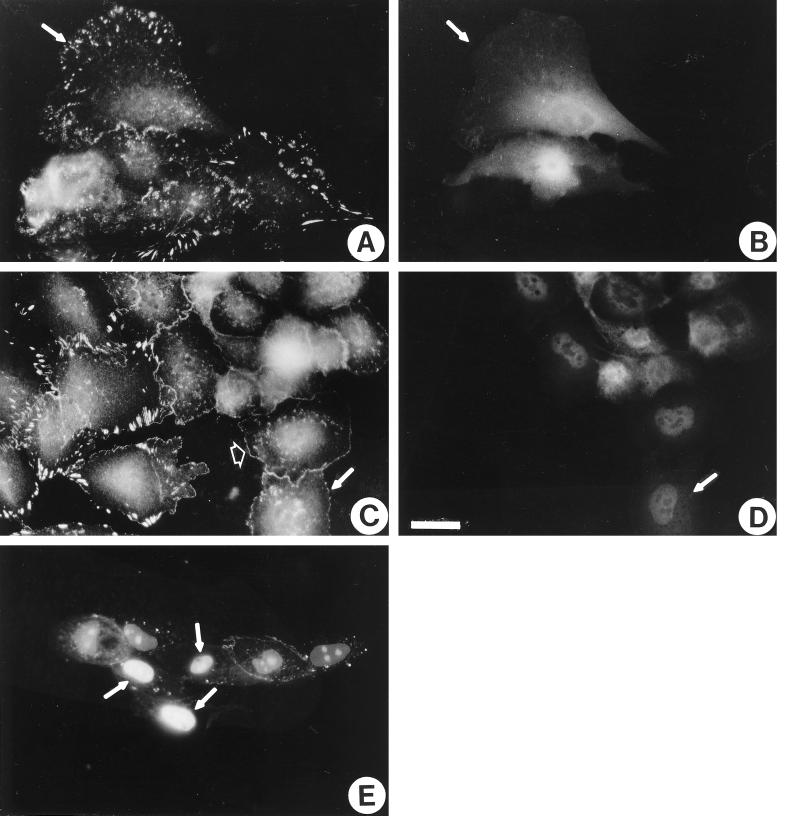
Because contractility is partly regulated by Rho and inhibition of contractility leads to restoration of adherens junctions, we were interested whether inhibition of Rho directly could restore adherens junctions in Ras-transformed cells. The activity of Rho was inhibited in the Ras-transformed MCF10A cells by microinjection of the C3 exotransferase (160 μg/ml) into these cells. By some criteria, C3 mimicked the action of the inhibitors of cell contractility. C3 caused the disassembly of focal adhesions (Figure 7C) and stress fibers (our unpublished observations). When the distribution of vinculin was examined (Figure 7C), it appeared to indicate the reformation of more normal continuous adherens junctions. However, this conclusion was not supported when the organization of E-cadherin was studied in C3-treated cells (Figure 7E). This indicated that E-cadherin-based adherens junctions had not reformed. The staining for vinculin was detected not only at sites of cell–cell contact but also along the free borders of the C3-injected cells. This organization is reminiscent of the “focal complexes” detected at the margins of lamellipodia in cells in which Rho has been inhibited by C3 treatment but in which Rac has been activated (Ridley and Hall, 1992; Nobes and Hall, 1995).
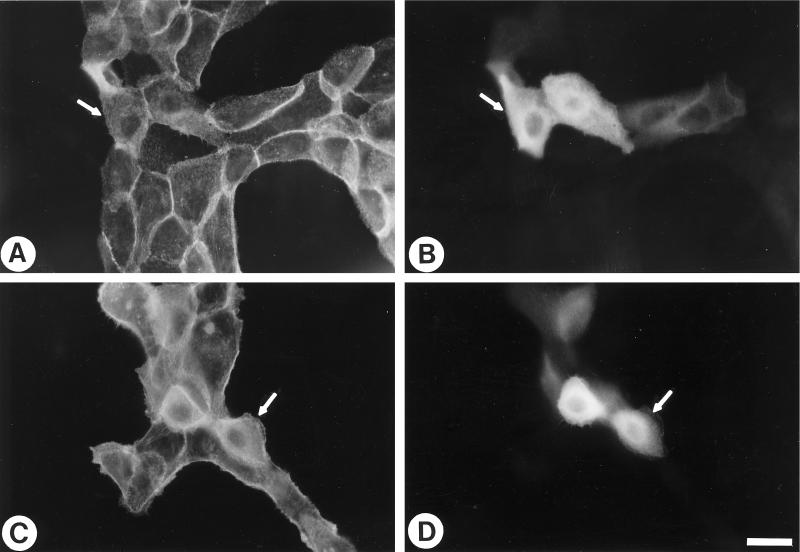
Figure 7.
Inhibition of Rho activity partially reverses the transformed phenotype of Ras-transformed MCF10A cells. Ras-transformed MCF10A cells were microinjected with coumarin-BSA (A and B) or coumarin-BSA and 160 μg/ml C3 (C and D). Twenty-five minutes after microinjection, the cells were fixed and stained for vinculin (A and C) or visualized for coumarin-BSA to reveal injected cells (B and D). Single examples of injected cells are indicated with arrows. The arrowhead in C indicates vinculin at the margin of a cell (in focal complexes). (E) Ras-transformed MCF10A cells were microinjected with 160 μg/ml C3 and propidium iodide to label the nuclei of injected cells. One hour later, cells were permeabilized, fixed, and stained with anti-E-cadherin antibody. Arrows indicate three injected cells. Note the lack of E-cadherin staining between the injected cells. Bar, 20 μm.
Because C3 has been criticized for its potential toxicity, we also explored the use of dominant negative Rho. Expression of dominant negative RhoA (19N-RhoA) in the Ras-transformed MCF10A cells led to a loss of focal adhesions as judged by vinculin staining (Figure 8D). However, vinculin was not seen in cell–cell junctions or at the margins of cells expressing dominant negative RhoA.
Figure 8.
Blocking Rho function by 19N-RhoA prevents focal adhesion formation but does not promote cell–cell adherens junction formation. Ras-transformed MCF10A cells were microinjected into the nucleus with plasmid pGreen Lantern either alone (A and B) or with a plasmid encoding 19N-RhoA (C and D). Twenty-four hours later, cells were fixed, permeabilized, and stained with antibody against vinculin (A and C). Injected cells were visualized by expression of green fluorescent protein (B and D). Arrows indicate microinjected cells. Bar, 20 μm.
Introduction of Activated Rho into Normal MCF10A Cells Is Insufficient to Induce the Ras-transformed Phenotype
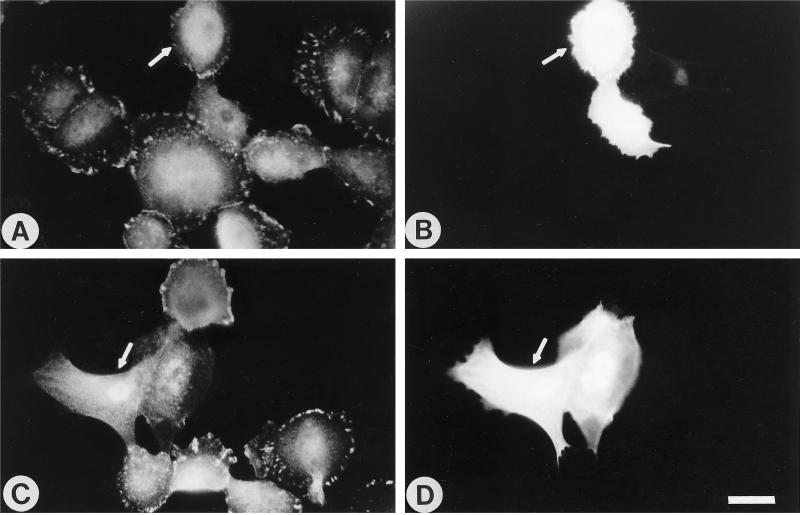
Because Rho is upstream of contractility and because many of the phenotypic characteristics of the Ras-transformed cells could be reversed by inhibition of Rho or inhibition of contractility, we asked whether introduction of constitutively active Rho into normal MCF10A cells would induce the morphological and cytoskeletal effects of the Ras-transformed phenotype. Subconfluent normal MCF10A cells were microinjected with constitutively active 14V Rho and analyzed at times from 30 min to 7 h for the development of stress fibers and other morphological characteristics consistent with the Ras-transformed phenotype. The cells injected with activated Rho often stained very brightly with phalloidin, consistent with increased actin polymerization. The cells appeared contracted (Figure 9C), whereas cells injected with buffer alone did not (Figure 9A). Frequently, the contracted cells exhibited retraction fibers that stained strongly with phalloidin. However, distinct stress fibers and focal adhesions were not detected. Within short periods of time (<30 min), 14V Rho did not affect cell–cell junctions (our unpublished observations). It was difficult to distinguish the state of adherens junctions at longer periods of time after microinjection, because the injected cells tended to round up, while remaining attached, probably reflecting their highly contracted state.
Figure 9.
Introduction of constitutively active Rho into normal MCF10A cells is not sufficient to promote the formation of stress fibers. Normal MCF10A cells were microinjected with either GST (A and B) or with 14V Rho-GST at 2.5 mg/ml (C and D) and returned to the incubator for 7 h, followed by staining for GST (B and D) and actin (A and C). Arrows indicate microinjected cells. Bar, 20 μm.
Rho Activity Is Required for Cell–Cell Adherens Junction Formation and Maintenance
The inability of activated Rho to disrupt adherens junctions in normal MCF10A cells and the fact that the C3 did not restore the adherens junctions of Ras-transformed MCF10A cells led us to ask whether Rho may actually have a role in the formation of adherens junctions. The relationship of Rho to adherens junctions was examined in cells that already had developed these structures and in cells that were in the process of assembling cell–cell junctions. Normal MCF10A cells that had been confluent for 2 d were microinjected with C3 and then examined at various time points for the state of adherens junction formation as judged by staining with an antibody against E-cadherin. Injected cells were revealed by including propidium iodide in the injection buffer to label the nuclei of the injected cells. Cells that had not been injected or had been injected with control proteins revealed the normal epithelial organization of E-cadherin (Figure 10A); E-cadherin was observed in a smooth continuous pattern at cell–cell contact sites after detergent extraction. Staining was absent from free borders. After introduction of C3, there was a deterioration of the E-cadherin staining pattern (Figure 10B). This was more obvious in cells that were first extracted with detergent (0.5% Triton X-100) before fixation and staining. The increased extraction of E-cadherin in nonionic detergents after C3 injection indicated that E-cadherin was less associated with the cytoskeleton, was failing to interact with E-cadherin molecules on the surface of adjacent cells, or both.
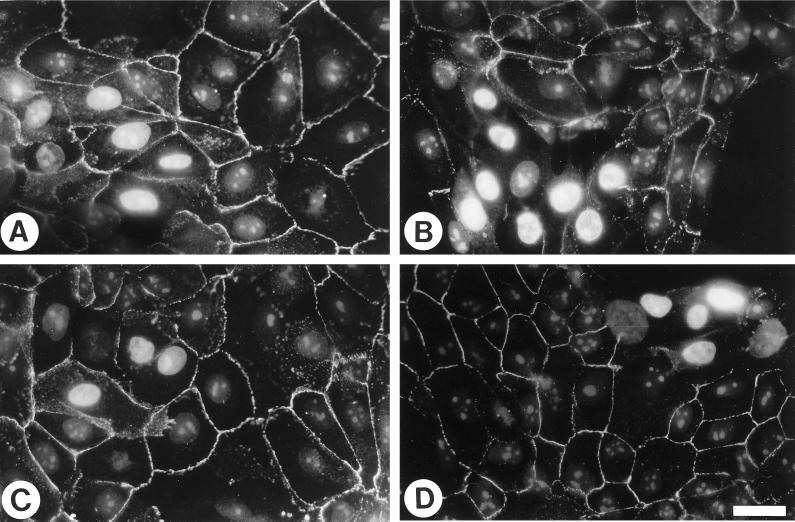
Figure 10.
Rho is required for the maintenance and formation of cell–cell adherens junctions in normal MCF10A cells. To examine the effect of C3 on existing adherens junctions, normal MCF10A cells plated for 2 d were either injected with control buffer (A) or 100 μg/ml C3 (B). To visualize the injected cells, the injection buffer contained propidium iodide to label the nuclei. After 30 min of incubation, cells were permeabilized first, then fixed, and stained with E-cadherin. To examine the effect of C3 on the formation of adherens junctions, cells were injected with buffer (C) or 100 μg/ml C3 (D), followed by addition of medium containing 4 mM ethylene glycol-bis(β-aminoethyl ether)-N,N,N′,N′-tetraacetic acid for 30 min to disrupt the adherens junctions. Ca2+ was then restored by changing the medium back to normal for another 45 min. Cells were then permeabilized, fixed, and stained for E-cadherin. Bar, 20 μm.
To examine the effect of inhibiting Rho activity on the assembly of adherens junctions, C3 was injected into normal MCF10A cells that lacked adherens junctions due to chelation of extracellular calcium. In normal circumstances, restoration of calcium stimulates the rapid assembly of adherens junctions. Cells microinjected with C3 and returned to high calcium to induce reassembly of adherens junctions were unable to assemble these structures (Figure 10D). These results lead us to conclude that activated Rho is required both for the assembly of adherens junctions and for their maintenance.
DISCUSSION
Several studies have provided evidence that Rho can be a downstream effector of activated Ras (Ridley and Hall, 1992; Khosravi-Far et al., 1995, 1996; Nobes and Hall, 1995; Joneson et al., 1996; Marshall, 1996; Taylor and Shalloway, 1996) and Rho plays an important role in Ras transformation of fibroblasts (Prendergast et al., 1995; Qiu et al., 1995b). In epithelial cells, oncogenic Ras activation of the Raf/MAPK pathway alone is not sufficient for full tumorigenic transformation, which may indicate the possible involvement of other Ras-related proteins (Oldham et al., 1996). How activated Ras leads to activation of Rho is poorly understood. It is possible that Ras may activate Rho via Ras-GTPase-activating protein and/or phosphatidylinositol 3-kinase (Settleman et al., 1992; Reif et al., 1996).
Ras-transformed MCF10A epithelial cells display the hallmarks of activated Rho. These cells exhibit prominent stress fibers and focal adhesions, whereas their normal counterparts usually lack these structures. In addition, Ras-transformed epithelia have disrupted cadherin-based adherens junctions (Kinch et al., 1995). Previously this laboratory has shown that the formation of stress fibers and focal adhesions induced by Rho results, at least in part, from a stimulation of contractility (Chrzanowska-Wodnicka and Burridge, 1996). In this work, we set out to determine whether the Ras-transformed epithelial phenotype results from Rho-mediated contractility. We have found that Ras-transformed epithelia are indeed more contractile than their nontransformed counterparts and this contractility is blocked by inhibiting Rho with C3 exotransferase. The increased contractility of the Ras-transformed epithelial cells is accompanied by an increase in the level of myosin light chain phosphorylation. Others have shown that activated Rho can elevate myosin light chain phosphorylation by two pathways involving the Rho-activated kinase termed Rho kinase. Rho kinase phosphorylates the myosin phosphatase, thereby inhibiting it (Kimura et al., 1996). The decreased phosphatase activity will elevate light chain phosphorylation. In addition, Rho kinase has been found to phosphorylate the regulatory light chain directly (Amano et al., 1996) and to induce stress fibers and focal adhesions when expressed in quiescent fibroblasts (Leung et al., 1996; Amano et al., 1997; Ishizaki et al., 1997).
Several different inhibitors of contractility that act on the myosin light chain kinase or on actin–myosin interaction restore a more normal epithelial phenotype to the Ras-transformed epithelial cells. Previous work has shown that inhibitors of contractility result in a decrease in or loss of focal adhesions and stress fibers (Volberg et al., 1994; Bershadsky et al., 1996; Chrzanowska-Wodnicka and Burridge, 1996). With the Ras-transformed epithelial cells, not only do agents that inhibit contractility lead to a loss of stress fibers and focal adhesions but they also restore a more normal organization of E-cadherin. Disruption of adherens junctions by chelation of extracellular calcium can similarly be blocked by inhibition of contractility (Citi et al., 1994; Volberg et al., 1994). In this earlier work, it was concluded that the contractility within cells contributed to the pulling apart of adherens junctions that had been weakened by removal of calcium from the cadherins that was involved in their homophilic interactions. Similarly, the increased contractility of the Ras-transformed MCF10A cells is likely to contribute to the disruption of the adherens junctions. However, Rho-induced contractility is unlikely to account for the complete Ras-transformed phenotype, because introduction of constitutively activated Rho seemed to be insufficient to mimic the Ras-transformed cytoskeletal and junctional organization, and inhibiting Rho activity in the Ras-transformed cells did not restore normal adherens junctions. Other effects of Ras transformation must contribute to the phenotype. One likely candidate is the increased tyrosine phosphorylation in these transformed cells, which may affect junction integrity (Kinch et al., 1995). A weakening of the links between E-cadherin and the cytoskeleton due to tyrosine phosphorylation may combine with the increased tension induced by activated Rho to disrupt the junctions.
We anticipated that blocking Rho function with C3 exotransferase or dominant negative 19N-RhoA would restore a more normal phenotype. This was partially true in that both C3 and 19N-RhoA caused a loss of stress fibers and focal adhesions, as has been observed with other cell types (Paterson et al., 1990). However, when we examined the state of the junctions in C3-injected cells, the situation was more complex. Although vinculin appeared to have been translocated from focal adhesions to adherens junctions (Figure 7C), we noted that the vinculin was prominent at the free margins of the cells and at sites of cell–cell contact. Examination of E-cadherin distribution revealed that the adherens junctions had not been restored (Figure 7E). The most likely interpretation of the vinculin organization in the C3-treated cells is that this reflects the “Rac” phenotype and that the vinculin is in “focal complexes” at the margins of the cells (Nobes and Hall, 1995). Focal complexes contain many of the same proteins as focal adhesions but are smaller structures found at the edge of lamellipodia or tips of filopodia. Both Rac and Rho have been shown to be downstream of Ras (Ridley et al., 1992; Khosravi-Far et al., 1995; Qiu et al., 1995a,b), and so Rac would be expected to be active in these Ras-transformed cells. Blocking Rho activation with C3 has previously been used to demonstrate Rac-induced lamellipodia, with focal complexes at the distal tips of these structures (Ridley et al., 1992; Nobes and Hall, 1995). Peripheral focal complexes staining for vinculin were not seen in cells expressing 19N-RhoA, possibly indicating that this dominant negative form of Rho may also affect the Rac pathway.
It is known that Rho controls a variety of actin-based structures in addition to focal adhesions and stress fibers. The inability of C3 to restore the epithelial organization of E-cadherin led us to ask whether Rho may also be needed for the normal assembly and maintenance of adherens junctions. In this regard, it is interesting that epitope-tagged Rho localizes to adherens junctions (Takaishi et al., 1995) and Rho has been shown previously to be involved in the regulation of tight junctions and the organization of perijunctional actin (Nusrat et al., 1995; Ridley et al., 1995). We have found that blocking Rho activity with C3 in normal epithelial cells inhibits the assembly of adherens junctions that are induced to form experimentally. Moreover, when C3 was introduced into epithelial cells that already had well formed adherens junctions, it led to the disassembly of these structures. The E-cadherin became more easily extracted in nonionic detergents, presumably indicating a decreased association with the cytoskeleton (Figure 10). How might Rho be regulating adherens junction assembly and integrity? It is unlikely that this involves the stimulation of myosin activity and contractility, because our data indicate that inhibitors of contractility enhance adherens junction formation. Rho binds to and activates at least two serine/threonine kinases, Rho kinase (Ishizaki et al., 1996; Leung et al., 1996; Matsui et al., 1996) and protein kinase N (Watanabe et al., 1996). In future work, it will be interesting to determine whether any of the adherens junction proteins are direct substrates for these kinases and whether serine/threonine phosphorylation of particular components enhances adherens junction assembly or stability. Other potential targets for the action of Rho in adherens junctions are members of the ERM (ezrin, radixin, moesin) family of proteins. These are found in adherens junctions and in other locations where actin filaments interact with the plasma membrane (Sato et al., 1992). Recent work has indicated that interaction of ERM proteins with some transmembrane proteins, such as CD44, is regulated by Rho activity (Hirao et al., 1996). This may be via phosphatidylinositol 4,5-bisphosphate, which was also shown to regulate ERM–CD44 interactions (Hirao et al., 1996). Consistent with the idea that Rho-mediated ERM protein activity may be critical for adherens junction integrity, perturbation of ERM proteins leads to disruption of cell–cell interactions (Takeuchi et al., 1994).
We conclude that in Ras-transformed epithelial cells, downstream activation of Rho contributes to the fibroblastic phenotype of these cells. The increased contractility induced by Rho leads to the development of stress fibers and focal adhesions and to the disruption of adherens junctions. However, high contractility induced by Rho appears not to be sufficient for the disruption of adherens junctions and probably other events such as elevated tyrosine phosphorylation of junctional components are required. Notably, we have found that Rho activity is necessary for both the assembly and maintenance of adherens junctions in normal cells.
Note. After submitting this work, Braga et al. (1997) demonstrated that both Rho and Rac are required for the assembly of adherens junctions in keratinocytes, consistent with our findings.
ACKNOWLEDGMENTS
We are grateful to Dr. Channing Der and Dr. Marc Symons for providing the normal and Ras-transformed MCF10A cells and the dominant negative RhoA plasmid, respectively. We thank Drs. A. Belkin, M. Chrzanowska-Wodnicka, and S. Sastry for critical reading of the manuscript and valuable discussion. This work was supported by National Institutes of Health grant GM-29860 and HL-45100 to K.B.
REFERENCES
- Aktories K, Hall A. Botulinum ADP-ribosyltransferase C3: a new tool to study low molecular weight GTP-binding proteins. Trends Pharmacol Sci. 1989;10:415–418. doi: 10.1016/0165-6147(89)90191-0. [DOI] [PubMed] [Google Scholar]
- Amano M, Chihara K, Kimura K, Fukata Y, Nakamura N, Matsuura Y, Kaibuchi K. Formation of actin stress fibers and focal adhesions enhanced by Rho-kinase. Science. 1997;275:1308–1311. doi: 10.1126/science.275.5304.1308. [DOI] [PubMed] [Google Scholar]
- Amano M, Ito M, Kimura K, Fukata Y, Chihara K, Nakano T, Matsuura Y, Kaibuchi K. Phosphorylation and activation of myosin by Rho-associated kinase (Rho-kinase) J Biol Chem. 1996;271:20246–20249. doi: 10.1074/jbc.271.34.20246. [DOI] [PubMed] [Google Scholar]
- Basolo F, Elliott J, Tait L, Chen XQ, Maloney T, Russo IH, Pauley R, Momiki S, Caamano J, Klein-Szanto AJP, Koszaika M, Russo J. Transformation of human breast epithelial cells by c-Ha-ras oncogene. Mol Carcinogen. 1991;4:25–35. doi: 10.1002/mc.2940040106. [DOI] [PubMed] [Google Scholar]
- Behrens JQ, Mareel MM, Van Roy FM, Birchmeier W. Dissection tumor cell invasion: epithelial cells acquire invasive properties after the loss of Uvomorulin-mediated cell-cell adhesion. J Cell Biol. 1989;108:2435–2447. doi: 10.1083/jcb.108.6.2435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bershadsky A, Chausovsky A, Becker E, Lyubimova A, Geiger B. Involvement of microtubles in the control of adhesion-dependent signal transduction. Curr Biol. 1996;6:1279–1289. doi: 10.1016/s0960-9822(02)70714-8. [DOI] [PubMed] [Google Scholar]
- Birchmeier W, Weidner KM, Behrens J. Molecular mechanisms leading to loss of differentiation and gain of invasiveness in epithelial cells. J Cell Sci Suppl. 1993;17:159–164. doi: 10.1242/jcs.1993.supplement_17.23. [DOI] [PubMed] [Google Scholar]
- Braga VMM, Machesky LM, Hall A, Hotchin NA. The small GTPases Rho and Rac are required for the establishment of cadherin-dependent cell-cell contacts. J Cell Biol. 1997;137(6):1421–1431. doi: 10.1083/jcb.137.6.1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burridge K. Are stress fiber contractile? Nature. 1981;294:691–692. doi: 10.1038/294691a0. [DOI] [PubMed] [Google Scholar]
- Burridge K, Chrzanowska-Wodnicka M. Focal adhesions, contractility and signaling. Annu Rev Cell Dev Biol. 1996;12:463–519. doi: 10.1146/annurev.cellbio.12.1.463. [DOI] [PubMed] [Google Scholar]
- Chrzanowska-Wodnicka M, Burridge K. Rho-stimulated contractility drives the formation of stress fibers and focal adhesions. J Cell Biol. 1996;133:1403–1415. doi: 10.1083/jcb.133.6.1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Citi S, Volberg T, Bershadsky AD, Denisenko N, Geiger B. Cytoskeletal involvement in the modulation of cell-cell junctions by the protein kinase inhibitor H-7. J Cell Sci. 1994;107:683–692. [PubMed] [Google Scholar]
- Clark GC, Der CJ. Aberrant function of the ras signal transduction pathway in human breast cancer. Breast Cancer Res Treat. 1995;35:133–144. doi: 10.1007/BF00694753. [DOI] [PubMed] [Google Scholar]
- Craig R, Smith R, Kendrick-Jones J. Light-chain phosphorylation controls the conformation of vertebrate non-muscle and smooth muscle myosin molecules. Nature. 1983;302:436–439. doi: 10.1038/302436a0. [DOI] [PubMed] [Google Scholar]
- Ewing CM, Ru N, Morton RA, Robinson JC, Wheelock MJ, Johnson KR, Barrett JC, Isaacs WB. Chromosome 5 suppresses tumorigenicity of PC3 prostate cancer cells: correlation with re-expression of alpha-catenin and restoration of E-cadherin function. Cancer Res. 1995;55:4813–4817. [PubMed] [Google Scholar]
- Glukhova MA, Frid MG, Shekhonin BV, Balabanov YV, Koteliansky VE. Expression of fibronectin variants in vascular and visceral smooth muscle. Dev Biol. 1990;141:193–202. doi: 10.1016/0012-1606(90)90114-x. [DOI] [PubMed] [Google Scholar]
- Goeckeler ZM, Wysolmerski RB. Myosin light chain kinase-regulated endothelial cell contraction: the relationship between isometric tension, actin polymerization, and myosin phosphorylation. J Cell Biol. 1995;130:613–627. doi: 10.1083/jcb.130.3.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graessmann A, Graessmann M, Mueller C. Microinjection of early SV40 DNA fragments and T antigen. Methods in Enzymology. Vol. 65. New York: Academic Press; 1980. pp. 816–825. [DOI] [PubMed] [Google Scholar]
- Hall A. Small GTP-binding proteins and the regulation of the actin cytoskeleton. Annu Rev Cell Biol. 1994;10:31–54. doi: 10.1146/annurev.cb.10.110194.000335. [DOI] [PubMed] [Google Scholar]
- Harris AK, Wild P, Stopak D. Silicone rubber substrata: a new wrinkle in the study of cell locomotion. Science. 1980;208:177–179. doi: 10.1126/science.6987736. [DOI] [PubMed] [Google Scholar]
- Hermann C, Wray J, Travers F, Barman T. The effect of 2,3-butanedione monoxime on myosin and myofibrillar ATPase. An example of an uncompetitive inhibitor. Biochemistry. 1993;31:12227–12232. doi: 10.1021/bi00163a036. [DOI] [PubMed] [Google Scholar]
- Higuchi H, Takemori S. Butanedione monoxime suppresses contraction and ATPase activity of rabbit skeletal muscle. J Biochem. 1989;105:638–643. doi: 10.1093/oxfordjournals.jbchem.a122717. [DOI] [PubMed] [Google Scholar]
- Hinck L, Nathke IS, Papkoff J, Nelson WJ. Dynamics of cadherin/catenin complex formation: novel protein interactions and pathways of complex assembly. J Cell Biol. 1994;125:1327–1340. doi: 10.1083/jcb.125.6.1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirao M, Sato N, Kondo T, Yonemura S, Monden M, Sasaki T, Takai Y, Tsukita S, Tsukita S. Regulation mechanism of ERM (Ezrin/Radixin/Moesin) protein/plasma membrane association: possible involvement of phosphatidylinositol turnover and Rho-dependent signalling pathway. J Cell Biol. 1996;135:37–51. doi: 10.1083/jcb.135.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishizaki T, Maekawa M, Fujisawa K, Okawa K, Iwamatsu A, Fujita A, Watanabe N, Saito Y, Kakizuka A, Morii N, Narumiya S. The small GTP-binding protein Rho binds to and activates a 160-kDa ser/thr protein kinase homologous myotonic dystrophy kinase. EMBO J. 1996;15:1885–1893. [PMC free article] [PubMed] [Google Scholar]
- Ishizaki T, Naito M, Fujisawa K, Maekawa M, Watanabe N, Saito Y, Narumiya S. P160ROCK, a Rho-associated coiled-coil forming protein kinase, works downstream of Rho and induces focal adhesions. FEBS Lett. 1997;404:118–124. doi: 10.1016/s0014-5793(97)00107-5. [DOI] [PubMed] [Google Scholar]
- Joneson T, White MA, Wigler MH, Bar-Sagi D. Stimulation of membrane ruffling and MAP kinase activation by distinct effectors of ras. Science. 1996;271:810–812. doi: 10.1126/science.271.5250.810. [DOI] [PubMed] [Google Scholar]
- Khosravi-Far R, Solski PA, Clark GJ, Kinch MS, Der CJ. Activation of Rac1, RhoA, and mitogen-activated protein kinases is required for ras transformation. Mol Cell Biol. 1995;15:6443–6453. doi: 10.1128/mcb.15.11.6443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khosravi-Far R, White MA, Westwick JK, Solski PA, Chrzanowska-Wodnicka M, Aelst LV, Wigler MH, Der CJ. Oncogenic ras activation of raf/mitogen-activated protein kinase-independent pathways is sufficient to cause tumorigenic transformation. Mol Cell Biol. 1996;16:3923–3933. doi: 10.1128/mcb.16.7.3923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura K, Ito M, Amano M, Chihara K, Fukata Y, Nakafuku M, Yamamori B, Feng J, Nakano T, Okawa K, Iwamatsu A, Kaibuchi K. Regulation of myosin phosphatase by rho and rho-associated kinase (rho-kinase) Science. 1996;273:245–248. doi: 10.1126/science.273.5272.245. [DOI] [PubMed] [Google Scholar]
- Kinch MS, Clark G, Der CJ, Burridge K. Tyrosine phosphorylation regulates the adhesions of ras-transformed breast epithelia. J Cell Biol. 1995;130:461–471. doi: 10.1083/jcb.130.2.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolodney MS, Elson EL. Correlation of myosin light chain phosphorylation with isometric contraction of fibroblasts. J Biol Chem. 1993;268:23850–23855. [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Leung T, Chen XQ, Manser E, Lim L. The P160RhoA-binding kinase ROK alpha is a member of a kinase family and is involved in the reorganization of the cytoskeleton. Mol Cell Biol. 1996;16:5313–5327. doi: 10.1128/mcb.16.10.5313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall CJ. Ras effectors. Curr Opin Cell Biol. 1996;8:197–204. doi: 10.1016/s0955-0674(96)80066-4. [DOI] [PubMed] [Google Scholar]
- Matsui T, Amano M, Yamamoto T, Chihara K, Nakafuku M, Ito M, Nakano T, Okawa K, Iwamatsu A, Kaibuchi K. Rho-associated kinase, a novel serine/threonine kinase, as a putative target for small GTP binding protein Rho. EMBO J. 1996;15:2208–2216. [PMC free article] [PubMed] [Google Scholar]
- McKillop DFA, Fortune NS, Ranatunga KW, Geeves MA. The influence of 2,3-butanedione 2-monoxime (BDM) on the interaction between actin and myosin in solution and in skinned muscle fibers. J Muscle Res Cell Motil. 1994;15:309–318. doi: 10.1007/BF00123483. [DOI] [PubMed] [Google Scholar]
- Nakanishi S, Yamada K, Iwanashi K, Kuroda K, Kase H. KT5926, a potent and selective inhibitor of myosin light chain kinase. Mol Pharmacol. 1990;37:482–488. [PubMed] [Google Scholar]
- Nobes CD, Hall A. Rho, rac and cdc42 GTPases regulate the assembly of stress fibers, lamellipodia, and filopodia. Cell. 1995;81:53–62. doi: 10.1016/0092-8674(95)90370-4. [DOI] [PubMed] [Google Scholar]
- Nusrat A, Giry M, Turner JR, Colgan SP, Parkos CA, Carnes D, Lemichez E, Boquet P, Madara JL. Rho protein regulates tight junctions and perijunctional actin organization in polarized epithelia. Proc Natl Acad Sci USA. 1995;92:10629–10633. doi: 10.1073/pnas.92.23.10629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldham SM, Clark GJ, Gangarosa LM, Coffey RJ, Jr, Der CJ. Activation of the raf-1/MAP kinase cascade is not sufficient for ras transformation of RIE-1 epithelial cells. Proc Natl Acad Sci USA. 1996;93:6924–6928. doi: 10.1073/pnas.93.14.6924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osterman A, Arner A, Malmqvist U. Effects of 2,3-butanedione monoxime on activation of contraction and crossbridge kinetics in intact and chemically skinned smooth muscle fibers from guinea pig taenia coli. J Muscle Res Cell Motil. 1993;14:186–194. doi: 10.1007/BF00115453. [DOI] [PubMed] [Google Scholar]
- Paterson HF, Self AJ, Garrett MD, Just I, Aktories K, Hall A. Microinjection of recombinant p21rho induces rapid changes in cell morphology. J Cell Biol. 1990;111:1001–1007. doi: 10.1083/jcb.111.3.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prendergast GC, Khosravi-Far R, Kurzawa H, Lebowitz PF, Der CJ. Critical role of RhoB in cell transformation by oncogenic Ras. Oncogene. 1995;10:2289–2296. [PubMed] [Google Scholar]
- Qiu R, Chen J, Kim D, McCormick F, Symons M. An essential role for Rac in Ras transformation. Nature. 1995a;374:457–459. doi: 10.1038/374457a0. [DOI] [PubMed] [Google Scholar]
- Qiu R, Chen J, McCormick F, Symons M. A role for rho in ras transformation. Proc Natl Acad Sci USA. 1995b;92:11781–11785. doi: 10.1073/pnas.92.25.11781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reif K, Nobes CD, Thomas G, Hall A, Cantrell DA. Phosphatidylinositol 3-kinase signals activate a selective subset of rac/rho-dependent effector pathways. Curr Biol. 1996;6:1445–1455. doi: 10.1016/s0960-9822(96)00749-x. [DOI] [PubMed] [Google Scholar]
- Ridley AJ, Comoglio PM, Hall A. Regulation of scatter factor/hepatocyte growth factor responses by ras, rac, and rho in MDCK cells. Mol Cell Biol. 1995;15:1110–1122. doi: 10.1128/mcb.15.2.1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridley AJ, Hall A. The small GTP-binding protein rho regulates the assembly of focal adhesions and actin stress fibers in response to growth factors. Cell. 1992;70:389–399. doi: 10.1016/0092-8674(92)90163-7. [DOI] [PubMed] [Google Scholar]
- Ridley AJ, Paterson HF, Johnston CL, Diekmann D, Hall A. The small GTP-binding protein rac regulates growth factor-induced membrane ruffling. Cell. 1992;70:401–410. doi: 10.1016/0092-8674(92)90164-8. [DOI] [PubMed] [Google Scholar]
- Roberts TM. A signal chain of events. Nature. 1992;360:534–535. doi: 10.1038/360534a0. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Viciana P, Warne PH, Dhand R, Vanhaesebroeck B, Gout I, Fry MJ, Waterfield MD, Downward J. Phosphatidylinositol-3-OH kinase as a direct target of ras. Nature. 1994;370:527–532. doi: 10.1038/370527a0. [DOI] [PubMed] [Google Scholar]
- Saitoh M, Ishikawa T, Matsushima S, Naka M, Hidaka H. Selective inhibition of catalytic activity of smooth muscle myosin light chain kinase. J Biol Chem. 1987;262:7796–7801. [PubMed] [Google Scholar]
- Sato N, Funayama N, Nagafuchi A, Yonemura S, Tsukita Sa, Tsukita Sh. A gene family consisting of ezrin, radixin, and moesin. Its specific localization at actin filament/plasma membrane association sites. J Cell Sci. 1992;103:131–143. doi: 10.1242/jcs.103.1.131. [DOI] [PubMed] [Google Scholar]
- Settleman J, Albright CF, Foster LC, Weinberg RA. Association between GTPase activators for rho and ras families. Nature. 1992;359:153–154. doi: 10.1038/359153a0. [DOI] [PubMed] [Google Scholar]
- Slamon DJ, deKernion JB, Verma IM, Cline MJ. Expression of cellular oncogenes in human malignancies. Science. 1984;224:256–262. doi: 10.1126/science.6538699. [DOI] [PubMed] [Google Scholar]
- Soule HD, Maliney TM, Wolman SR, Peterson WD, Jr, Brenz R, McGrath CM, Russo J, Pauley RJ, Jones RF, Brooks SC. Isolation and characterization of a spontaneously immortalized human breast epithelial cell line, MCF10. Cancer Res. 1990;50:6075–6086. [PubMed] [Google Scholar]
- Spaargaren M, Bischoff JR. Identification of the guanine nucleotide dissociation stimulator for Ral as a putative effector molecule of R-ras, H-ras, K-ras, and Rap. Proc Natl Acad Sci USA. 1994;91:12609–12613. doi: 10.1073/pnas.91.26.12609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takaishi K, Sasaki T, Kameyama T, Tsukita Sa, Takai Y. Translocation of activated Rho from the cytoplasm to membrane ruffling area, cell-cell adhesion sites and cleavage furrows. Oncogene. 1995;11:39–48. [PubMed] [Google Scholar]
- Takeichi M. Morphogenetic roles of classic cadherins. Curr Opin Cell Biol. 1995;7:619–627. doi: 10.1016/0955-0674(95)80102-2. [DOI] [PubMed] [Google Scholar]
- Takeuchi K, Sato N, Kasahara H, Funayama N, Nagafuchi A, Yonemura S, Tsukita S, Tsukita S. Perturbation of cell adhesion and microvilli formation by antisense oligonucleotides to ERM family members. J Cell Biol. 1994;125:1371–1384. doi: 10.1083/jcb.125.6.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor SJ, Shalloway D. Cell cycle-dependent activation of Ras. Curr Biol. 1996;6:1621–1627. doi: 10.1016/s0960-9822(02)70785-9. [DOI] [PubMed] [Google Scholar]
- Thor A, Ohuchi N, Hand PH, Callahan R, Weeks MO, Theillet C, Lidereau R, Escot C, Page DL, Vilasi V, Scholm J. Biology of disease: ras gene alterations and enhanced levels of ras p21 expression in a spectrum of benign and malignant human mammary tumors. Lab Invest. 1986;6:603–615. [PubMed] [Google Scholar]
- Turner CE, Glenney JR, Burridge K. Paxillin: a new vinculin-binding protein present in focal adhesions. J Cell Biol. 1990;111:1059–1069. doi: 10.1083/jcb.111.3.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volberg T, Geiger B, Citi S, Bershadsky AD. Effect of protein kinase inhibitor H-7 on the contractility, integrity, and membrane anchorage of the microfilament system. Cell Motil Cytoskeleton. 1994;29:321–338. doi: 10.1002/cm.970290405. [DOI] [PubMed] [Google Scholar]
- Watanabe G, Saito Y, Madaule P, Ishizaki T, Fujisawa K, Morri N, Mukai H, Ono Y, Kakizuka A, Narumiya S. Protein kinase N (PKN) and PKN-related protein rhophilin as targets of small GTPase rho. Science. 1996;271:645–648. doi: 10.1126/science.271.5249.645. [DOI] [PubMed] [Google Scholar]
- Zhong, C., Kinch, M., and Burridge, K. (1996). Rho-stimulated contractility contributes to the fibroblastic phenotype of Ras-transformed epithelial cells. Mol. Biol. Cell 7, (suppl), 525a. [DOI] [PMC free article] [PubMed]