Abstract
The P-8 proteoglycolipid complex (P-8 PGLC), an amastigote antigen of L. pifanoi, has been demonstrated to induce protection in mouse models, as well as to induce Tc1/Th1-like cellular responses in American cutaneous leishmaniasis patients. Because the immunization with P-8 PGLC in the murine model does not appear to be genetically restricted, we have studied the reactivity of the P-8 PGLC in L. infantum infected dogs. In this study, it is shown that PBMC from experimentally infected dogs (asymptomatic, oligosymptomatic) significantly proliferated in response to soluble leishmanial antigen (SLA) or the P-8 PGLC. Further, quantification of the gene expression induced by the stimulation with P-8 in asymptomatically infected dogs showed an up-regulation of IFN-γ and TNF-α, which were three to four-fold higher than that induced by soluble Leishmania antigen (SLA). While no measurable induction of IL-10 was observed, low levels of IL-4 mRNA were observed in response to both P-8 and SLA antigens. Thus, our studies establish that P-8 is recognized by infected canines and elicits a potentially curative/protective Th1-like immune response. The identification of Leishmania antigens that elicit appropriate immune responses across different host species (humans, canine) and disease manifestations (cutaneous or visceral) could be an advantage in generating a general vaccine for leishmaniasis.
Keywords: Leishmania, canine, cytokine, immunogenicity, P-8
1. Introduction
Zoonotic visceral leishmaniasis (ZVL), caused by Leishmania infantum/Leishmania chagasi is a progressive wasting disease of dogs and humans that is often fatal if untreated. The disease is endemic in parts of the Mediterranean basin, Asia, Central and South America, where it is widespread. ZVL disease incidence is increasing, representing a serious public health problem [1].
Dogs are the main domestic reservoirs for the parasite, which is transmitted from dogs to humans by phlebotomine sand flies (Lutzomyia or Phlebotomus spp.). The use of molecular techniques (such as the polymerase chain reaction, PCR) has determined that prevalence of infected dogs in endemic areas can be high [2]; further, there exists a high level of subclinical infection in canines in endemic areas that can, at least in part, have infective capability [3,4]. Chemotherapy is able to reduce or eliminate clinical symptoms [5–8] but does not consistently eliminate infectivity to sand flies, indicating the difficulty of achieving parasitological cure in dogs [9]. This situation might explain the failure of treatment and culling of seropositive animals as strategies to control ZVL [10]. Since dogs constitute the major source of parasites transmitted to humans, successful canine immunization could significantly reduce the incidence of human visceral leishmaniasis. This epidemiological feature has promoted the development of vaccines against canine leishmaniasis as an important tool and a cost-effective strategy for controlling visceral leishmaniasis caused by L. infantum/L. chagasi [11].
The use of a laboratory canine model of leishmaniasis allows the longitudinal study of the immune response to infection and has been used to evaluate both infection treatment and vaccine efficacy. The canine model has helped to improve understanding of the natural history of leishmaniasis and the underlying events occurring during the prepatent/asymptomatic stage of the disease [12]. Experimentally infected animals can also be used to study the immunogenic capability of defined leishmanial antigens. Vaccines against canine leishmaniasis must be safe and should induce strong and long-lasting cell mediated immunity [13]. Many Leishmania vaccine candidates have been identified in murine models [14], but conclusions obtained in this model might not be directly applicable to dogs. To date, only a small number of Leishmania proteins have been investigated in the canine model of visceral leishmaniasis. The fucose-mannose ligand [15], protein Q [16], purified excreted/secreted antigens from L. infantum [17], H1 and HASPB1 [18], TSA-LmsT11-LeIF trifusion protein [19], Leishmania homologue of receptors for activated C kinase (LACK) [20] and cysteine proteinases [21,22] have been used in vaccine trials with variable success in providing protection to dogs against a parasite challenge.
However, further vaccine studies in the canine reservoir, examining the immunogenicity and protective capacity of different antigens, and the identification of adequate adjuvants are still required; also, the efficacy of these vaccines in blocking transmission should be considered. Among the many Leishmania antigens isolated and characterized, molecules that are specific to or up-regulated in the amastigote stage are relevant for study since this stage is the progressive form found in the infected mammalian host. The P-8 antigen is a proteoglycolipid-complex associated with the external surface membrane of the amastigote [23]. Immunization with P-8 has been shown to induce significant cross- protection against infection with either L. amazonensis or L. pifanoi in mice with different H-2 haplotypes [24,25]. Further, when the P-8 proteoglycolipid complex was tested in vitro using PBMCs from American cutaneous leishmaniasis patients infected with L. (Viannia) braziliensis, it was found to induce Tc1/Th1-like responsiveness in CD8+ and CD4+ T cells, respectively [25–27].
Further studies are required to evaluate the potential of the P-8 PGLC as vaccine candidate against canine visceral leishmaniasis. In this paper we report the antigenicity of P-8 PGLC in the experimental canine model of visceral leishmaniasis. We have evaluated the lymphoproliferative and cytokine responses of PBMCs and determined serum levels of P-8 specific subclass antibodies in these animals.
2. Materials and Methods
2.1. Animals
Two different groups of dogs were used in this study, which was conducted at two sites (Colombia, Spain) and utilized either outbred or inbred animals, respectively.
Group 1 Mixed-breed, 4–5 month old female dogs were obtained from the Centro de Zoonosis of Cali, Colombia. All these animals were collected in areas not endemic for visceral leishmaniasis. Animals were maintained in CIDEIM’s kennel near Cali where neither Leishmania transmission nor sand fly activity has been recorded. Dogs were housed and handled according to local and federal regulations, following international and Colombian guidelines (Law 84/89). The research protocols were approved by the animal care and use committee at CIDEIM. Prior to the experimental infection the animals were quarantined, subjected to treatment for common intestinal parasites (Triantelm®, Intervet; Rondel®, Virbac labs.; Ivomec®, Merial) and vaccinated against frequent dog pathogens (Galaxy DH2PPiL, Wyeth-Fort Dodge Labs; Novicac Rabia, Intervet). Dogs were negative for anti-leishmanial antibody by ELISA.
The Leishmania chagasi strain (MCAN/COL/98/CATIRE) used for all experimental infections was isolated previously from a polysymptomatic dog. Metacyclic promastigotes (104–105) obtained from experimentally infected Lutzomyia longipalpis were inoculated either intravenously or intradermally in the ear, which lead to different clinical presentations varying from asymptomatic to polysymptomatic dogs. Dogs representative of each clinical group (asymptomatic, oligosymptomatic and polysymptomatic) [28,29] were examined in terms of their lymphoproliferative responses to concanavalin A (ConA), soluble leishmanial antigen (SLA) and P-8. In group 1, 24 dogs were studied: 3 asymptomatic, 8 oligosymptomatic, 6 polysymptomatic and 7 non-infected control dogs.
Group 2 consisted of beagle dogs ranging in age from 24–30 months, purchased to a local breeder. These animals were kept at the facilities of the National Center for Microbiology in Majadahonda (Madrid). All dogs received routine vaccinations. These dogs were experimentally infected by intravenous (iv) inoculation with 108 L. infantum promastigotes (MCAN/ES/98/LLM-722). Prior to experimental infection, both the antibody and cellular immune responses to soluble leishmanial antigen (SLA) were confirmed to be negative in all animals. After infection, dogs were monitored for 1.5 years, and a complete characterization of the animals was carried out, including haematological, parasitological and immunological aspects. The animals showed a variable evolution of disease and presented with a range of different clinical conditions: asymptomatic, oligosymptomatic or polysymptomatic, as previously defined [28,29]. These animals were kept and handled in the facilities under veterinary care, following ethical guidelines in accordance with national and European Union regulations. The various analyses employed a total of 14 dogs representative of each clinical group: 5 asymptomatic, 4 oligosymptomatic and 5 polysymptomatic dogs. In addition, 2 non-infected) dogs were employed as controls. Control animals were negative for anti-leishmanial antibody, lymphoproliferative response to SLA and parasitological studies performed.
It should be noted that the species employed at the two sites, L. infantum and L. chagasi, are considered indistinguishable [30] and cause similar disease in all animal models (mouse, dog, hamster). The approach of this study was to compare the immune responses of animals in different defined clinical states, as determined using standardized observational methods for canine visceral leishmaniasis [28,29] in both inbred and outbred canine subjects and to determine if the responses were consistent across breeds based on clinical status.
2.2 P-8 purification
The P-8 antigen was purified as described previously [23,25]. Briefly, surface membranes of axenically cultured L. pifanoi amastigotes were isolated using nitrogen cavitation and differential centrifugation. Membrane proteins were solubilized with 1% decanoyl-N-methylglucamide (Mega-10, Sigma). The solubilized membranes were reduced and alkylated and then fractionated on Sephadex G-25 (Pharmacia, Piscataway, NJ) gel exclusion chromatography to remove the excess of reagents. The sample was then diluted with PBS and subjected to P-8 immunoaffinity chromatography. The various chromatography columns used in the purification of P-8 PGLC were performed with solutions made using endotoxin-free water; further, column eluents are assessed for endotoxin using a LAL-assay before and after each use. The fractions eluted from the affinity column were assessed for protein by measuring the absorbance at 280 nm and 320 nm; protein fractions were pooled, concentrated and then stored at −70°C. P-8 preparations had <0.05ng/mL of endotoxin using a standard assay for LPS.
2.3. ELISA tests
Maxisorp microtiter plates (Nunc, Roskilde, Denmark) were coated overnight with 250 ng of P-8 or 1000 ng of SLA in carbonate buffer (15 mM Na2CO3, 28mM NaHCO3, pH 9.6) and blocked with 200 μl of blocking buffer (PBS containing 1 BSA% and 0.1% Tween 20, pH 7.4) for 1 hour at room temperature. Plates were washed three times using PBS (pH 7.4) containing 0.01% Tween 20; then 100 μL of sera diluted 1:100 in dilution buffer (PBS containing 0.1 BSA% and 0.1% Tween 20) were incubated for 30 minutes. Plates were subsequently washed and then incubated for 30 minutes with either horseradish peroxidase (HRP)-conjugated sheep anti-canine IgG (Bethyl Laboratories, Montgomery TX; 1:2500), anti- canine IgG1 (Bethyl Laboratories; 1:800), or anti- canine IgG2 (Bethyl Laboratories; 1:2500). Immune complexes were revealed with 2,2′-azino-bis (3-ethylbenzthioline-6-sulfonic acid) diluted in phosphate-citrate buffer (ABTS; (Sigma-Aldrich, St. Louis MO, USA); absorbance values (405 nm) were read using a microelisa reader (Benchmark, Biorad, USA). Results are expressed as mean values of duplicate samples.
2.4. Cell isolation
PBMC were isolated from heparinized blood samples using standard Ficoll-hypaque gradient centrifugation (Lymphocyte Isolation Solution, RAFER, Spain). Erythrocytes were removed after treatment with ACK erythrocyte lysis buffer (0.15 M NH4Cl, 1.0 mM KHCO3, 0.1 mM Na2 EDTA, pH 7.4). PBMC were washed twice in PBS (0.15 M NaCl, 0.05 M Na2PO4, pH 7.4), counted and adjusted up to 2.5×106 cells/ml in complete medium (RPMI 1640 supplemented with 100 U/ml penicillin, 100 μg/ml streptomycin, 2 mM L-glutamine, 25 mM HEPES and 10% heat-inactivated fetal calf serum).
2.5. Cell-proliferation assay
Lymphocyte proliferation was evaluated in 96 well plates using 2 × 105 PBMCs stimulated for 6 days with either P-8 PGLC (5 μg/mL) or SLA (10 μg/mL) or for 3 days with 0.2 μg of concanavalin A (ConA) in a final volume of 200μl per well. Cells were pulsed for the final 8 h of culture with 1 μCi of methyl-3H thymidine and counted in a scintillation counter. Results were expressed as stimulation index (net counts per min of stimulated cells/net counts per min of unstimulated cells).
2.6. PBMC stimulation and RNA extraction
PBMC (5×106 cells/well) from infected animals (3 asymptomatic, 4 oligosymptomatic and 4 polysymptomatic dogs) and controls (2 non-infected) were incubated with either complete media (unstimulated), 10 μg/ml SLA, 5 μg/ml P-8 or 10 μg/ml ConA in a 6-well plate (Nunc, Roskilde, Denmark) at 37°C for 24 h in 5% CO2 atmosphere. Total RNA was extracted using a RNA extraction kit according to the manufacturer’s recommendations (SV-total RNA isolation system, Promega, Madison, USA). The concentration of total RNA was determined spectrophotometrically (ND-1000 UV-V Spectrophotometer, NanoDrop Technology, USA) and each sample was adjusted to a final working concentration of 5 ng per μl with nuclease-free H2O and stored at −80ºC until use. RNA was free of genomic DNA as determined by PCR.
2.7. Cytokine mRNA quantification by real-time QRT-PCR
Due to the lack of specific antibodies for various canine cytokines, comparative studies of cytokine levels utilized the determination of mRNA rather than protein levels. Although variation between cytokines (in the efficiency of transcription, translation and post-transcriptional processing of cytokine mRNA) does not allow a direct correlation between mRNA and protein levels, for comparisons for a single cytokine (between groups), it would be expected that relative mRNA levels would, in fact, reflect overall relative protein levels. Quantitative Real-Time-PCR analyses were performed by using an Applied Biosystems ABI Prism 7000 DNA sequence detection system (PE Applied Biosystems, Foster City, CA, USA). Reverse transcription (RT) and PCR amplifications were carried out in a single well by using TaqMan PCR Core Reagent Kit (PE Applied Biosystems). The amplification conditions, the gene-specific primers and probes are as described previously [31]. Each 25 μl reaction mixture contained 50 ng of template RNA, 5 mM MgCl2, 2.5 mM dNTPs, 0.625 U of AmpliTaq Gold, 6.25 U of MultiScribe reverse transcriptase, 10 U of RNase inhibitor, each primer at 200 nM and 100 nM of TaqMan probe. Parallel reactions were performed for the detection of canine IL-10, TNF-α, IFN-γ, IL-4 and IL-18 transcripts from PBMC.
When the response to SLA was examined in different groups of infected dogs, the amount of the target RNA relative to the control gene β-actin, is presented as the 2−ΔCT value. Data presented are the averaged mRNA levels of PBMC from three asymptomatic, four oligosymptomatic, four polysymptomatic and two control non-infected dogs. To examine the effect of specific antigen stimulation (P-8 or SLA) in asymptomatic infected animals, the cytokine expression of individual animals (V-25, V-38 and V-27) is shown. For these determinations, the β-actin gene was used as the control gene (for calculation of ΔCt) and unstimulated samples for each animal were used as the calibrator (for calculation of ΔΔCt). Differences in gene transcription between stimulated and unstimulated cells are expressed as n-fold difference relative to the calibrator. Basal expression of IL-4 was not detected but IL-4 mRNA gene expression was measurable after stimulation with SLA, P-8 or ConA. Therefore, for calculation of ΔΔCt in IL-4 gene expression, a Ct value of 35 was assigned to the undetected unstimulated PBMCs.
2.8. Statistical analysis
The effect of antigen stimulation on cytokine gene expression of PBMC from different groups of infected dogs was analyzed using the Mann-Whitney U-test. Logarithmic transformation was performed for all mRNA levels before the data were analyzed. Significance was set at P ≤ 0.05.
3. Results
3.1. Antibody responses to leishmanial antigens
The sera from Leishmania experimentally infected dogs (Group 2: asymptomatic, oligosymptomatic and polysymptomatic) collected at 2, 6, 11 and 16 months post-infection, were examined. Serum antibody levels to P-8 and SLA measured by ELISA are shown in Figure 1. In addition, 2 control non-infected dogs (data not shown) were examined; these animals were negative for anti-leishmanial antibody, as well as in their lymphoproliferative responses to leishmanial antigens.
Figure 1.
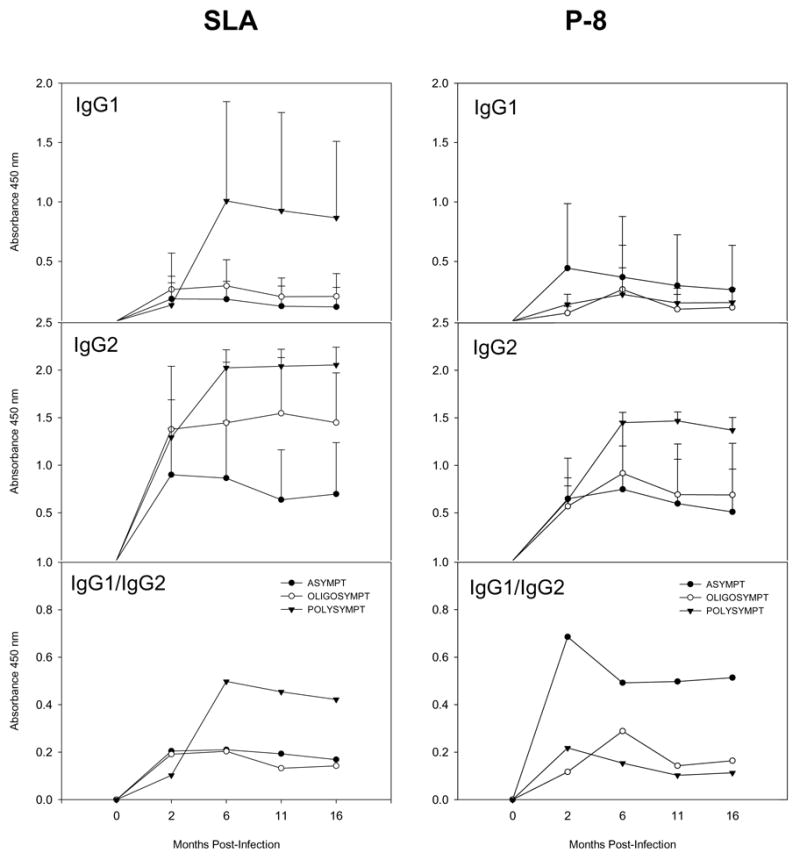
Antibody responses in infected dogs (Group 2) to soluble leishmanianial antigens (SLA) and the P-8 proteoglycolipid complex (P-8 PGLC). Shown are the antibody responses A) IgG1; B) IgG2 and C) Ratio: IgG1/IgG2 of: ▼ — ▼, polysymptomatic; ○— ○, oligosymptomatic; and ●— ● asymptomatic infected dogs at the indicated times post-infection. The antibody responses were examined in 5 asymptomatic, 3 oligosymptomatic and 3 polysymptomatic dogs (P-8) and for 5 asymptomatic, 4 oligosymptomatic and 5 polysymptomatic dogs for SLA antigen.
SLA-specific IgG antibodies were found in all dogs screened. However, polysymptomatic and oligosymptomatic dogs, in general, showed higher levels than asymptomatic animals; these results may reflect the relative parasite burdens present. Interestingly, high levels of IgG1 antibodies against SLA (Figure 1) were found only in polysymptomatic animals (2 of 3) and appeared to correlate with the progression of disease. In addition, the levels of IgG2 antibodies to SLA trended to be lower in asymptomatic dogs; however, overall the IgG1/IgG2 ratios were higher for the polysymptomatic animals. Although the correlation of IgG subclass responses has been controversial [32–37], these data are consistent with previous studies [35,36]. The pattern of P-8-specific IgG antibody response (Figure 1) with time post-infection was similar to that observed to SLA in the dogs studied; the IgG2 subclass predominated in the sera of the infected animals. Notably although not statistically significant, asymptomatic dogs appeared to develop lower specific P-8- IgG2 antibody responses. Overall, these results indicated an ongoing response to both SLA and the P-8 PGLC during infection.
3.2. Lymphoproliferative response to leishmanial antigens
The antibody responses to P-8 as well as SLA lead to the question of the nature of this response at the cellular level. Hence, the proliferative responses of dogs at 6, 8 and 10 months post-infection were examined and related to their clinical status (asymptomatic, oligosymptomatic and polysymptomatic); results were comparable across the time points. As shown in Figure 2, at 10 months post-infection, PBMC from asymptomatic and oligosymptomatic infected dogs showed antigen specific proliferation to SLA and P-8. In contrast, lymphocyte proliferation in presence of SLA and P-8 was at basal levels in most of the polysymptomatic dogs. Stimulation with ConA produced high lymphoproliferative responses in both asymptomatic and oligosymptomatic infected dogs; however, as they progressed to the polysymptomatic stage, this response decreased along with the response to leishmanial antigens (SLA). As previously observed, the pattern of cellular response to leishmanial antigens appears to be increasingly impaired with progression of illness in canine visceral leishmaniasis [13,38].
Figure 2.
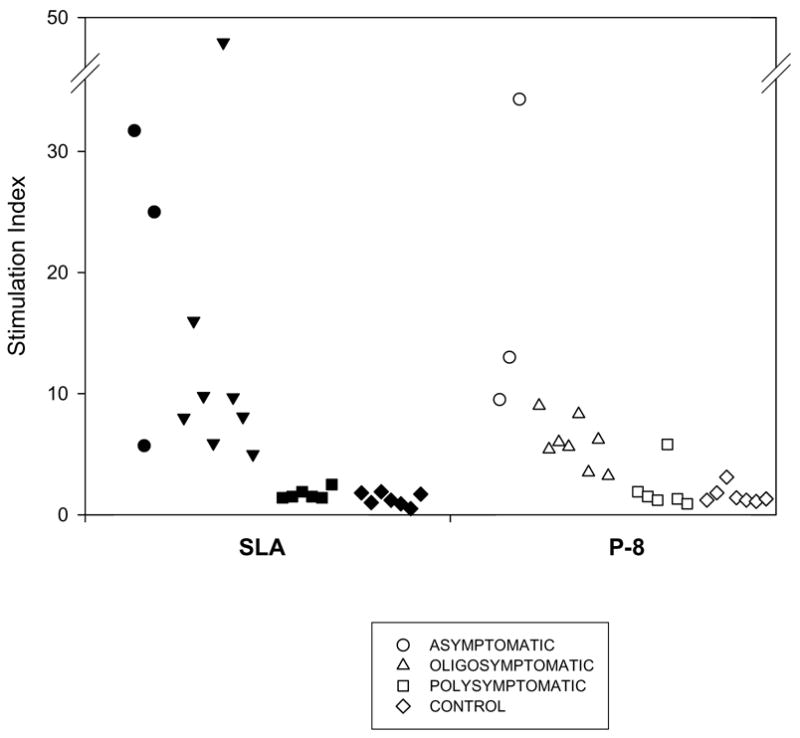
Proliferative responses of infected dogs with different clinical outcomes. Shown are the proliferative responses at 10 month post-infection of PBMCs from polysymptomatic (6; ■, □), oligosymptomatic (8; ▲, △) and asymptomatic (3; ●, ○) infected dogs and control (7; ◆, ◇) dogs at 10 months post-infection to either soluble leishmanial antigens (SLA) or the P-8 proteoglycolipid complex. The responses observed at 10 months post-infection were similar to those observed for these animals at both 6 and 8 months post-infection.
3.3. Expression of cytokine transcripts in SLA-stimulated PBMC from L. infantum experimentally infected dogs
The study of the cytokine pattern elicited after 24 hours in SLA- stimulated PBMC from dogs with different status of the illness showed that cytokine expression of asymptomatic and oligosymptomatic dogs were, in general, similar. Asymptomatic (P= 0.05) and oligosymptomatic (P= 0.021) dogs significantly up-regulated IFN-γ in comparison to unstimulated PBMC (Figure 3). An up-regulation of TNF-α mRNA in the SLA- stimulated PBMC also occurred in asymptomatic and oligosymptomatic dogs; however, this was only significant in the oligosymptomatic group (P= 0.021). After stimulation with SLA, asymptomatic and oligosymptomatic animals showed a tendency to decrease the IL-10 transcripts below basal levels of the control cells; however, this was not significant. The expression of IL-4 by unstimulated PBMC was below the detection limit. Further, although antigen stimulation produced detectable IL-4 in PBMCs from infected animals; the levels of IL-4 did not differ significantly among clinical groups (data not shown). In the case of IL-18, elevated levels were observed in polysymptomatic animals; however these differences did not appear to be statistically significant (Figure 3).
Figure 3.
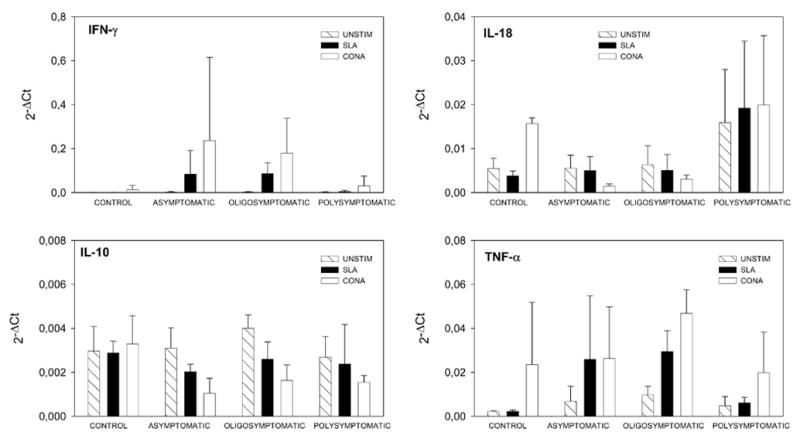
Cytokine gene expression in PBMC from L. infantum experimentally infected dogs and healthy control dogs. PBMC were stimulated with soluble leishmanial antigen (SLA) from L. infantum and ConA. RNA isolation and QRT-PCR were performed after 24 hours of stimulation as described in Material and Methods. QRT-PCR data were analysed by using the 2−ΔCt method. The average of the mRNA levels of PBMC from three asymptomatic, four oligosymptomatic, four polysymptomatic and two control non-infected dogs are presented. Striped bars represent unstimulated PBMCs, black bars represent SLA and open bars represent ConA. A) IFNγ; B) TNF-α; C) IL-10; D) IL-18. Cytokine responses were examined in: 3 asymptomatic, 4 oligosymptomatic, 4 polysymptomatic dogs and 2 control dogs.
3.4. Expression of cytokine transcripts in P-8 antigen-stimulated PBMC from L. infantum experimentally infected and control dogs
To investigate whether the L. pifanoi P-8 antigen could stimulate cells from L. infantum infected dogs to produce specific cytokines, we examined the induction of mRNA for cytokines known to be relevant to the pathogenesis or control of visceral leishmaniasis. The capability of P-8 to induce a specific cytokine profile was studied in asymptomatic dogs (Group 2) and compared to the cytokine profile induced by SLA, as asymptomatically infected dogs presented with a distinct but preferential Th1-like response. This provided an internal control (standard) for this response and allowed a comparison of the mixture of leishmanial antigens to that of the isolated P-8 proteoglycolipid complex. PBMC from three asymptomatic dogs were stimulated with SLA or P-8 antigen for 24 hours and the levels of cytokine mRNA were determined.
The stimulation with leishmanial antigens produced an up-regulation of the IFN-γ mRNA abundance in PBMC from asymptomatic animals (Figure 4). The incubation with P-8 induced 168.9 to 442.6- fold increase in the IFN-γ gene expression compared to unstimulated cells. However, the IFN-γ transcript abundance of SLA- stimulated cells increased 34.8 to 138.1- fold (Figure 3). Therefore, L. pifanoi P-8 antigen induced a 3 to 4-fold higher level of IFN-γ expression than the L. infantum soluble antigen (SLA) in the asymptomatic L. infantum infected dogs.
Figure 4.
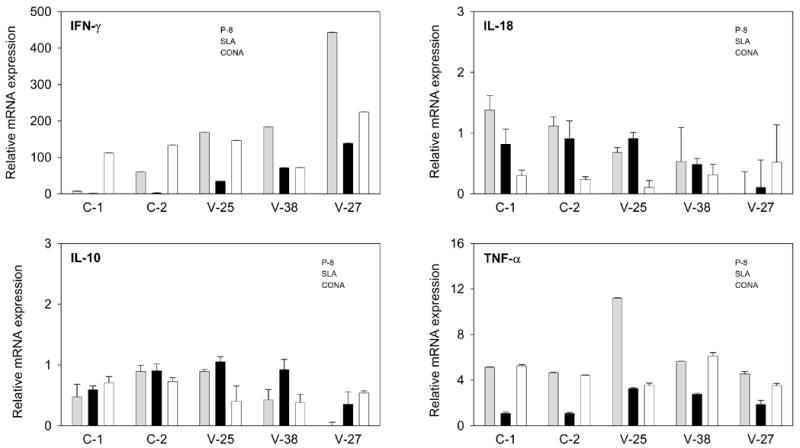
Cytokine gene expression in PBMC from asymptomatic L. infantum infected dogs and healthy control dogs (Group 2) after stimulation with P-8 antigen from L. pifanoi. QRT-PCR data were analysed by using the 2−ΔΔCt method. Differences in gene transcription after stimulation are expressed as n-fold difference relative to the calibrator (unstimulated cells). Shown are the cytokine expression (IFN-γ; TNF-α; IL-10; IL-18) for each infected animal (V-25, V-38 and V-27) as well as controls (C-1, C-2). Grey bars represent results from P-8 stimulation, black bars represent SLA stimulation and open bars represent ConA stimulation. Standard deviations are indicated and range from 0.0001 to 0.300.
P-8 antigen also produced an up-regulation of the TNF-α gene expression in PBMC from asymptomatic dogs (4.6–11.2-fold increase) (Figure 4). This enhancement of expression was again higher than that observed for SLA stimulation of these PBMC (1.9–3.3- fold increase). Surprisingly, healthy (non-infected) dogs presented an unspecific mRNA production after P-8 stimulation (4.4 to 5.0- fold increase), whereas no TNF-α expression was observed in response to incubation with SLA (1.1- fold increase in both C-1 and C-2).
In general IL-10 and IL-18 mRNA transcript abundances in PBMCs stimulated with either SLA or P-8 were similar to those found for unstimulated cells. IL-4 gene expression levels (data not shown) after stimulation with P-8 showed 7.4–65.8- fold increases compared to unstimulated PBMCs; these were comparable to those found for SLA stimulation (14.8–61.8- fold increase).
4. Discussion
The ability of vaccine candidate molecules to elicit an immune response in infected hosts is indicative of antigen presentation during infection and might be considered to be a minimal requirement for any vaccine candidate molecule. If such molecules also elicit a Th1-like cytokine response (IFN-γ, TNF-α) during an ongoing infection, it might be indicative of protection. In the present study, P-8 PGLC has been found to elicit such a response. This is similar to observations for other vaccine candidates which induce the expansion of Th1-type T-cells producing interferon (IFN)-γ cytokine in vaccinated dogs [17] and are protective against infection.
The stimulation with the leishmanial antigens P-8 PGLC and SLA produced an up-regulation of the IFN-γ mRNA abundance in PBMC from asymptomatic animals. The increase in IFN-γ transcripts in SLA- stimulated PBMC, contrasts with the lack of IFN-γ induction in polysymptomatic dogs. Experimentally L. infantum infected beagle dogs that exhibited mild clinical signs (oligosymptomatic) showed strong PBMC proliferation, IFN-γ production and mRNA expression in response to stimulation with L. infantum antigen (SLA) [39]. While PBMC from naturally infected polysymptomatic dogs still proliferate in response to SLA, this is minimal and IFN-γ production in response to Leishmania antigen was completely abolished [39]. In the canine model, the expression of IFN-γ from PBMCs correlates with disease resistance/asymptomatic status in non-vaccinated animals [17,40]; further, IFN-γ is observed to increase and correlate with protection in vaccinated dogs [17,22,41]. In humans, IFN-γ has been shown to be a mediator of resistance to the parasites because of its ability to induce killing of the parasite by macrophages; further, IFN-γ levels increase after treatment [42]. These results are consistent with murine model studies where IFN-γ has been shown to be associated with control of cutaneous [43] and visceral leishmaniasis [44].
The effect on IL-4 gene expression after stimulation with P-8 and SLA was low in asymptomatic dogs. In the current study, we have found IL-4 mRNA transcription after SLA stimulation in all the stages of the infection. Although previous reports have found that early detection of IL-4 appeared to be correlated with disease progression [13,38], the role of this cytokine in canine VL remains controversial [38]. Further studies are necessary in order to determine the role of IL-4 in susceptibility. In asymptomatic, experimentally infected dogs we have found that values of IL-10 transcription after P-8 or SLA stimulation were near to those of unstimulated dogs. IL-10 has been related to progressive disease in human visceral leishmaniasis [45] and was shown to play a role in susceptibility to VL in hamster and murine models [46]. However in dogs experimentally infected with L. infantum, IL-10 transcripts were have not been generally observed except occasionally late in infection [47]. Furthermore, IL-10 mRNA accumulation in infected tissues of naturally infected dogs, including those with severe disease, was comparable with that of uninfected control dogs [38]. Thus, IL-10 does not seem to have a predominant negative immunoregulatory role in canine visceral leishmaniasis, as has been previously described in Indian kalaazar [48,49] or the murine model [50].
Abundance of IL-18 mRNA transcripts was similar in stimulated and unstimulated cells, suggesting that this cytokine may not to be necessary for the development of a protective cellular response in canine leishmaniasis. IL-18 alone cannot induce Th1 differentiation, but does facilitate/accelerate it [51]. Although IL-18 was initially identified as a potent IFN-γ-inducing factor in T cells and NK cells [52], recently it has been shown to enhance both innate immunity and promote both Th1- and Th2- driven immune responses, leading to protective immunity in murine VL [53–55]. However, in canine leishmaniasis, a negative relationship between IL-18 expression and clinical status was found in bone marrow aspirates from naturally infected dogs [38]. In the current study, no clear associations could be established between dog resistance or susceptibility to VL and IL-18 expression levels.
P-8 antigen elicited a higher expression of TNF-α in PBMC from asymptomatic dogs compared to SLA. Surprisingly, healthy non-infected dogs also produced TNF-α mRNA after P-8 stimulation. However, it has been observed that P-8 PGLC is capable of inducing TNF-α and IL-1 (but not IL-10, IL-8) from uninfected murine macrophages (Whitaker, Colmenares and McMahon-Pratt, unpublished data). Consequently, although the precise cell population has not been determined here, it is possible that a similar induction within canine PBMCs has occurred. As observed in the current study, higher TNF-α levels have been observed during asymptomatic infection in the canine model [40,56,57]. These higher levels of TNF-α are consistent with murine leishmaniasis model studies of both cutaneous and visceral diseases [58,59], where TNF-α has been shown to enhance the effects of IFN-γ in mediating parasite killing. These results suggest that TNF-α is important in the resolution of canine visceral leishmaniasis and may be useful for evaluation of potential vaccine candidate molecules.
While it is generally accepted that cellular, rather than humoral immunity, plays an important role in host defense against leishmaniasis a few studies have shown that antibodies are instrumental in providing resistance to many intracellular pathogens [60]. The actual contribution of antibodies to the protective response in leishmaniasis is still a question hotly debated. In murine leishmaniasis a negative regulatory role at the level of the macrophage has been associated with IL-10 production [61]; however, recent studies have suggested a potentially positive role for the modulation of dendritic cell function [62]. Further, specific subclasses of immunoglobulin (IgG2a, IgG1) are considered to correlate with Th1-like and Th2-like responses. It is known that IgG2a elicitation is regulated by IL-12 induced production of IFN-γ [63], and IgG1 by IL-4 [64]. However, this dichotomy is not absolute and it has been observed that IL-12 can induce enhanced IgG1 production [65–67], suggesting the possibility that IgG1 and IgG2a could work in tandem rather than acting antagonistically. Given the lack of regulation by IL-10 in the canine model, the role of antibody responses in mounting successful protective immune response against visceral leishmaniasis requires further investigation. The challenge ahead in terms of vaccine development is to understand how the various parts of the immune system work collectively [68] in the canine model.
In this study, we have examined the antigenicity of the antigen P-8 in canine visceral leishmaniasis. The higher lymphoproliferative response in asymptomatic dogs in comparison to symptomatic dogs and level of IFN-γ expression induced by P-8 in asymptomatic dogs, suggest that this antigen may be involved in protection and thus, represents a potential vaccine candidate for the control of canine leishmaniasis.
Acknowledgments
This work was supported by the Ministerio de Ciencia y Tecnologia grant AGL 2000–0284 and a Grant from the NIH (AI27811). E. Carrillo was supported by a FPI fellowship from the Ministerio de Ciencia y Tecnologia. S. Ahmed was supported by fellowships from the Howard Hughes Medical Institute and Yale Downs International Health Fellowship. J. Moreno holds a “Ramon y Cajal” contract from MEC.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gramiccia M, Gradoni L. The current status of zoonotic leishmaniases and approaches to disease control. Int J Parasitol. 2005;35(11–12):1169–1180. doi: 10.1016/j.ijpara.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 2.Solano-Gallego L, Morell P, Arboix M, Alberola J, Ferrer L. Prevalence of Leishmania infantum infection in dogs living in an area of canine leishmaniasis endemicity using PCR on several tissues and serology. J Clin Microbiol. 2001;39(2):560–563. doi: 10.1128/JCM.39.2.560-563.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Travi BL, Tabares CJ, Cadena H, Ferro C, Osorio Y. Canine visceral leishmaniasis in Colombia: relationship between clinical and parasitologic status and infectivity for sand flies. Am J Trop Med Hyg. 2001;64(3–4):119–124. doi: 10.4269/ajtmh.2001.64.119. [DOI] [PubMed] [Google Scholar]
- 4.Courtenay O, Quinnell RJ, Garcez LM, Shaw JJ, Dye C. Infectiousness in a cohort of brazilian dogs: why culling fails to control visceral leishmaniasis in areas of high transmission. J Infect Dis. 2002;186(9):1314–1320. doi: 10.1086/344312. [DOI] [PubMed] [Google Scholar]
- 5.Moreno J, Nieto J, Chamizo C, et al. The immune response and PBMC subsets in canine visceral leishmaniasis before, and after, chemotherapy. Vet Immunol Immunopathol. 1999;71(3–4):181–195. doi: 10.1016/s0165-2427(99)00096-3. [DOI] [PubMed] [Google Scholar]
- 6.Baneth G, Shaw SE. Chemotherapy of canine leishmaniosis. Vet Parasitol. 2002;106(4):315–324. doi: 10.1016/s0304-4017(02)00115-2. [DOI] [PubMed] [Google Scholar]
- 7.Pasa S, Toz SO, Voyvoda H, Ozbel Y. Clinical and serological follow-up in dogs with visceral leishmaniosis treated with allopurinol and sodium stibogluconate. Vet Parasitol. 2005;128(3–4):243–249. doi: 10.1016/j.vetpar.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 8.Pennisi MG, De Majo M, Masucci M, Britti D, Vitale F, Del Maso R. Efficacy of the treatment of dogs with leishmaniosis with a combination of metronidazole and spiramycin. Vet Rec. 2005;156(11):346–349. doi: 10.1136/vr.156.11.346. [DOI] [PubMed] [Google Scholar]
- 9.Alvar J, Molina R, San Andres M, et al. Canine leishmaniasis: clinical, parasitological and entomological follow-up after chemotherapy. Ann Trop Med Parasitol. 1994;88(4):371–378. doi: 10.1080/00034983.1994.11812879. [DOI] [PubMed] [Google Scholar]
- 10.Alvar J, Canavate C, Molina R, Moreno J, Nieto J. Canine leishmaniasis. 2004;57:1–88. doi: 10.1016/S0065-308X(04)57001-X. [DOI] [PubMed] [Google Scholar]
- 11.Tesh RB. Control of zoonotic visceral leishmaniasis: is it time to change strategies? Am J Trop Med Hyg. 1995;52(3):287–292. doi: 10.4269/ajtmh.1995.52.287. [DOI] [PubMed] [Google Scholar]
- 12.Moreno J, Alvar J. Canine leishmaniasis: epidemiological risk and the experimental model. 2002;18(9):399–405. doi: 10.1016/s1471-4922(02)02347-4. [DOI] [PubMed] [Google Scholar]
- 13.Gradoni L. An update on antileishmanial vaccine candidates and prospects for a canine Leishmania vaccine. Vet Parasitol. 2001;100(1–2):87–103. doi: 10.1016/s0304-4017(01)00486-1. [DOI] [PubMed] [Google Scholar]
- 14.Kubar J, Fragaki K. Recombinant DNA-derived leishmania proteins: from the laboratory to the field. Lancet Infect Dis. 2005;5(2):107–114. doi: 10.1016/S1473-3099(05)01282-X. [DOI] [PubMed] [Google Scholar]
- 15.da Silva VO, Borja-Cabrera GP, Correia Pontes NN, et al. A phase III trial of efficacy of the FML-vaccine against canine kala-azar in an endemic area of Brazil (Sao Goncalo do Amaranto, RN) Vaccine. 2000;19(9–10):1082–1092. doi: 10.1016/s0264-410x(00)00339-x. [DOI] [PubMed] [Google Scholar]
- 16.Molano I, Alonso MG, Miron C, et al. A Leishmania infantum multi-component antigenic protein mixed with live BCG confers protection to dogs experimentally infected with L. infantum. Vet Immunol Immunopathol. 2003;92(1–2):1–13. doi: 10.1016/s0165-2427(02)00315-x. [DOI] [PubMed] [Google Scholar]
- 17.Lemesre JL, Holzmuller P, Cavaleyra M, Goncalves RB, Hottin G, Papierok G. Protection against experimental visceral leishmaniasis infection in dogs immunized with purified excreted secreted antigens of Leishmania infantum promastigotes. Vaccine. 2005;23(22):2825–2840. doi: 10.1016/j.vaccine.2004.11.061. [DOI] [PubMed] [Google Scholar]
- 18.Moreno J, Nieto J, Masina S, et al. Immunization with H1 and HASPB1 leishmania proteins protect dogs against experimental canine leishmaniasis. Third World Congress on Leishmaniasis; Palermo, Italy. 2005. p. 178. [Google Scholar]
- 19.Gradoni L, Foglia Manzillo V, Pagano A, et al. Failure of a multi-subunit recombinant leishmanial vaccine (MML) to protect dogs from Leishmania infantum infection and to prevent disease progression in infected animals. Vaccine. 2005;23(45):5245–5251. doi: 10.1016/j.vaccine.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 20.Ramiro MJ, Zarate JJ, Hanke T, et al. Protection in dogs against visceral leishmaniasis caused by Leishmania infantum is achieved by immunization with a heterologous prime-boost regime using DNA and vaccinia recombinant vectors expressing LACK. Vaccine. 2003;21(19–20):2474–2484. doi: 10.1016/s0264-410x(03)00032-x. [DOI] [PubMed] [Google Scholar]
- 21.Rafati S, Nakhaee A, Taheri T, et al. Protective vaccination against experimental canine visceral leishmaniasis using a combination of DNA and protein immunization with cysteine proteinases type I and II of L. infantum. Vaccine. 2005;23(28):3716–3725. doi: 10.1016/j.vaccine.2005.02.009. [DOI] [PubMed] [Google Scholar]
- 22.Poot J, Spreeuwenberg K, Sanderson SJ, et al. Vaccination with a preparation based on recombinant cysteine peptidases and canine IL-12 does not protect dogs from infection with Leishmania infantum. Vaccine. 2006;24(14):2460–2468. doi: 10.1016/j.vaccine.2005.12.039. [DOI] [PubMed] [Google Scholar]
- 23.Colmenares M, Tiemeyer M, Kima P, McMahon-Pratt D. Biochemical and biological characterization of the protective Leishmania pifanoi amastigote antigen P-8. Infect Immun. 2001;69(11):6776–6784. doi: 10.1128/IAI.69.11.6776-6784.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Colmenares M, Kima PE, Samoff E, Soong L, McMahon-Pratt D. Perforin and gamma interferon are critical CD8+ T-cell-mediated responses in vaccine-induced immunity against Leishmania amazonensis infection. Infect Immun. 2003;71(6):3172–3182. doi: 10.1128/IAI.71.6.3172-3182.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Soong L, Duboise SM, Kima P, McMahon-Pratt D. Leishmania pifanoi amastigote antigens protect mice against cutaneous leishmaniasis. Infect Immun. 1995;63(9):3559–3566. doi: 10.1128/iai.63.9.3559-3566.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Coutinho SG, Oliveira MP, Da Cruz AM, et al. T-cell responsiveness of American cutaneous leishmaniasis patients to purified Leishmania pifanoi amastigote antigens and Leishmania braziliensis promastigote antigens: immunologic patterns associated with cure. 1996;84(2):144–155. doi: 10.1006/expr.1996.0100. [DOI] [PubMed] [Google Scholar]
- 27.Haberer JE, Da-Cruz AM, Soong L, et al. Leishmania pifanoi amastigote antigen P-4: epitopes involved in T-cell responsiveness in human cutaneous leishmaniasis. Infect Immun. 1998;66(7):3100–3105. doi: 10.1128/iai.66.7.3100-3105.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mancianti F, Gramiccia M, Gradoni L, Pieri S. Studies on canine leishmaniasis control. 1. Evolution of infection of different clinical forms of canine leishmaniasis following antimonial treatment. Trans R Soc Trop Med Hyg. 1988;82(4):566–567. doi: 10.1016/0035-9203(88)90510-x. [DOI] [PubMed] [Google Scholar]
- 29.Abranches P, Silva-Pereira MC, Conceicao-Silva FM, Santos-Gomes GM, Janz JG. Canine leishmaniasis: pathological and ecological factors influencing transmission of infection. J Parasitol. 1991;77(4):557–561. [PubMed] [Google Scholar]
- 30.Kuhls K, Mauricio IL, Pratlong F, Presber W, Schonian G. Analysis of ribosomal DNA internal transcribed spacer sequences of the Leishmania donovani complex. Microbes Infect. 2005;7(11–12):1224–1234. doi: 10.1016/j.micinf.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 31.Fujiwara S, Yasunaga S, Iwabuchi S, Masuda K, Ohno K, Tsujimoto H. Cytokine profiles of peripheral blood mononuclear cells from dogs experimentally sensitized to Japanese cedar pollen. 2003;93(1–2):9–20. doi: 10.1016/s0165-2427(03)00049-7. [DOI] [PubMed] [Google Scholar]
- 32.Solano-Gallego L, Riera C, Roura X, et al. Leishmania infantum-specific IgG, IgG1 and IgG2 antibody responses in healthy and ill dogs from endemic areas. Evolution in the course of infection and after treatment. Vet Parasitol. 2001;96(4):265–276. doi: 10.1016/s0304-4017(00)00446-5. [DOI] [PubMed] [Google Scholar]
- 33.Bourdoiseau G, Bonnefont C, Hoareau E, Boehringer C, Stolle T, Chabanne L. Specific IgG1 and IgG2 antibody and lymphocyte subset levels in naturally Leishmania infantum-infected treated and untreated dogs. Vet Immunol Immunopathol. 1997;59(1–2):21–30. doi: 10.1016/s0165-2427(97)00072-x. [DOI] [PubMed] [Google Scholar]
- 34.Cordeiro-da-Silva A, Cardoso L, Araujo N, et al. Identification of antibodies to Leishmania silent information regulatory 2 (SIR2) protein homologue during canine natural infections: pathological implications. Immunol Lett. 2003;86(2):155–162. doi: 10.1016/s0165-2478(03)00020-8. [DOI] [PubMed] [Google Scholar]
- 35.Quinnell RJ, Courtenay O, Garcez LM, et al. IgG subclass responses in a longitudinal study of canine visceral leishmaniasis. Vet Immunol Immunopathol. 2003;91 (3–4):161–168. doi: 10.1016/s0165-2427(02)00311-2. [DOI] [PubMed] [Google Scholar]
- 36.Leandro C, Santos-Gomes GM, Campino L, et al. Cell mediated immunity and specific IgG1 and IgG2 antibody response in natural and experimental canine leishmaniosis. Vet Immunol Immunopathol. 2001;79(3–4):273–284. doi: 10.1016/s0165-2427(01)00270-7. [DOI] [PubMed] [Google Scholar]
- 37.Reis AB, Teixeira-Carvalho A, Vale AM, et al. Isotype patterns of immunoglobulins: Hallmarks for clinical status and tissue parasite density in brazilian dogs naturally infected by Leishmania (Leishmania) chagasi. Vet Immunol Immunopathol. 2006 doi: 10.1016/j.vetimm.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 38.Quinnell RJ, Courtenay O, Shaw MA, et al. Tissue cytokine responses in canine visceral leishmaniasis. 2001;183(9):1421–1424. doi: 10.1086/319869. [DOI] [PubMed] [Google Scholar]
- 39.Strauss-Ayali D, Baneth G, Shor S, Okano F, Jaffe CL. Interleukin-12 augments a Th1-type immune response manifested as lymphocyte proliferation and interferon gamma production in Leishmania infantum-infected dogs. 2005;35(1):63–73. doi: 10.1016/j.ijpara.2004.10.015. [DOI] [PubMed] [Google Scholar]
- 40.Chamizo C, Moreno J, Alvar J. Semi-quantitative analysis of cytokine expression in asymptomatic canine leishmaniasis. 2005;103(1–2):67–75. doi: 10.1016/j.vetimm.2004.08.010. [DOI] [PubMed] [Google Scholar]
- 41.Rafati S, Zahedifard F, Nazgouee F. Prime-boost vaccination using cysteine proteinases type I and II of Leishmania infantum confers protective immunity in murine visceral leishmaniasis. Vaccine. 2006;24(12):2169–2175. doi: 10.1016/j.vaccine.2005.11.011. [DOI] [PubMed] [Google Scholar]
- 42.Kumar R, Pai K, Sundar S. Reactive oxygen intermediates, nitrite and IFN-gamma in Indian visceral leishmaniasis. Clin Exp Immunol. 2001;124(2):262–265. doi: 10.1046/j.1365-2249.2001.01551.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Heinzel FP, Sadick MD, Holaday BJ, Coffman RL, Locksley RM. Reciprocal expression of interferon gamma or interleukin 4 during the resolution or progression of murine leishmaniasis. Evidence for expansion of distinct helper T cell subsets. 1989;169(1):59–72. doi: 10.1084/jem.169.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Squires KE, Schreiber RD, McElrath MJ, Rubin BY, Anderson SL, Murray HW. Experimental visceral leishmaniasis: role of endogenous IFN-gamma in host defense and tissue granulomatous response. 1989;143(12):4244–4249. [PubMed] [Google Scholar]
- 45.Karp CL, el Safi SH, Wynn TA, et al. In vivo cytokine profiles in patients with kala-azar. Marked elevation of both interleukin-10 and interferon-gamma. 1993;91(4):1644–1648. doi: 10.1172/JCI116372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Melby PC, Tryon VV, Chandrasekar B, Freeman GL. Cloning of Syrian hamster (Mesocricetus auratus) cytokine cDNAs and analysis of cytokine mRNA expression in experimental visceral leishmaniasis. 1998;66(5):2135–2142. doi: 10.1128/iai.66.5.2135-2142.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Santos-Gomes GM, Rosa R, Leandro C, Cortes S, Romao P, Silveira H. Cytokine expression during the outcome of canine experimental infection by Leishmania infantum. 2002;88(1–2):21–30. doi: 10.1016/s0165-2427(02)00134-4. [DOI] [PubMed] [Google Scholar]
- 48.Sundar S, Reed SG, Sharma S, Mehrotra A, Murray HW. Circulating T helper 1 (Th1) cell- and Th2 cell-associated cytokines in Indian patients with visceral leishmaniasis. Am J Trop Med Hyg. 1997;56(5):522–525. doi: 10.4269/ajtmh.1997.56.522. [DOI] [PubMed] [Google Scholar]
- 49.Kenney RT, Sacks DL, Gam AA, Murray HW, Sundar S. Splenic cytokine responses in Indian kala-azar before and after treatment. J Infect Dis. 1998;177(3):815–818. doi: 10.1086/517817. [DOI] [PubMed] [Google Scholar]
- 50.Murphy ML, Wille U, Villegas EN, Hunter CA, Farrell JP. IL-10 mediates susceptibility to Leishmania donovani infection. Eur J Immunol. 2001;31(10):2848–2856. doi: 10.1002/1521-4141(2001010)31:10<2848::aid-immu2848>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 51.Yoshimoto T, Takeda K, Tanaka T, et al. IL-12 up-regulates IL-18 receptor expression on T cells, Th1 cells, and B cells: synergism with IL-18 for IFN-gamma production. 1998;161(7):3400–3407. [PubMed] [Google Scholar]
- 52.Okamura H, Tsutsi H, Komatsu T, et al. Cloning of a new cytokine that induces IFN-gamma production by T cells. 1995;378(6552):88–91. doi: 10.1038/378088a0. [DOI] [PubMed] [Google Scholar]
- 53.Stager S, Alexander J, Kirby AC, et al. Natural antibodies and complement are endogenous adjuvants for vaccine-induced CD8+ T-cell responses. Nat Med. 2003;9(10):1287–1292. doi: 10.1038/nm933. [DOI] [PubMed] [Google Scholar]
- 54.Stager S, Alexander J, Carter KC, Brombacher F, Kaye PM. Both interleukin-4 (IL-4) and IL-4 receptor alpha signaling contribute to the development of hepatic granulomas with optimal antileishmanial activity. Infect Immun. 2003;71(8):4804–4807. doi: 10.1128/IAI.71.8.4804-4807.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Alexander J, Bryson K. T helper (h)1/Th2 and Leishmania: paradox rather than paradigm. Immunol Lett. 2005;99(1):17–23. doi: 10.1016/j.imlet.2005.01.009. [DOI] [PubMed] [Google Scholar]
- 56.Pinelli E, Killick-Kendrick R, Wagenaar J, Bernadina W, Del Real G, Ruitenberg J. Cellular and humoral immune responses in dogs experimentally and naturally infected with Leishmania infantum. 1994;62(1):229–235. doi: 10.1128/iai.62.1.229-235.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Barbieri CL. Immunology of canine leishmaniasis. Parasite Immunol. 2006;28(7):329–337. doi: 10.1111/j.1365-3024.2006.00840.x. [DOI] [PubMed] [Google Scholar]
- 58.Tumang MC, Keogh C, Moldawer LL, et al. Role and effect of TNF-alpha in experimental visceral leishmaniasis. 1994;153(2):768–775. [PubMed] [Google Scholar]
- 59.Liew FY, Li Y, Millott S. Tumor necrosis factor-alpha synergizes with IFN-gamma in mediating killing of Leishmania major through the induction of nitric oxide. J Immunol. 1990;145(12):4306–4310. [PubMed] [Google Scholar]
- 60.Casadevall A. Antibody-mediated protection against intracellular pathogens. Trends Microbiol. 1998;6(3):102–107. doi: 10.1016/s0966-842x(98)01208-6. [DOI] [PubMed] [Google Scholar]
- 61.Miles SA, Conrad SM, Alves RG, Jeronimo SM, Mosser DM. A role for IgG immune complexes during infection with the intracellular pathogen Leishmania. J Exp Med. 2005;201(5):747–754. doi: 10.1084/jem.20041470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Woelbing F, Kostka SL, Moelle K, et al. Uptake of Leishmania major by dendritic cells is mediated by Fcgamma receptors and facilitates acquisition of protective immunity. J Exp Med. 2006;203(1):177–188. doi: 10.1084/jem.20052288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Morris SC, Madden KB, Adamovicz JJ, et al. Effects of IL-12 on in vivo cytokine gene expression and Ig isotype selection. J Immunol. 1994;152(3):1047–1056. [PubMed] [Google Scholar]
- 64.Snapper CM, Paul WE. Interferon-gamma and B cell stimulatory factor-1 reciprocally regulate Ig isotype production. Science. 1987;236(4804):944–947. doi: 10.1126/science.3107127. [DOI] [PubMed] [Google Scholar]
- 65.Wynn TA, Reynolds A, James S, et al. IL-12 enhances vaccine-induced immunity to schistosomes by augmenting both humoral and cell-mediated immune responses against the parasite. J Immunol. 1996;157(9):4068–4078. [PubMed] [Google Scholar]
- 66.Jankovic D, Caspar P, Zweig M, et al. Adsorption to aluminum hydroxide promotes the activity of IL-12 as an adjuvant for antibody as well as type 1 cytokine responses to HIV-1 gp120. J Immunol. 1997;159(5):2409–2417. [PubMed] [Google Scholar]
- 67.Gurunathan S, Irvine KR, Wu CY, et al. CD40 ligand/trimer DNA enhances both humoral and cellular immune responses and induces protective immunity to infectious and tumor challenge. J Immunol. 1998;161(9):4563–4571. [PMC free article] [PubMed] [Google Scholar]
- 68.Ravindran R, Ali N. Progress in vaccine research and possible effector mechanisms in visceral leishmaniasis. Curr Mol Med. 2004;4(6):697–709. doi: 10.2174/1566524043360212. [DOI] [PubMed] [Google Scholar]


