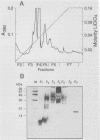
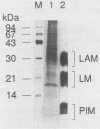
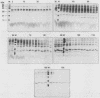
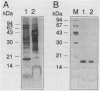
Abstract
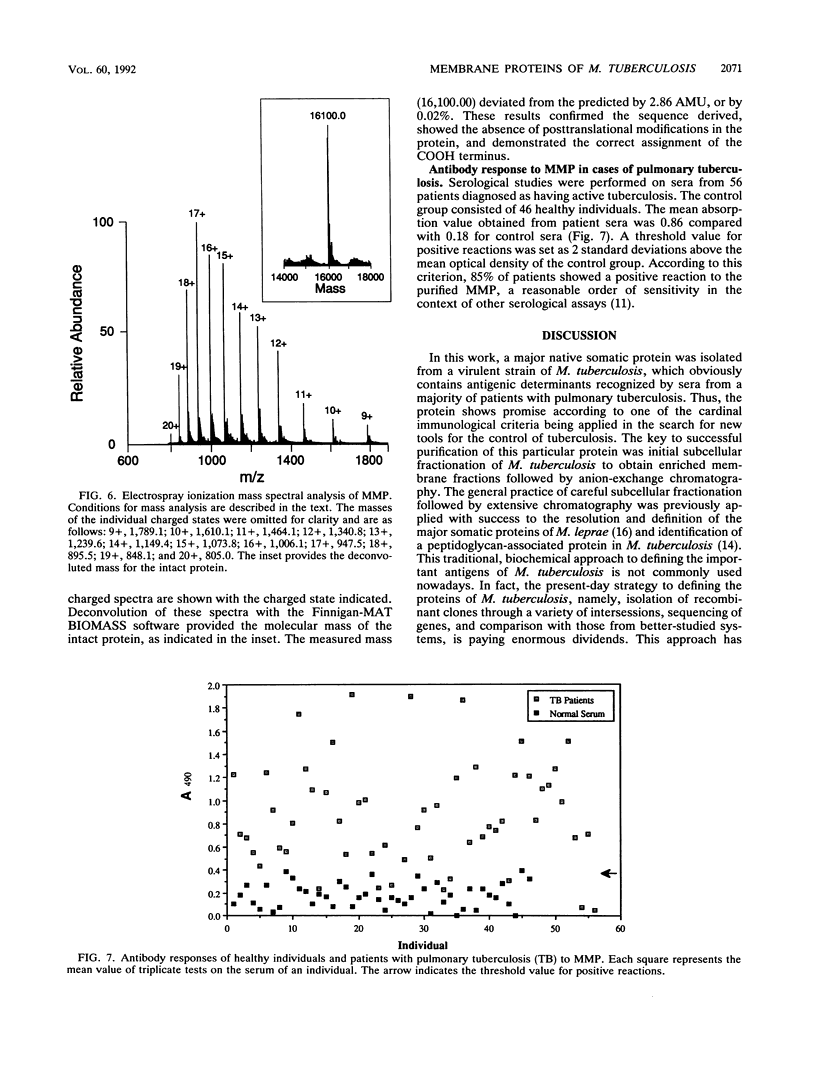
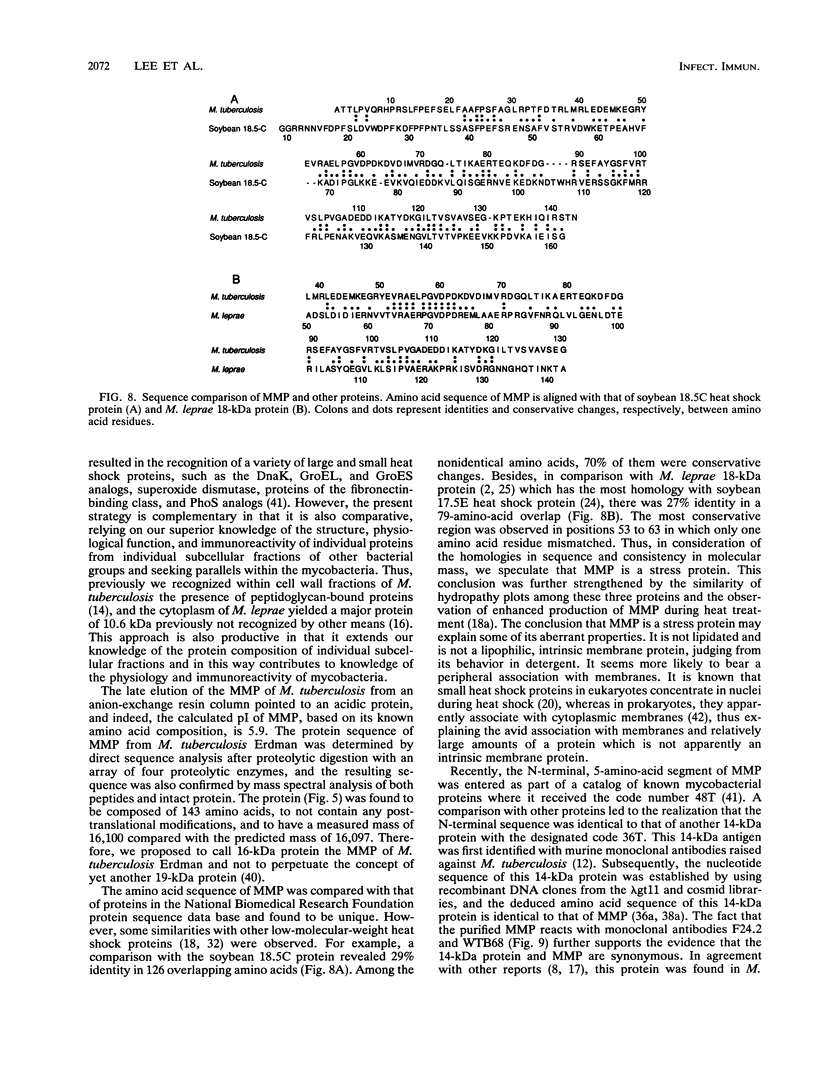
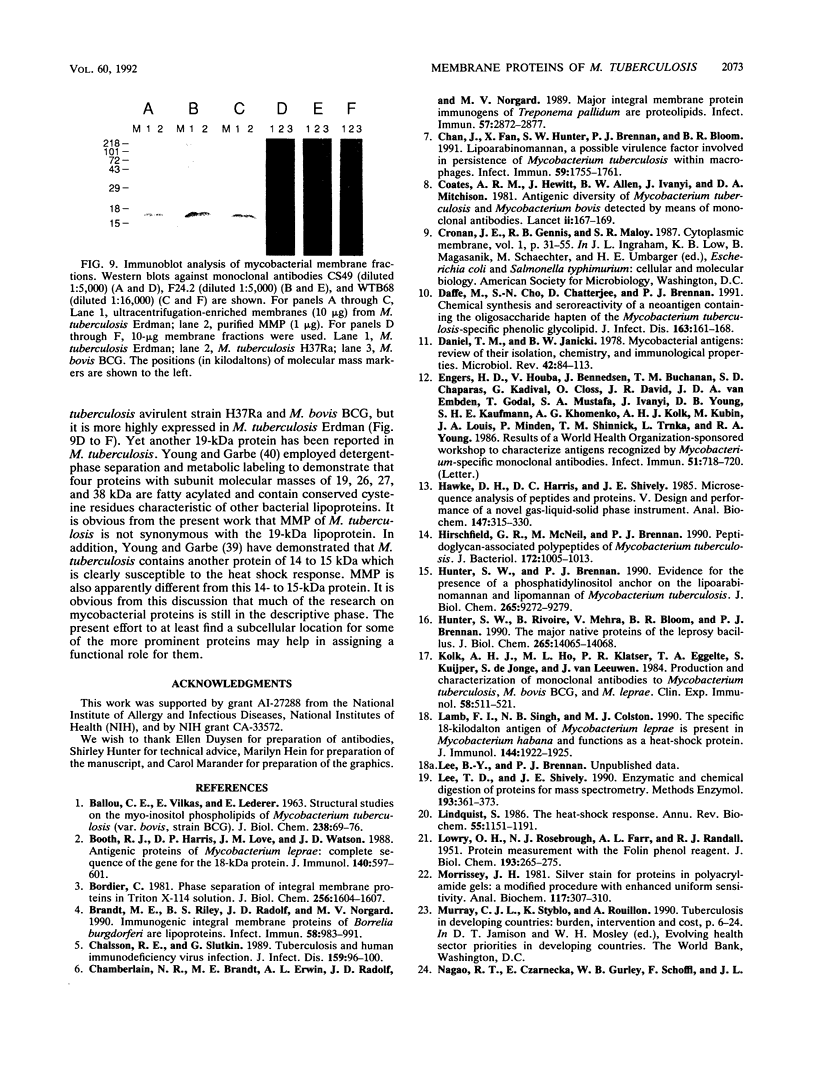
A protein with a molecular mass of 19 kDa was isolated and purified from enriched membrane fractions of the virulent Erdman strain of Mycobacterium tuberculosis. The protein is different from another 19-kDa protein, a lipoprotein, that was recently described (D. B. Young and T. R. Garbe, Res. Microbiol. 142:55-65, 1991). The sequencing strategy applied to this major membrane protein employed four different endoproteinases and resulted in sufficient overlapping peptide sequences for assignment of the entire protein sequence. Electron spray ionization mass spectrometry demonstrated a measured mass of 16,100, deviating from the predicted mass by only 2.86 atomic mass units. The sequence of this protein is unique. However, some similarities with other low-molecular-weight heat shock proteins were observed. Immunoblotting indicated that this protein is highly expressed in the virulent strains of M. tuberculosis. Its application to sera from patients with pulmonary tuberculosis showed promise as a serodiagnostic tool.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BALLOU C. E., VILKAS E., LEDERER E. Structural studies on the myo-inositol phospholipids of Mycobacterium tuberculosis (var. bovis, strain BCG). J Biol Chem. 1963 Jan;238:69–76. [PubMed] [Google Scholar]
- Booth R. J., Harris D. P., Love J. M., Watson J. D. Antigenic proteins of Mycobacterium leprae. Complete sequence of the gene for the 18-kDa protein. J Immunol. 1988 Jan 15;140(2):597–601. [PubMed] [Google Scholar]
- Bordier C. Phase separation of integral membrane proteins in Triton X-114 solution. J Biol Chem. 1981 Feb 25;256(4):1604–1607. [PubMed] [Google Scholar]
- Brandt M. E., Riley B. S., Radolf J. D., Norgard M. V. Immunogenic integral membrane proteins of Borrelia burgdorferi are lipoproteins. Infect Immun. 1990 Apr;58(4):983–991. doi: 10.1128/iai.58.4.983-991.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaisson R. E., Slutkin G. Tuberculosis and human immunodeficiency virus infection. J Infect Dis. 1989 Jan;159(1):96–100. doi: 10.1093/infdis/159.1.96. [DOI] [PubMed] [Google Scholar]
- Chamberlain N. R., Brandt M. E., Erwin A. L., Radolf J. D., Norgard M. V. Major integral membrane protein immunogens of Treponema pallidum are proteolipids. Infect Immun. 1989 Sep;57(9):2872–2877. doi: 10.1128/iai.57.9.2872-2877.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan J., Fan X. D., Hunter S. W., Brennan P. J., Bloom B. R. Lipoarabinomannan, a possible virulence factor involved in persistence of Mycobacterium tuberculosis within macrophages. Infect Immun. 1991 May;59(5):1755–1761. doi: 10.1128/iai.59.5.1755-1761.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coates A. R., Hewitt J., Allen B. W., Ivanyi J., Mitchison D. A. Antigenic diversity of Mycobacterium tuberculosis and Mycobacterium bovis detected by means of monoclonal antibodies. Lancet. 1981 Jul 25;2(8239):167–169. doi: 10.1016/s0140-6736(81)90355-x. [DOI] [PubMed] [Google Scholar]
- Daffe M., Cho S. N., Chatterjee D., Brennan P. J. Chemical synthesis and seroreactivity of a neoantigen containing the oligosaccharide hapten of the Mycobacterium tuberculosis-specific phenolic glycolipid. J Infect Dis. 1991 Jan;163(1):161–168. doi: 10.1093/infdis/163.1.161. [DOI] [PubMed] [Google Scholar]
- Daniel T. M., Janicki B. W. Mycobacterial antigens: a review of their isolation, chemistry, and immunological properties. Microbiol Rev. 1978 Mar;42(1):84–113. doi: 10.1128/mr.42.1.84-113.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawke D. H., Harris D. C., Shively J. E. Microsequence analysis of peptides and proteins. V. Design and performance of a novel gas-liquid-solid phase instrument. Anal Biochem. 1985 Jun;147(2):315–330. doi: 10.1016/0003-2697(85)90278-7. [DOI] [PubMed] [Google Scholar]
- Hirschfield G. R., McNeil M., Brennan P. J. Peptidoglycan-associated polypeptides of Mycobacterium tuberculosis. J Bacteriol. 1990 Feb;172(2):1005–1013. doi: 10.1128/jb.172.2.1005-1013.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter S. W., Brennan P. J. Evidence for the presence of a phosphatidylinositol anchor on the lipoarabinomannan and lipomannan of Mycobacterium tuberculosis. J Biol Chem. 1990 Jun 5;265(16):9272–9279. [PubMed] [Google Scholar]
- Hunter S. W., Rivoire B., Mehra V., Bloom B. R., Brennan P. J. The major native proteins of the leprosy bacillus. J Biol Chem. 1990 Aug 25;265(24):14065–14068. [PubMed] [Google Scholar]
- Kolk A. H., Ho M. L., Klatser P. R., Eggelte T. A., Kuijper S., de Jonge S., van Leeuwen J. Production and characterization of monoclonal antibodies to Mycobacterium tuberculosis, M. bovis (BCG) and M. leprae. Clin Exp Immunol. 1984 Dec;58(3):511–521. [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lamb F. I., Singh N. B., Colston M. J. The specific 18-kilodalton antigen of Mycobacterium leprae is present in Mycobacterium habana and functions as a heat-shock protein. J Immunol. 1990 Mar 1;144(5):1922–1925. [PubMed] [Google Scholar]
- Lee T. D., Shively J. E. Enzymatic and chemical digestion of proteins for mass spectrometry. Methods Enzymol. 1990;193:361–374. doi: 10.1016/0076-6879(90)93427-m. [DOI] [PubMed] [Google Scholar]
- Lindquist S. The heat-shock response. Annu Rev Biochem. 1986;55:1151–1191. doi: 10.1146/annurev.bi.55.070186.005443. [DOI] [PubMed] [Google Scholar]
- Morrissey J. H. Silver stain for proteins in polyacrylamide gels: a modified procedure with enhanced uniform sensitivity. Anal Biochem. 1981 Nov 1;117(2):307–310. doi: 10.1016/0003-2697(81)90783-1. [DOI] [PubMed] [Google Scholar]
- Murray C. J., Styblo K., Rouillon A. Tuberculosis in developing countries: burden, intervention and cost. Bull Int Union Tuberc Lung Dis. 1990 Mar;65(1):6–24. [PubMed] [Google Scholar]
- Nagao R. T., Czarnecka E., Gurley W. B., Schöffl F., Key J. L. Genes for low-molecular-weight heat shock proteins of soybeans: sequence analysis of a multigene family. Mol Cell Biol. 1985 Dec;5(12):3417–3428. doi: 10.1128/mcb.5.12.3417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nerland A. H., Mustafa A. S., Sweetser D., Godal T., Young R. A. A protein antigen of Mycobacterium leprae is related to a family of small heat shock proteins. J Bacteriol. 1988 Dec;170(12):5919–5921. doi: 10.1128/jb.170.12.5919-5921.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborn M. J., Munson R. Separation of the inner (cytoplasmic) and outer membranes of Gram-negative bacteria. Methods Enzymol. 1974;31:642–653. doi: 10.1016/0076-6879(74)31070-1. [DOI] [PubMed] [Google Scholar]
- Osborn M. J., Wu H. C. Proteins of the outer membrane of gram-negative bacteria. Annu Rev Microbiol. 1980;34:369–422. doi: 10.1146/annurev.mi.34.100180.002101. [DOI] [PubMed] [Google Scholar]
- Results of a World Health Organization-sponsored workshop to characterize antigens recognized by mycobacterium-specific monoclonal antibodies. Infect Immun. 1986 Feb;51(2):718–720. doi: 10.1128/iai.51.2.718-720.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieder H. L., Cauthen G. M., Kelly G. D., Bloch A. B., Snider D. E., Jr Tuberculosis in the United States. JAMA. 1989 Jul 21;262(3):385–389. [PubMed] [Google Scholar]
- Saito S., Tsuchiya T. Characteristics of n-octyl beta-D-thioglucopyranoside, a new non-ionic detergent useful for membrane biochemistry. Biochem J. 1984 Sep 15;222(3):829–832. doi: 10.1042/bj2220829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schöffl F., Key J. L. An analysis of mRNAs for a group of heat shock proteins of soybean using cloned cDNAs. J Mol Appl Genet. 1982;1(4):301–314. [PubMed] [Google Scholar]
- Shively J. E., Miller P., Ronk M. Microsequence analysis of peptides and proteins. VI. A continuous flow reactor for sample concentration and sequence analysis. Anal Biochem. 1987 Jun;163(2):517–529. doi: 10.1016/0003-2697(87)90257-0. [DOI] [PubMed] [Google Scholar]
- Takayama K., Schnoes H. K., Armstrong E. L., Boyle R. W. Site of inhibitory action of isoniazid in the synthesis of mycolic acids in Mycobacterium tuberculosis. J Lipid Res. 1975 Jul;16(4):308–317. [PubMed] [Google Scholar]
- Titani K., Sasagawa T., Resing K., Walsh K. A. A simple and rapid purification of commercial trypsin and chymotrypsin by reverse-phase high-performance liquid chromatography. Anal Biochem. 1982 Jul 1;123(2):408–412. doi: 10.1016/0003-2697(82)90465-1. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verbon A., Hartskeerl R. A., Schuitema A., Kolk A. H., Young D. B., Lathigra R. The 14,000-molecular-weight antigen of Mycobacterium tuberculosis is related to the alpha-crystallin family of low-molecular-weight heat shock proteins. J Bacteriol. 1992 Feb;174(4):1352–1359. doi: 10.1128/jb.174.4.1352-1359.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiker H. G., Harboe M., Bennedsen J., Closs O. The antigens of Mycobacterium tuberculosis, H37Rv, studied by crossed immunoelectrophoresis. Comparison with a reference system for Mycobacterium bovis, BCG. Scand J Immunol. 1988 Feb;27(2):223–239. doi: 10.1111/j.1365-3083.1988.tb02342.x. [DOI] [PubMed] [Google Scholar]
- Wise K. S., Kim M. F. Major membrane surface proteins of Mycoplasma hyopneumoniae selectively modified by covalently bound lipid. J Bacteriol. 1987 Dec;169(12):5546–5555. doi: 10.1128/jb.169.12.5546-5555.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young D. B., Garbe T. R. Heat shock proteins and antigens of Mycobacterium tuberculosis. Infect Immun. 1991 Sep;59(9):3086–3093. doi: 10.1128/iai.59.9.3086-3093.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young D. B., Garbe T. R. Lipoprotein antigens of Mycobacterium tuberculosis. Res Microbiol. 1991 Jan;142(1):55–65. doi: 10.1016/0923-2508(91)90097-t. [DOI] [PubMed] [Google Scholar]
- Young D. B., Kaufmann S. H., Hermans P. W., Thole J. E. Mycobacterial protein antigens: a compilation. Mol Microbiol. 1992 Jan;6(2):133–145. doi: 10.1111/j.1365-2958.1992.tb01994.x. [DOI] [PubMed] [Google Scholar]
- Zeilstra-Ryalls J., Fayet O., Georgopoulos C. The universally conserved GroE (Hsp60) chaperonins. Annu Rev Microbiol. 1991;45:301–325. doi: 10.1146/annurev.mi.45.100191.001505. [DOI] [PubMed] [Google Scholar]