Abstract
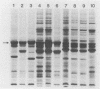
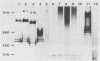
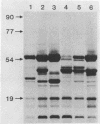
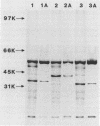
We investigated the phenotypic, structural, and pathogenic properties of 11 Aeromonas schubertii strains recovered from extraintestinal sites. Most A. schubertii strains were autoagglutination positive, possessed a high surface charge but low hydrophobicity, and fell into one or two biogroups on the basis of carbon substrate utilization patterns. Fatty acid methyl ester analysis of A. schubertii revealed this species to contain a relatively high percentage of branched fatty acids (i-13:0, i-15:0, i-17:1, i-17:0) compared with A. hydrophila. Immunologic and biochemical analysis of the lipopolysaccharides of A. schubertii strains allowed for two groups to be distinguished, namely, (i) a collection of six strains belonging to serogroup O:11 that possessed a characteristic homogeneous O polysaccharide side chain profile by silver staining and immunoblotting techniques and (ii) a second antigenically diverse group (five strains) that either exhibited a heterogeneous side chain profile or were side chain deficient. A, schubertii O:11 strains were all found to contain a 55-kDa major protein associated with the outer membrane fraction which was glycine-hydrochloride extractable; non-O:11 strains did not harbor a similar protein molecule. Screening of A. schubertii strains for reputed virulence factors indicated (i) that slightly more than half of the isolates produce an apparent contact-dependent hemolysin that is not cell associated or released extracellularly, (ii) a potent cytotoxin active against HEp-2 cells that is devoid of hemolytic activity, and (iii) lack of enterotoxigeniclike activity as determined by suckling mouse assays. All A. schubertii strains were pathogenic for mice as determined by 50% lethal dose assays, although no single factor correlated with mouse pathogenicity.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abbott S. L., Kokka R. P., Janda J. M. Laboratory investigations on the low pathogenic potential of Plesiomonas shigelloides. J Clin Microbiol. 1991 Jan;29(1):148–153. doi: 10.1128/jcm.29.1.148-153.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carnahan A. M., Chakraborty T., Fanning G. R., Verma D., Ali A., Janda J. M., Joseph S. W. Aeromonas trota sp. nov., an ampicillin-susceptible species isolated from clinical specimens. J Clin Microbiol. 1991 Jun;29(6):1206–1210. doi: 10.1128/jcm.29.6.1206-1210.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carnahan A. M., Joseph S. W., Janda J. M. Species identification of Aeromonas strains based on carbon substrate oxidation profiles. J Clin Microbiol. 1989 Sep;27(9):2128–2129. doi: 10.1128/jcm.27.9.2128-2129.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carnahan A. M., Marii M. A., Fanning G. R., Pass M. A., Joseph S. W. Characterization of Aeromonas schubertii strains recently isolated from traumatic wound infections. J Clin Microbiol. 1989 Aug;27(8):1826–1830. doi: 10.1128/jcm.27.8.1826-1830.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carnahan A., Fanning G. R., Joseph S. W. Aeromonas jandaei (formerly genospecies DNA group 9 A. sobria), a new sucrose-negative species isolated from clinical specimens. J Clin Microbiol. 1991 Mar;29(3):560–564. doi: 10.1128/jcm.29.3.560-564.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicmanec J. F., Holder I. A. Growth of Pseudomonas aeruginosa in normal and burned skin extract: role of extracellular proteases. Infect Immun. 1979 Aug;25(2):477–483. doi: 10.1128/iai.25.2.477-483.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen W., Freney J., Labbe M., Renaud F., Yourassowsky E., Fleurette J. Gas-liquid chromatographic analysis of cellular fatty acid methyl esters in Aeromonas species. Zentralbl Bakteriol. 1991 Apr;275(1):1–10. doi: 10.1016/s0934-8840(11)80762-0. [DOI] [PubMed] [Google Scholar]
- Hickman-Brenner F. W., Fanning G. R., Arduino M. J., Brenner D. J., Farmer J. J., 3rd Aeromonas schubertii, a new mannitol-negative species found in human clinical specimens. J Clin Microbiol. 1988 Aug;26(8):1561–1564. doi: 10.1128/jcm.26.8.1561-1564.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickman-Brenner F. W., MacDonald K. L., Steigerwalt A. G., Fanning G. R., Brenner D. J., Farmer J. J., 3rd Aeromonas veronii, a new ornithine decarboxylase-positive species that may cause diarrhea. J Clin Microbiol. 1987 May;25(5):900–906. doi: 10.1128/jcm.25.5.900-906.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honma Y., Nakasone N. Pili of Aeromonas hydrophila: purification, characterization, and biological role. Microbiol Immunol. 1990;34(2):83–98. doi: 10.1111/j.1348-0421.1990.tb00995.x. [DOI] [PubMed] [Google Scholar]
- Janda J. M., Abbott S. L., Oshiro L. S. Penetration and replication of Edwardsiella spp. in HEp-2 cells. Infect Immun. 1991 Jan;59(1):154–161. doi: 10.1128/iai.59.1.154-161.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janda J. M. Biochemical and exoenzymatic properties of Aeromonas species. Diagn Microbiol Infect Dis. 1985 May;3(3):223–232. doi: 10.1016/0732-8893(85)90034-3. [DOI] [PubMed] [Google Scholar]
- Janda J. M., Kokka R. P. The pathogenicity of Aeromonas strains relative to genospecies and phenospecies identification. FEMS Microbiol Lett. 1991 Dec 15;69(1):29–33. doi: 10.1111/j.1574-6968.1991.tb05120.x. [DOI] [PubMed] [Google Scholar]
- Janda J. M., Oshiro L. S., Abbott S. L., Duffey P. S. Virulence markers of mesophilic aeromonads: association of the autoagglutination phenomenon with mouse pathogenicity and the presence of a peripheral cell-associated layer. Infect Immun. 1987 Dec;55(12):3070–3077. doi: 10.1128/iai.55.12.3070-3077.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janda J. M. Recent advances in the study of the taxonomy, pathogenicity, and infectious syndromes associated with the genus Aeromonas. Clin Microbiol Rev. 1991 Oct;4(4):397–410. doi: 10.1128/cmr.4.4.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokka R. P., Janda J. M., Oshiro L. S., Altwegg M., Shimada T., Sakazaki R., Brenner D. J. Biochemical and genetic characterization of autoagglutinating phenotypes of Aeromonas species associated with invasive and noninvasive disease. J Infect Dis. 1991 Apr;163(4):890–894. doi: 10.1093/infdis/163.4.890. [DOI] [PubMed] [Google Scholar]
- Kokka R. P., Vedros N. A., Janda J. M. Characterization of classic and atypical serogroup O:11 Aeromonas: evidence that the surface array protein is not directly involved in mouse pathogenicity. Microb Pathog. 1991 Jan;10(1):71–79. doi: 10.1016/0882-4010(91)90067-k. [DOI] [PubMed] [Google Scholar]
- Kokka R. P., Vedros N. A., Janda J. M. Electrophoretic analysis of the surface components of autoagglutinating surface array protein-positive and surface array protein-negative Aeromonas hydrophila and Aeromonas sobria. J Clin Microbiol. 1990 Oct;28(10):2240–2247. doi: 10.1128/jcm.28.10.2240-2247.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozaki S., Asao T., Kamata Y., Sakaguchi G. Characterization of Aeromonas sobria hemolysin by use of monoclonal antibodies against Aeromonas hydrophila hemolysins. J Clin Microbiol. 1989 Aug;27(8):1782–1786. doi: 10.1128/jcm.27.8.1782-1786.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuijper E. J., van Alphen L., Leenders E., Zanen H. C. Typing of Aeromonas strains by DNA restriction endonuclease analysis and polyacrylamide gel electrophoresis of cell envelopes. J Clin Microbiol. 1989 Jun;27(6):1280–1285. doi: 10.1128/jcm.27.6.1280-1285.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Reilly T., Day D. F. Effects of cultural conditions on protease production by Aeromonas hydrophila. Appl Environ Microbiol. 1983 Mar;45(3):1132–1135. doi: 10.1128/aem.45.3.1132-1135.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orskov I., Orskov F. Escherichia coli in extra-intestinal infections. J Hyg (Lond) 1985 Dec;95(3):551–575. doi: 10.1017/s0022172400060678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paula S. J., Duffey P. S., Abbott S. L., Kokka R. P., Oshiro L. S., Janda J. M., Shimada T., Sakazaki R. Surface properties of autoagglutinating mesophilic aeromonads. Infect Immun. 1988 Oct;56(10):2658–2665. doi: 10.1128/iai.56.10.2658-2665.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert R. H., Hegazi M. Aeromonas eucrenophila species nova Aeromonas caviae a later and illegitimate synonym of Aeromonas punctata. Zentralbl Bakteriol Mikrobiol Hyg A. 1988 Mar;268(1):34–39. doi: 10.1016/s0176-6724(88)80112-3. [DOI] [PubMed] [Google Scholar]
- Stephenson J. R., Millership S. E., Tabaqchali S. Typing of Aeromonas species by polyacrylamide-gel electrophoresis of radiolabelled cell proteins. J Med Microbiol. 1987 Sep;24(2):113–118. doi: 10.1099/00222615-24-2-113. [DOI] [PubMed] [Google Scholar]
- Wong J. D., Miller M. A., Janda J. M. Surface properties and ultrastructure of Edwardsiella species. J Clin Microbiol. 1989 Aug;27(8):1797–1801. doi: 10.1128/jcm.27.8.1797-1801.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]