Abstract
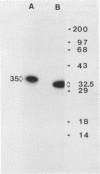
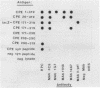
Studies were conducted to allow construction of an initial map of the structure-versus-function relationship of the Clostridium perfringens type A enterotoxin (CPE). Removal of the N-terminal 25 amino acids of CPE increased the primary cytotoxic effect of CPE but did not affect binding. CPE sequences required for at least four epitopes were also identified.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Granum P. E. Inhibition of protein synthesis by a tryptic polypeptide of Clostridium perfringens type A enterotoxin. Biochim Biophys Acta. 1982 Oct 20;708(1):6–11. doi: 10.1016/0167-4838(82)90196-0. [DOI] [PubMed] [Google Scholar]
- Granum P. E., Whitaker J. R., Skjelkvåle R. Trypsin activation of enterotoxin from Clostridium perfringens type A: fragmentation and some physicochemical properties. Biochim Biophys Acta. 1981 May 29;668(3):325–332. doi: 10.1016/0005-2795(81)90165-3. [DOI] [PubMed] [Google Scholar]
- Hanna P. C., McClane B. A. A recombinant C-terminal toxin fragment provides evidence that membrane insertion is important for Clostridium perfringens enterotoxin cytotoxicity. Mol Microbiol. 1991 Jan;5(1):225–230. doi: 10.1111/j.1365-2958.1991.tb01843.x. [DOI] [PubMed] [Google Scholar]
- Hanna P. C., Mietzner T. A., Schoolnik G. K., McClane B. A. Localization of the receptor-binding region of Clostridium perfringens enterotoxin utilizing cloned toxin fragments and synthetic peptides. The 30 C-terminal amino acids define a functional binding region. J Biol Chem. 1991 Jun 15;266(17):11037–11043. [PubMed] [Google Scholar]
- Hanna P. C., Wnek A. P., McClane B. A. Molecular cloning of the 3' half of the Clostridium perfringens enterotoxin gene and demonstration that this region encodes receptor-binding activity. J Bacteriol. 1989 Dec;171(12):6815–6820. doi: 10.1128/jb.171.12.6815-6820.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horiguchi Y., Akai T., Sakaguchi G. Isolation and function of a Clostridium perfringens enterotoxin fragment. Infect Immun. 1987 Dec;55(12):2912–2915. doi: 10.1128/iai.55.12.2912-2915.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulkower K. I., Wnek A. P., McClane B. A. Evidence that alterations in small molecule permeability are involved in the Clostridium perfringens type A enterotoxin-induced inhibition of macromolecular synthesis in Vero cells. J Cell Physiol. 1989 Sep;140(3):498–504. doi: 10.1002/jcp.1041400314. [DOI] [PubMed] [Google Scholar]
- Hwang J., Fitzgerald D. J., Adhya S., Pastan I. Functional domains of Pseudomonas exotoxin identified by deletion analysis of the gene expressed in E. coli. Cell. 1987 Jan 16;48(1):129–136. doi: 10.1016/0092-8674(87)90363-1. [DOI] [PubMed] [Google Scholar]
- Jackson M. P. Structure-function analyses of Shiga toxin and the Shiga-like toxins. Microb Pathog. 1990 Apr;8(4):235–242. doi: 10.1016/0882-4010(90)90050-z. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Ludwig D. S., Holmes R. K., Schoolnik G. K. Chemical and immunochemical studies on the receptor binding domain of cholera toxin B subunit. J Biol Chem. 1985 Oct 15;260(23):12528–12534. [PubMed] [Google Scholar]
- Matsuda M., Ozutsumi K., Iwahashi H., Sugimoto N. Primary action of Clostridium perfringens type A enterotoxin on HeLa and Vero cells in the absence of extracellular calcium: rapid and characteristic changes in membrane permeability. Biochem Biophys Res Commun. 1986 Dec 15;141(2):704–710. doi: 10.1016/s0006-291x(86)80229-7. [DOI] [PubMed] [Google Scholar]
- McClane B. A., Hanna P. C., Wnek A. P. Clostridium perfringens enterotoxin. Microb Pathog. 1988 May;4(5):317–323. doi: 10.1016/0882-4010(88)90059-9. [DOI] [PubMed] [Google Scholar]
- McClane B. A., McDonel J. L. Characterization of membrane permeability alterations induced in Vero cells by Clostridium perfringens enterotoxin. Biochim Biophys Acta. 1980 Aug 14;600(3):974–985. doi: 10.1016/0005-2736(80)90499-x. [DOI] [PubMed] [Google Scholar]
- McClane B. A. Osmotic stabilizers differentially inhibit permeability alterations induced in Vero cells by Clostridium perfringens enterotoxin. Biochim Biophys Acta. 1984 Oct 17;777(1):99–106. doi: 10.1016/0005-2736(84)90501-7. [DOI] [PubMed] [Google Scholar]
- McClane B. A., Wnek A. P., Hulkower K. I., Hanna P. C. Divalent cation involvement in the action of Clostridium perfringens type A enterotoxin. Early events in enterotoxin action are divalent cation-independent. J Biol Chem. 1988 Feb 15;263(5):2423–2435. [PubMed] [Google Scholar]
- McClane B. A., Wnek A. P. Studies of Clostridium perfringens enterotoxin action at different temperatures demonstrate a correlation between complex formation and cytotoxicity. Infect Immun. 1990 Sep;58(9):3109–3115. doi: 10.1128/iai.58.9.3109-3115.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonel J. L. Binding of Clostridium perfringens [125I]enterotoxin to rabbit intestinal cells. Biochemistry. 1980 Oct 14;19(21):4801–4807. doi: 10.1021/bi00562a014. [DOI] [PubMed] [Google Scholar]
- McDonel J. L., McClane B. A. Production, purification, and assay of Clostridium perfringens enterotoxin. Methods Enzymol. 1988;165:94–103. doi: 10.1016/s0076-6879(88)65018-x. [DOI] [PubMed] [Google Scholar]
- Richardson M., Granum P. E. Sequence of the amino-terminal part of enterotoxin from Clostridium perfringens type A: identification of points of trypsin activation. Infect Immun. 1983 Jun;40(3):943–949. doi: 10.1128/iai.40.3.943-949.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommer I., Schachner M. Monoclonal antibodies (O1 to O4) to oligodendrocyte cell surfaces: an immunocytological study in the central nervous system. Dev Biol. 1981 Apr 30;83(2):311–327. doi: 10.1016/0012-1606(81)90477-2. [DOI] [PubMed] [Google Scholar]
- Thelestam M., Möllby R. Sensitive assay for detection of toxin-induced damage to the cytoplasmic membrane of human diploid fibroblasts. Infect Immun. 1975 Aug;12(2):225–232. doi: 10.1128/iai.12.2.225-232.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wick M. J., Hamood A. N., Iglewski B. H. Analysis of the structure-function relationship of Pseudomonas aeruginosa exotoxin A. Mol Microbiol. 1990 Apr;4(4):527–535. doi: 10.1111/j.1365-2958.1990.tb00620.x. [DOI] [PubMed] [Google Scholar]
- Wnek A. P., McClane B. A. Comparison of receptors for Clostridium perfringens type A and cholera enterotoxins in isolated rabbit intestinal brush border membranes. Microb Pathog. 1986 Feb;1(1):89–100. doi: 10.1016/0882-4010(86)90035-5. [DOI] [PubMed] [Google Scholar]
- Wnek A. P., McClane B. A. Preliminary evidence that Clostridium perfringens type A enterotoxin is present in a 160,000-Mr complex in mammalian membranes. Infect Immun. 1989 Feb;57(2):574–581. doi: 10.1128/iai.57.2.574-581.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wnek A. P., Strouse R. J., McClane B. A. Production and characterization of monoclonal antibodies against Clostridium perfringens type A enterotoxin. Infect Immun. 1985 Nov;50(2):442–448. doi: 10.1128/iai.50.2.442-448.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]