Abstract
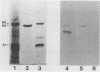
Helicobacter pylori urease is an extracellular, cell-bound enzyme with a molecular weight of approximately 600,000 (600K enzyme) comprising six 66K and six 31K subunits. A 62K protein is closely associated with the H. pylori urease, both in crude preparations and after gel filtration; this protein can be removed from the urease by ion-exchange chromatography without inactivating the enzyme. We purified this urease-associated protein and determined its N-terminal amino acid sequence. The sequence is 80% homologous (identical plus conserved amino acid residues) to the Escherichia coli GroEL heat shock protein (HSP), 75% homologous to the human homolog, and 84% homologous to the HSP homolog found in species of Chlamydia. Thus, the 62K urease-associated protein of H. pylori belongs to the HSP60 family of stress proteins known as chaperonins. Evidently this protein, HSP62, participates in the extracellular assembly and/or protection of the urease against inactivation in the hostile environment of the stomach.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blaser M. J. Epidemiology and pathophysiology of Campylobacter pylori infections. Rev Infect Dis. 1990 Jan-Feb;12 (Suppl 1):S99–106. doi: 10.1093/clinids/12.supplement_1.s99. [DOI] [PubMed] [Google Scholar]
- Born W., Happ M. P., Dallas A., Reardon C., Kubo R., Shinnick T., Brennan P., O'Brien R. Recognition of heat shock proteins and gamma delta cell function. Immunol Today. 1990 Feb;11(2):40–43. doi: 10.1016/0167-5699(90)90015-2. [DOI] [PubMed] [Google Scholar]
- Buchmeier N. A., Heffron F. Induction of Salmonella stress proteins upon infection of macrophages. Science. 1990 May 11;248(4956):730–732. doi: 10.1126/science.1970672. [DOI] [PubMed] [Google Scholar]
- Burns D. L., Gould-Kostka J. L., Kessel M., Arciniega J. L. Purification and immunological characterization of a GroEL-like protein from Bordetella pertussis. Infect Immun. 1991 Apr;59(4):1417–1422. doi: 10.1128/iai.59.4.1417-1422.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carreiro M. M., Laux D. C., Nelson D. R. Characterization of the heat shock response and identification of heat shock protein antigens of Borrelia burgdorferi. Infect Immun. 1990 Jul;58(7):2186–2191. doi: 10.1128/iai.58.7.2186-2191.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerrone M. C., Ma J. J., Stephens R. S. Cloning and sequence of the gene for heat shock protein 60 from Chlamydia trachomatis and immunological reactivity of the protein. Infect Immun. 1991 Jan;59(1):79–90. doi: 10.1128/iai.59.1.79-90.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudani A. K., Gupta R. S. Immunological characterization of a human homolog of the 65-kilodalton mycobacterial antigen. Infect Immun. 1989 Sep;57(9):2786–2793. doi: 10.1128/iai.57.9.2786-2793.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn B. E., Campbell G. P., Perez-Perez G. I., Blaser M. J. Purification and characterization of urease from Helicobacter pylori. J Biol Chem. 1990 Jun 5;265(16):9464–9469. [PubMed] [Google Scholar]
- Ellis J. Proteins as molecular chaperones. 1987 Jul 30-Aug 5Nature. 328(6129):378–379. doi: 10.1038/328378a0. [DOI] [PubMed] [Google Scholar]
- Evans D. G., Evans D. J., Jr, Graham D. Y. Receptor-mediated adherence of Campylobacter pylori to mouse Y-1 adrenal cell monolayers. Infect Immun. 1989 Aug;57(8):2272–2278. doi: 10.1128/iai.57.8.2272-2278.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans D. G., Evans D. J., Jr, Moulds J. J., Graham D. Y. N-acetylneuraminyllactose-binding fibrillar hemagglutinin of Campylobacter pylori: a putative colonization factor antigen. Infect Immun. 1988 Nov;56(11):2896–2906. doi: 10.1128/iai.56.11.2896-2906.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans D. J., Jr, Evans D. G., Graham D. Y., Klein P. D. A sensitive and specific serologic test for detection of Campylobacter pylori infection. Gastroenterology. 1989 Apr;96(4):1004–1008. doi: 10.1016/0016-5085(89)91616-8. [DOI] [PubMed] [Google Scholar]
- Evans D. J., Jr, Evans D. G., Kirkpatrick S. S., Graham D. Y. Characterization of the Helicobacter pylori urease and purification of its subunits. Microb Pathog. 1991 Jan;10(1):15–26. doi: 10.1016/0882-4010(91)90062-f. [DOI] [PubMed] [Google Scholar]
- Fayet O., Ziegelhoffer T., Georgopoulos C. The groES and groEL heat shock gene products of Escherichia coli are essential for bacterial growth at all temperatures. J Bacteriol. 1989 Mar;171(3):1379–1385. doi: 10.1128/jb.171.3.1379-1385.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields P. I., Swanson R. V., Haidaris C. G., Heffron F. Mutants of Salmonella typhimurium that cannot survive within the macrophage are avirulent. Proc Natl Acad Sci U S A. 1986 Jul;83(14):5189–5193. doi: 10.1073/pnas.83.14.5189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin C. S. Duodenal ulcer, Campylobacter pylori, and the "leaking roof" concept. Lancet. 1988 Dec 24;2(8626-8627):1467–1469. doi: 10.1016/s0140-6736(88)90942-7. [DOI] [PubMed] [Google Scholar]
- Hawtin P. R., Stacey A. R., Newell D. G. Investigation of the structure and localization of the urease of Helicobacter pylori using monoclonal antibodies. J Gen Microbiol. 1990 Oct;136(10):1995–2000. doi: 10.1099/00221287-136-10-1995. [DOI] [PubMed] [Google Scholar]
- Hemmingsen S. M., Woolford C., van der Vies S. M., Tilly K., Dennis D. T., Georgopoulos C. P., Hendrix R. W., Ellis R. J. Homologous plant and bacterial proteins chaperone oligomeric protein assembly. Nature. 1988 May 26;333(6171):330–334. doi: 10.1038/333330a0. [DOI] [PubMed] [Google Scholar]
- Houston L. S., Cook R. G., Norris S. J. Isolation and characterization of a Treponema pallidum major 60-kilodalton protein resembling the groEL protein of Escherichia coli. J Bacteriol. 1990 Jun;172(6):2862–2870. doi: 10.1128/jb.172.6.2862-2870.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanyi J., Sinha S., Aston R., Cussell D., Keen M., Sengupta U. Definition of species specific and cross-reactive antigenic determinants of Mycobacterium leprae using monoclonal antibodies. Clin Exp Immunol. 1983 Jun;52(3):528–536. [PMC free article] [PubMed] [Google Scholar]
- Jenkins D. E., Auger E. A., Matin A. Role of RpoH, a heat shock regulator protein, in Escherichia coli carbon starvation protein synthesis and survival. J Bacteriol. 1991 Mar;173(6):1992–1996. doi: 10.1128/jb.173.6.1992-1996.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jindal S., Dudani A. K., Singh B., Harley C. B., Gupta R. S. Primary structure of a human mitochondrial protein homologous to the bacterial and plant chaperonins and to the 65-kilodalton mycobacterial antigen. Mol Cell Biol. 1989 May;9(5):2279–2283. doi: 10.1128/mcb.9.5.2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann S. H. Heat shock proteins and the immune response. Immunol Today. 1990 Apr;11(4):129–136. doi: 10.1016/0167-5699(90)90050-j. [DOI] [PubMed] [Google Scholar]
- Lehner T., Lavery E., Smith R., van der Zee R., Mizushima Y., Shinnick T. Association between the 65-kilodalton heat shock protein, Streptococcus sanguis, and the corresponding antibodies in Behçet's syndrome. Infect Immun. 1991 Apr;59(4):1434–1441. doi: 10.1128/iai.59.4.1434-1441.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loffeld R. J., Willems I., Flendrig J. A., Arends J. W. Helicobacter pylori and gastric carcinoma. Histopathology. 1990 Dec;17(6):537–541. doi: 10.1111/j.1365-2559.1990.tb00793.x. [DOI] [PubMed] [Google Scholar]
- Morrison R. P., Belland R. J., Lyng K., Caldwell H. D. Chlamydial disease pathogenesis. The 57-kD chlamydial hypersensitivity antigen is a stress response protein. J Exp Med. 1989 Oct 1;170(4):1271–1283. doi: 10.1084/jem.170.4.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison R. P., Lyng K., Caldwell H. D. Chlamydial disease pathogenesis. Ocular hypersensitivity elicited by a genus-specific 57-kD protein. J Exp Med. 1989 Mar 1;169(3):663–675. doi: 10.1084/jem.169.3.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips G. J., Silhavy T. J. Heat-shock proteins DnaK and GroEL facilitate export of LacZ hybrid proteins in E. coli. Nature. 1990 Apr 26;344(6269):882–884. doi: 10.1038/344882a0. [DOI] [PubMed] [Google Scholar]
- Reading D. S., Hallberg R. L., Myers A. M. Characterization of the yeast HSP60 gene coding for a mitochondrial assembly factor. Nature. 1989 Feb 16;337(6208):655–659. doi: 10.1038/337655a0. [DOI] [PubMed] [Google Scholar]
- Sampson J. S., O'Connor S. P., Holloway B. P., Plikaytis B. B., Carlone G. M., Mayer L. W. Nucleotide sequence of htpB, the Legionella pneumophila gene encoding the 58-kilodalton (kDa) common antigen, formerly designated the 60-kDa common antigen. Infect Immun. 1990 Sep;58(9):3154–3157. doi: 10.1128/iai.58.9.3154-3157.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinnick T. M. The 65-kilodalton antigen of Mycobacterium tuberculosis. J Bacteriol. 1987 Mar;169(3):1080–1088. doi: 10.1128/jb.169.3.1080-1088.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidebotham R. L., Batten J. J., Karim Q. N., Spencer J., Baron J. H. Breakdown of gastric mucus in presence of Helicobacter pylori. J Clin Pathol. 1991 Jan;44(1):52–57. doi: 10.1136/jcp.44.1.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stover C. K., Marana D. P., Dasch G. A., Oaks E. V. Molecular cloning and sequence analysis of the Sta58 major antigen gene of Rickettsia tsutsugamushi: sequence homology and antigenic comparison of Sta58 to the 60-kilodalton family of stress proteins. Infect Immun. 1990 May;58(5):1360–1368. doi: 10.1128/iai.58.5.1360-1368.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vodkin M. H., Williams J. C. A heat shock operon in Coxiella burnetti produces a major antigen homologous to a protein in both mycobacteria and Escherichia coli. J Bacteriol. 1988 Mar;170(3):1227–1234. doi: 10.1128/jb.170.3.1227-1234.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb R., Reddy K. J., Sherman L. A. Regulation and sequence of the Synechococcus sp. strain PCC 7942 groESL operon, encoding a cyanobacterial chaperonin. J Bacteriol. 1990 Sep;172(9):5079–5088. doi: 10.1128/jb.172.9.5079-5088.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young D., Lathigra R., Hendrix R., Sweetser D., Young R. A. Stress proteins are immune targets in leprosy and tuberculosis. Proc Natl Acad Sci U S A. 1988 Jun;85(12):4267–4270. doi: 10.1073/pnas.85.12.4267. [DOI] [PMC free article] [PubMed] [Google Scholar]