Abstract
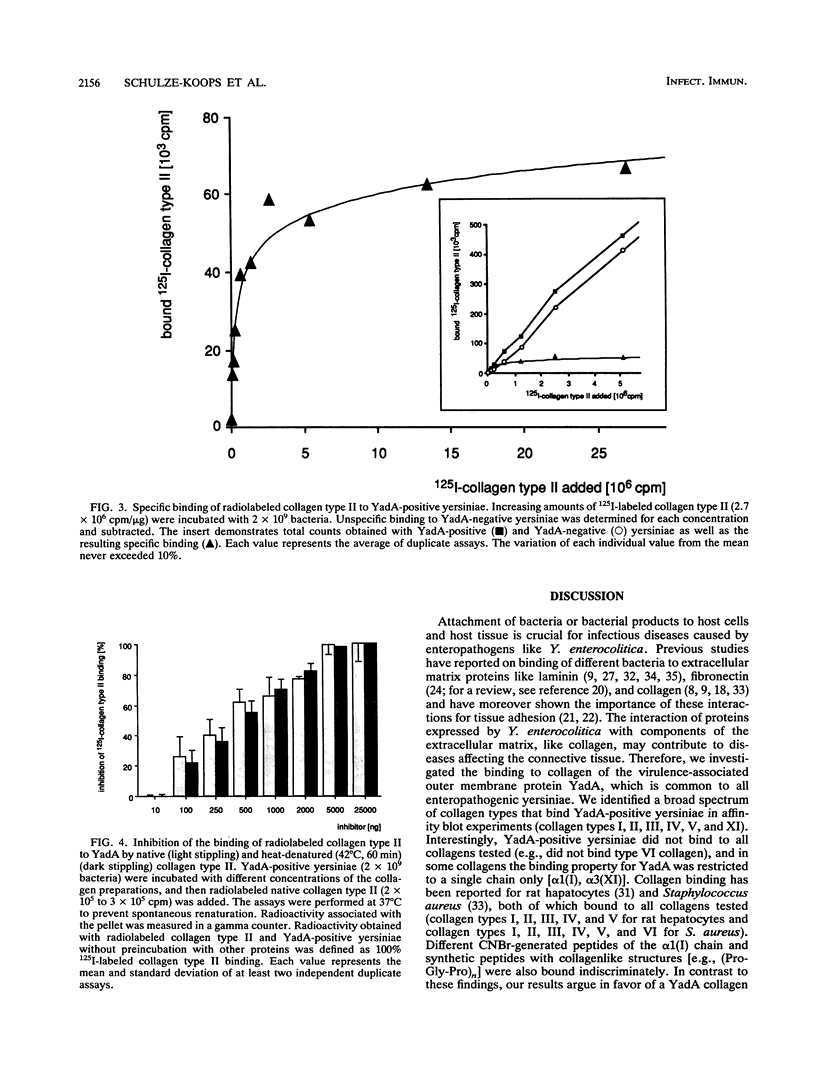
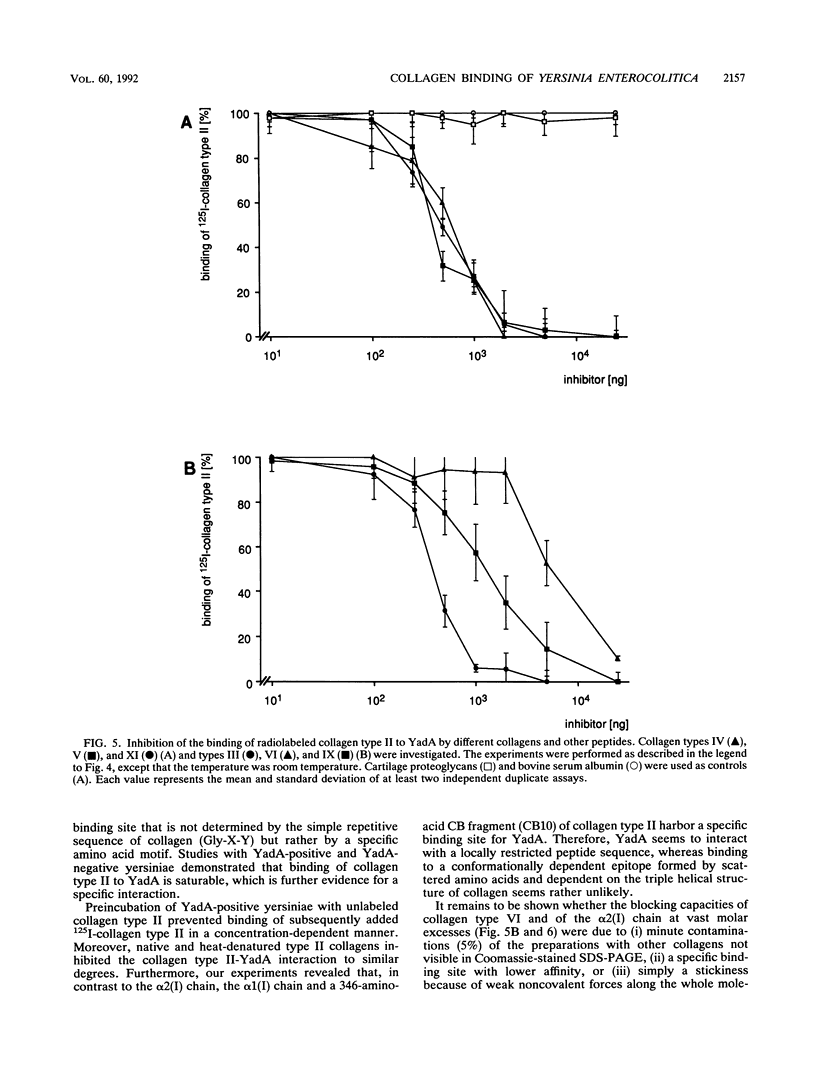
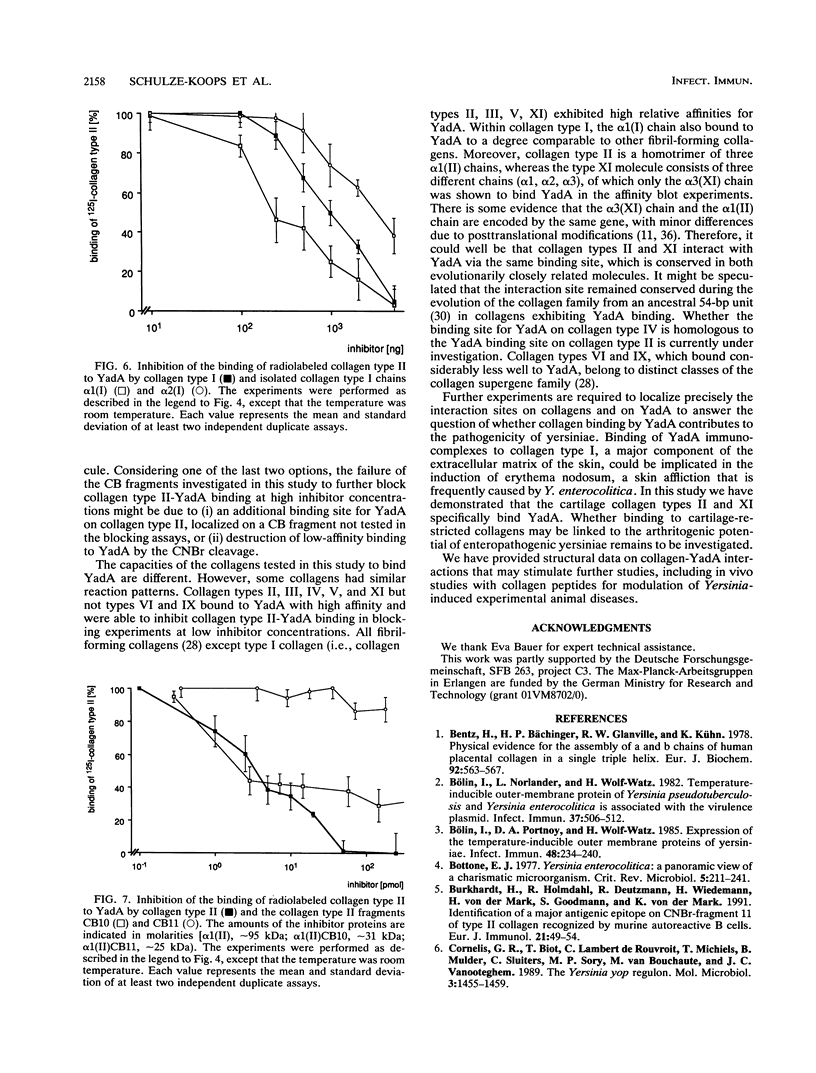
The plasmid-encoded outer membrane protein YadA of enteropathogenic yersiniae is associated with pathogenicity. Recently, collagen binding of YadA-positive yersiniae was reported without detailed characterization (L. Emödy, J. Heesemann, H. Wolf-Watz, M. Skurnik, G. Kapperud, P. O'Toole, and T. Wadström, J. Bacteriol. 171:6674-6679, 1989). To elucidate the nature of collagen binding to YadA, we used a recombinant Yersinia strain expressing the cloned YadA gene. Direct binding of YadA-positive yersiniae to collagens was demonstrated in affinity blot experiments on nitrocellulose filters. A spectrum of collagen types in a wide concentration range were tested for their ability to block binding of 125I-labeled collagen type II to YadA-positive yersiniae. The results indicate a specific binding site(s) for YadA in collagen types I, II, III, IV, V, and XI. In contrast, collagen type VI did not bind to YadA. To characterize the binding site(s) more precisely, isolated collagen chains and cyanogen bromide fragments were investigated. These studies revealed that binding of YadA to collagen type I is confined to the alpha 1(I) chain, whereas the binding site within collagen type XI is localized in the alpha 3(XI) chain. alpha 2(I), alpha 1(XI), and alpha 2(XI) did not bind to YadA. Most interestingly, in the alpha 1(II) chain the specific binding site for YadA resides in the cyanogen bromide fragment CB10. The latter might indicate a binding site that does not depend on conformation. Based on these findings, further fragmentation and the synthesis of peptides may allow definition of the peptide sequence(s) relevant for YadA binding.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bentz H., Bächinger H. P., Glanville R., Kühn K. Physical evidence for the assembly of A and B chains of human placental collagen in a single triple helix. Eur J Biochem. 1978 Dec;92(2):563–567. doi: 10.1111/j.1432-1033.1978.tb12778.x. [DOI] [PubMed] [Google Scholar]
- Bottone E. J. Yersinia enterocolitica: a panoramic view of a charismatic microorganism. CRC Crit Rev Microbiol. 1977;5(2):211–241. doi: 10.3109/10408417709102312. [DOI] [PubMed] [Google Scholar]
- Burkhardt H., Holmdahl R., Deutzmann R., Wiedemann H., von der Mark H., Goodman S., von der Mark K. Identification of a major antigenic epitope on CNBr-fragment 11 of type II collagen recognized by murine autoreactive B cells. Eur J Immunol. 1991 Jan;21(1):49–54. doi: 10.1002/eji.1830210109. [DOI] [PubMed] [Google Scholar]
- Bölin I., Norlander L., Wolf-Watz H. Temperature-inducible outer membrane protein of Yersinia pseudotuberculosis and Yersinia enterocolitica is associated with the virulence plasmid. Infect Immun. 1982 Aug;37(2):506–512. doi: 10.1128/iai.37.2.506-512.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bölin I., Portnoy D. A., Wolf-Watz H. Expression of the temperature-inducible outer membrane proteins of yersiniae. Infect Immun. 1985 Apr;48(1):234–240. doi: 10.1128/iai.48.1.234-240.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornelis G. R., Biot T., Lambert de Rouvroit C., Michiels T., Mulder B., Sluiters C., Sory M. P., Van Bouchaute M., Vanooteghem J. C. The Yersinia yop regulon. Mol Microbiol. 1989 Oct;3(10):1455–1459. doi: 10.1111/j.1365-2958.1989.tb00129.x. [DOI] [PubMed] [Google Scholar]
- Cover T. L., Aber R. C. Yersinia enterocolitica. N Engl J Med. 1989 Jul 6;321(1):16–24. doi: 10.1056/NEJM198907063210104. [DOI] [PubMed] [Google Scholar]
- Emödy L., Heesemann J., Wolf-Watz H., Skurnik M., Kapperud G., O'Toole P., Wadström T. Binding to collagen by Yersinia enterocolitica and Yersinia pseudotuberculosis: evidence for yopA-mediated and chromosomally encoded mechanisms. J Bacteriol. 1989 Dec;171(12):6674–6679. doi: 10.1128/jb.171.12.6674-6679.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald T. J., Repesh L. A., Blanco D. R., Miller J. N. Attachment of Treponema pallidum to fibronectin, laminin, collagen IV, and collagen I, and blockage of attachment by immune rabbit IgG. Br J Vener Dis. 1984 Dec;60(6):357–363. doi: 10.1136/sti.60.6.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraker P. J., Speck J. C., Jr Protein and cell membrane iodinations with a sparingly soluble chloroamide, 1,3,4,6-tetrachloro-3a,6a-diphrenylglycoluril. Biochem Biophys Res Commun. 1978 Feb 28;80(4):849–857. doi: 10.1016/0006-291x(78)91322-0. [DOI] [PubMed] [Google Scholar]
- Furuto D. K., Miller E. J. Different levels of glycosylation contribute to the heterogeneity of alpha 1(II) collagen chains derived from a transplantable rat chondrosarcoma. Arch Biochem Biophys. 1983 Oct 15;226(2):604–611. doi: 10.1016/0003-9861(83)90329-6. [DOI] [PubMed] [Google Scholar]
- Glanville R. W., Rauter A. Pepsin fragments of human placental basement-membrane collagens showing interrupted triple-helical amino acid sequences. Hoppe Seylers Z Physiol Chem. 1981 Jul;362(7):943–951. doi: 10.1515/bchm2.1981.362.2.943. [DOI] [PubMed] [Google Scholar]
- Guan K. L., Dixon J. E. Protein tyrosine phosphatase activity of an essential virulence determinant in Yersinia. Science. 1990 Aug 3;249(4968):553–556. doi: 10.1126/science.2166336. [DOI] [PubMed] [Google Scholar]
- Heesemann J., Algermissen B., Laufs R. Genetically manipulated virulence of Yersinia enterocolitica. Infect Immun. 1984 Oct;46(1):105–110. doi: 10.1128/iai.46.1.105-110.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heesemann J., Gaede K. Mechanisms involved in the pathogenesis of Yersinia infections. Rheumatol Int. 1989;9(3-5):213–217. doi: 10.1007/BF00271883. [DOI] [PubMed] [Google Scholar]
- Heesemann J., Laufs R. Construction of a mobilizable Yersinia enterocolitica virulence plasmid. J Bacteriol. 1983 Aug;155(2):761–767. doi: 10.1128/jb.155.2.761-767.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holderbaum D., Spech R. A., Ehrhart L. A. Specific binding of collagen to Staphylococcus aureus. Coll Relat Res. 1985 Jun;5(3):261–271. doi: 10.1016/s0174-173x(85)80016-9. [DOI] [PubMed] [Google Scholar]
- Holmdahl R., Rubin K., Klareskog L., Larsson E., Wigzell H. Characterization of the antibody response in mice with type II collagen-induced arthritis, using monoclonal anti-type II collagen antibodies. Arthritis Rheum. 1986 Mar;29(3):400–410. doi: 10.1002/art.1780290314. [DOI] [PubMed] [Google Scholar]
- Isberg R. R. Discrimination between intracellular uptake and surface adhesion of bacterial pathogens. Science. 1991 May 17;252(5008):934–938. doi: 10.1126/science.1674624. [DOI] [PubMed] [Google Scholar]
- Isberg R. R., Leong J. M. Cultured mammalian cells attach to the invasin protein of Yersinia pseudotuberculosis. Proc Natl Acad Sci U S A. 1988 Sep;85(18):6682–6686. doi: 10.1073/pnas.85.18.6682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirsch T., von der Mark K. Isolation of bovine type X collagen and immunolocalization in growth-plate cartilage. Biochem J. 1990 Jan 15;265(2):453–459. doi: 10.1042/bj2650453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuusela P. Fibronectin binds to Staphylococcus aureus. Nature. 1978 Dec 14;276(5689):718–720. doi: 10.1038/276718a0. [DOI] [PubMed] [Google Scholar]
- Lachica R. V., Zink D. L., Ferris W. R. Association of fibril structure formation with cell surface properties of Yersinia enterocolitica. Infect Immun. 1984 Oct;46(1):272–275. doi: 10.1128/iai.46.1.272-275.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lopes J. D., dos Reis M., Brentani R. R. Presence of laminin receptors in Staphylococcus aureus. Science. 1985 Jul 19;229(4710):275–277. doi: 10.1126/science.3160113. [DOI] [PubMed] [Google Scholar]
- Miller E. J., Gay S. The collagens: an overview and update. Methods Enzymol. 1987;144:3–41. doi: 10.1016/0076-6879(87)44170-0. [DOI] [PubMed] [Google Scholar]
- Portnoy D. A., Martinez R. J. Role of a plasmid in the pathogenicity of Yersinia species. Curr Top Microbiol Immunol. 1985;118:29–51. doi: 10.1007/978-3-642-70586-1_3. [DOI] [PubMed] [Google Scholar]
- Rubin K., Hök M., Obrink B., Timpl R. Substrate adhesion of rat hepatocytes: mechanism of attachment to collagen substrates. Cell. 1981 May;24(2):463–470. doi: 10.1016/0092-8674(81)90337-8. [DOI] [PubMed] [Google Scholar]
- Speziale P., Hök M., Wadström T., Timpl R. Binding of the basement membrane protein laminin to Escherichia coli. FEBS Lett. 1982 Sep 6;146(1):55–58. doi: 10.1016/0014-5793(82)80704-7. [DOI] [PubMed] [Google Scholar]
- Speziale P., Raucci G., Visai L., Switalski L. M., Timpl R., Hök M. Binding of collagen to Staphylococcus aureus Cowan 1. J Bacteriol. 1986 Jul;167(1):77–81. doi: 10.1128/jb.167.1.77-81.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Switalski L. M., Murchison H., Timpl R., Curtiss R., 3rd, Hök M. Binding of laminin to oral and endocarditis strains of viridans streptococci. J Bacteriol. 1987 Mar;169(3):1095–1101. doi: 10.1128/jb.169.3.1095-1101.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Switalski L. M., Speziale P., Hök M., Wadström T., Timpl R. Binding of Streptococcus pyogenes to laminin. J Biol Chem. 1984 Mar 25;259(6):3734–3738. [PubMed] [Google Scholar]
- Swoboda B., Holmdahl R., Stöss H., von der Mark K. Cellular heterogeneity in cultured human chondrocytes identified by antibodies specific for alpha 2(XI) collagen chains. J Cell Biol. 1989 Sep;109(3):1363–1369. doi: 10.1083/jcb.109.3.1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toivanen A., Granfors K., Lahesmaa-Rantala R., Leino R., Ståhlberg T., Vuento R. Pathogenesis of Yersinia-triggered reactive arthritis: immunological, microbiological and clinical aspects. Immunol Rev. 1985 Aug;86:47–70. doi: 10.1111/j.1600-065x.1985.tb01137.x. [DOI] [PubMed] [Google Scholar]
- Wolf-Watz H. Plasmid-mediated virulence in Yersiniae. Prog Vet Microbiol Immunol. 1987;3:159–178. [PubMed] [Google Scholar]
- von der Mark H., Aumailley M., Wick G., Fleischmajer R., Timpl R. Immunochemistry, genuine size and tissue localization of collagen VI. Eur J Biochem. 1984 Aug 1;142(3):493–502. doi: 10.1111/j.1432-1033.1984.tb08313.x. [DOI] [PubMed] [Google Scholar]
- von der Mark K., van Menxel M., Wiedemann H. Isolation and characterization of new collagens from chick cartilage. Eur J Biochem. 1982 May;124(1):57–62. doi: 10.1111/j.1432-1033.1982.tb05905.x. [DOI] [PubMed] [Google Scholar]