Abstract
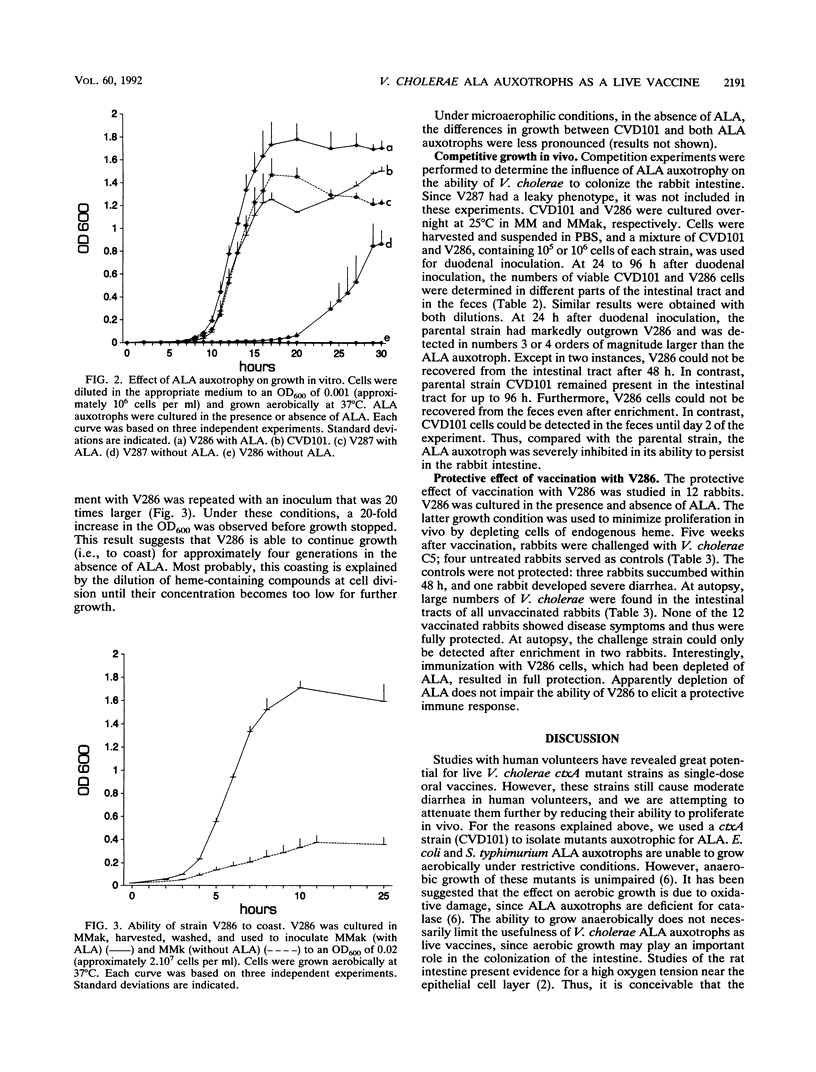
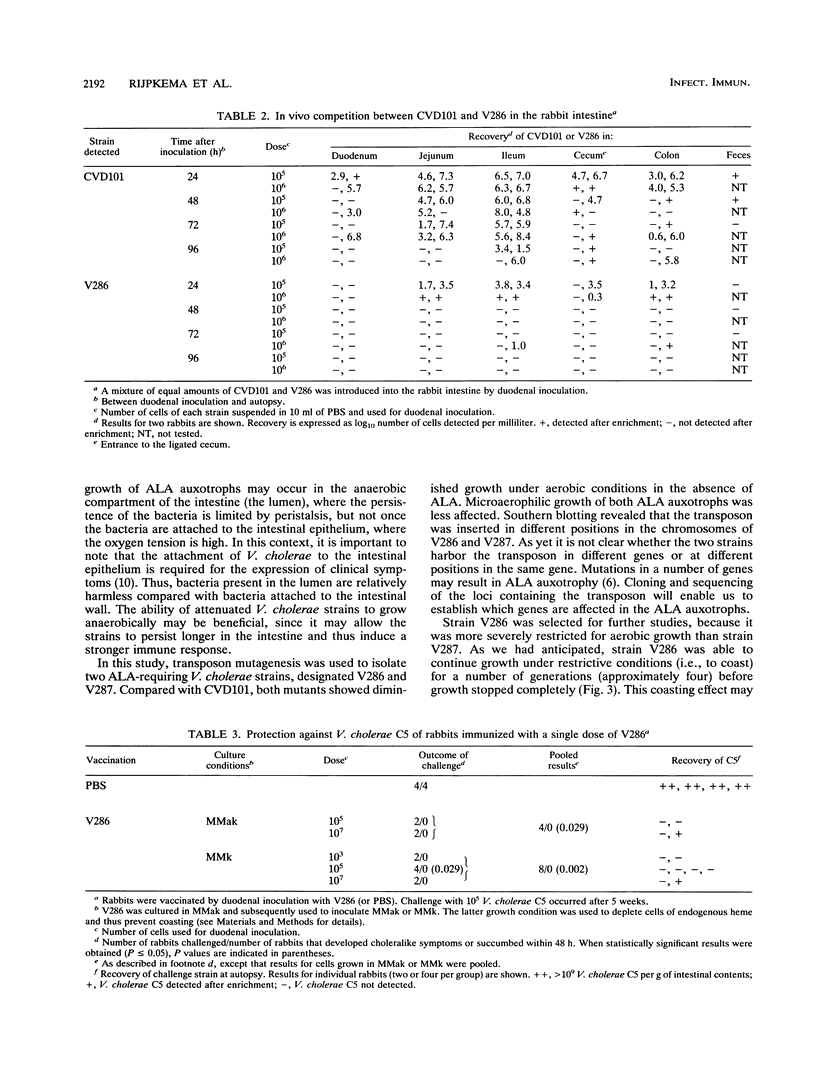
Vibrio cholerae CVD101 is a very effective live vaccine. Although this strain does not produce active cholera toxin because of a mutation in the gene for the cholera toxin A subunit, it still shows residual pathogenicity. To attenuate CVD101 further, we set out to isolate derivatives of CVD101 which were limited in their ability to proliferate in vivo. Two delta-aminolevulinic acid auxotrophs of CVD101, designated V286 and V287, were isolated by transposon mutagenesis and penicillin enrichment. Southern blotting revealed that the mutants differed with respect to the location of the transposon insertion. Under aerobic conditions, in the absence of delta-aminolevulinic acid, both mutants showed diminished growth compared with CVD101. The growth of V286 was most severely affected. Microaerophilic growth of both mutants was less affected. Competition experiments with a rabbit model showed that strain V286 was found in numbers 10(3)- to 10(4)-fold lower than its parental strain. This observation indicates that strain V286 is impaired in its ability to colonize the rabbit intestine. It also supports an important role for aerobic growth in the colonization of the intestine by V. cholerae. Vaccination of rabbits with a single dose of strain V286 resulted in full protection against challenge with a virulent strain. Strain V286 was not shed from rabbits in a cultivatable form. Our results suggest that delta-aminolevulinic acid auxotrophy can attenuate V. cholerae by limiting its ability to colonize without affecting its capacity to induce protective immunity. Furthermore, this type of mutation may prevent the spread of V. cholerae vaccine strains in the environment.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bornside G. H., Donovan W. E., Myers M. B. Intracolonic tensions of oxygen and carbon dioxide in germfree, conventional, and gnotobiotic rats. Proc Soc Exp Biol Med. 1976 Feb;151(2):437–441. doi: 10.3181/00379727-151-39229. [DOI] [PubMed] [Google Scholar]
- Clemens J. D., Sack D. A., Harris J. R., Van Loon F., Chakraborty J., Ahmed F., Rao M. R., Khan M. R., Yunus M., Huda N. Field trial of oral cholera vaccines in Bangladesh: results from three-year follow-up. Lancet. 1990 Feb 3;335(8684):270–273. doi: 10.1016/0140-6736(90)90080-o. [DOI] [PubMed] [Google Scholar]
- Cryz S. J., Jr, Levine M. M., Kaper J. B., Fürer E., Althaus B. Randomized double-blind placebo controlled trial to evaluate the safety and immunogenicity of the live oral cholera vaccine strain CVD 103-HgR in Swiss adults. Vaccine. 1990 Dec;8(6):577–580. doi: 10.1016/0264-410x(90)90012-b. [DOI] [PubMed] [Google Scholar]
- Elliott T., Roth J. R. Heme-deficient mutants of Salmonella typhimurium: two genes required for ALA synthesis. Mol Gen Genet. 1989 Apr;216(2-3):303–314. doi: 10.1007/BF00334369. [DOI] [PubMed] [Google Scholar]
- Guinée P. A., Jansen W. H., Gielen H., Rijpkema S. G., Peters P. W. Protective immunity against Vibrio cholerae infection in the rabbit. Zentralbl Bakteriol Mikrobiol Hyg A. 1987 Oct;266(3-4):552–562. doi: 10.1016/s0176-6724(87)80237-7. [DOI] [PubMed] [Google Scholar]
- Guinée P. A., Jansen W. H., Peters P. W. Vibrio cholerae infection and acquired immunity in an adult rabbit model. Zentralbl Bakteriol Mikrobiol Hyg A. 1985 Feb;259(1):118–131. doi: 10.1016/s0176-6724(85)80013-4. [DOI] [PubMed] [Google Scholar]
- Herrington D. A., Hall R. H., Losonsky G., Mekalanos J. J., Taylor R. K., Levine M. M. Toxin, toxin-coregulated pili, and the toxR regulon are essential for Vibrio cholerae pathogenesis in humans. J Exp Med. 1988 Oct 1;168(4):1487–1492. doi: 10.1084/jem.168.4.1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ketley J. M., Kaper J. B., Herrington D. A., Losonsky G., Levine M. M. Diminished immunogenicity of a recombination-deficient derivative of Vibrio cholerae vaccine strain CVD103. Infect Immun. 1990 May;58(5):1481–1484. doi: 10.1128/iai.58.5.1481-1484.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine M. M., Kaper J. B., Herrington D., Ketley J., Losonsky G., Tacket C. O., Tall B., Cryz S. Safety, immunogenicity, and efficacy of recombinant live oral cholera vaccines, CVD 103 and CVD 103-HgR. Lancet. 1988 Aug 27;2(8609):467–470. doi: 10.1016/s0140-6736(88)90120-1. [DOI] [PubMed] [Google Scholar]
- Levine M. M., Kaper J. B., Herrington D., Losonsky G., Morris J. G., Clements M. L., Black R. E., Tall B., Hall R. Volunteer studies of deletion mutants of Vibrio cholerae O1 prepared by recombinant techniques. Infect Immun. 1988 Jan;56(1):161–167. doi: 10.1128/iai.56.1.161-167.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manoil C., Beckwith J. TnphoA: a transposon probe for protein export signals. Proc Natl Acad Sci U S A. 1985 Dec;82(23):8129–8133. doi: 10.1073/pnas.82.23.8129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Migasena S., Pitisuttitham P., Prayurahong B., Suntharasamai P., Supanaranond W., Desakorn V., Vongsthongsri U., Tall B., Ketley J., Losonsky G. Preliminary assessment of the safety and immunogenicity of live oral cholera vaccine strain CVD 103-HgR in healthy Thai adults. Infect Immun. 1989 Nov;57(11):3261–3264. doi: 10.1128/iai.57.11.3261-3264.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spira W. M., Sack R. B., Froehlich J. L. Simple adult rabbit model for Vibrio cholerae and enterotoxigenic Escherichia coli diarrhea. Infect Immun. 1981 May;32(2):739–747. doi: 10.1128/iai.32.2.739-747.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor R. K., Manoil C., Mekalanos J. J. Broad-host-range vectors for delivery of TnphoA: use in genetic analysis of secreted virulence determinants of Vibrio cholerae. J Bacteriol. 1989 Apr;171(4):1870–1878. doi: 10.1128/jb.171.4.1870-1878.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Bruijn F. J. Transposon Tn5 mutagenesis to map genes. Methods Enzymol. 1987;154:175–196. doi: 10.1016/0076-6879(87)54077-0. [DOI] [PubMed] [Google Scholar]