Abstract
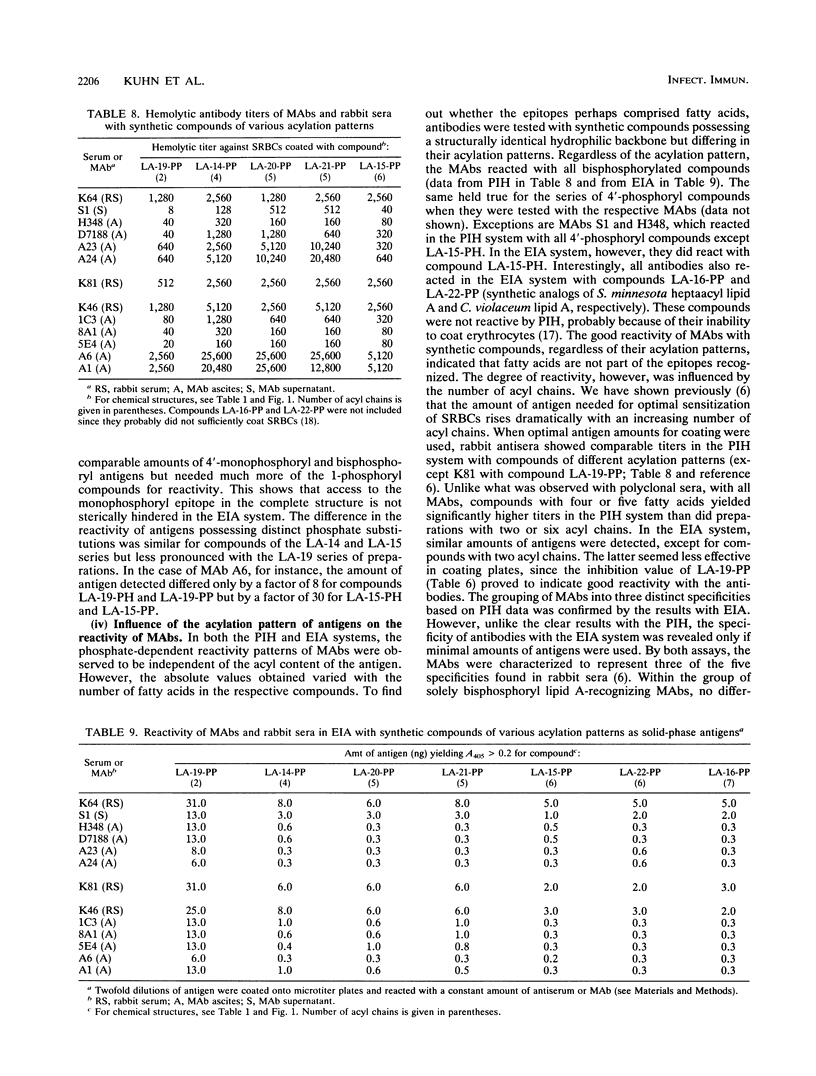
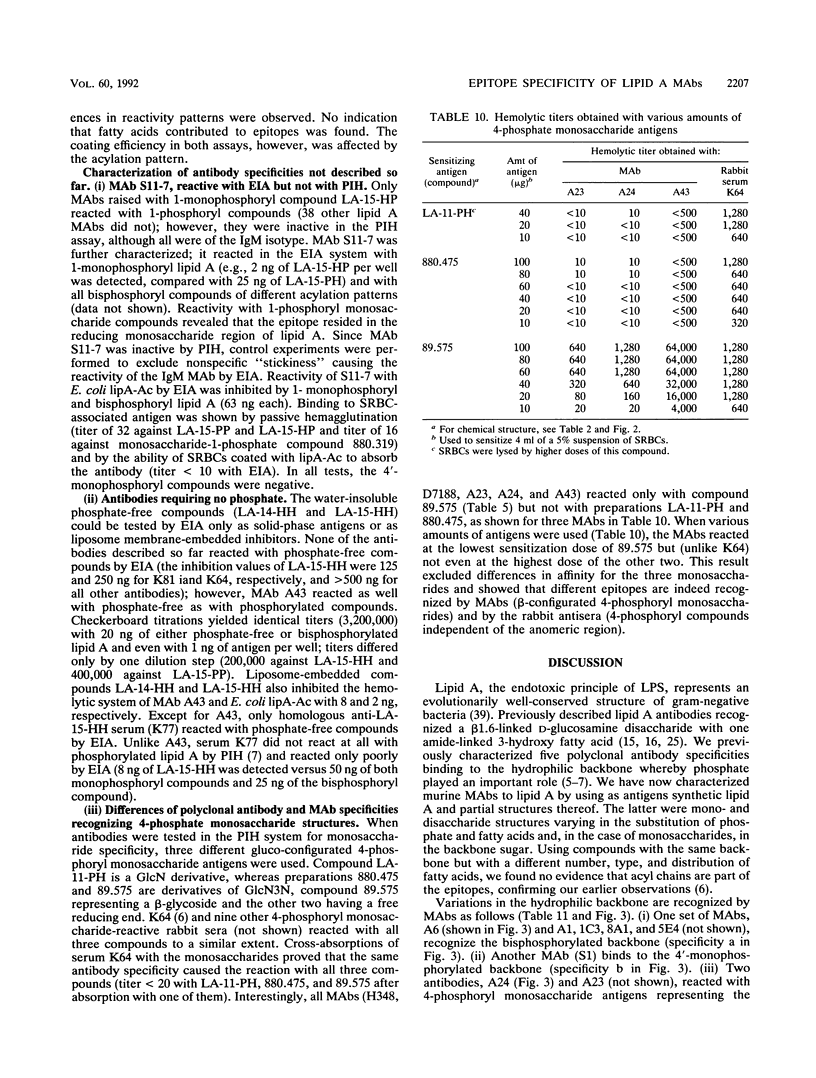
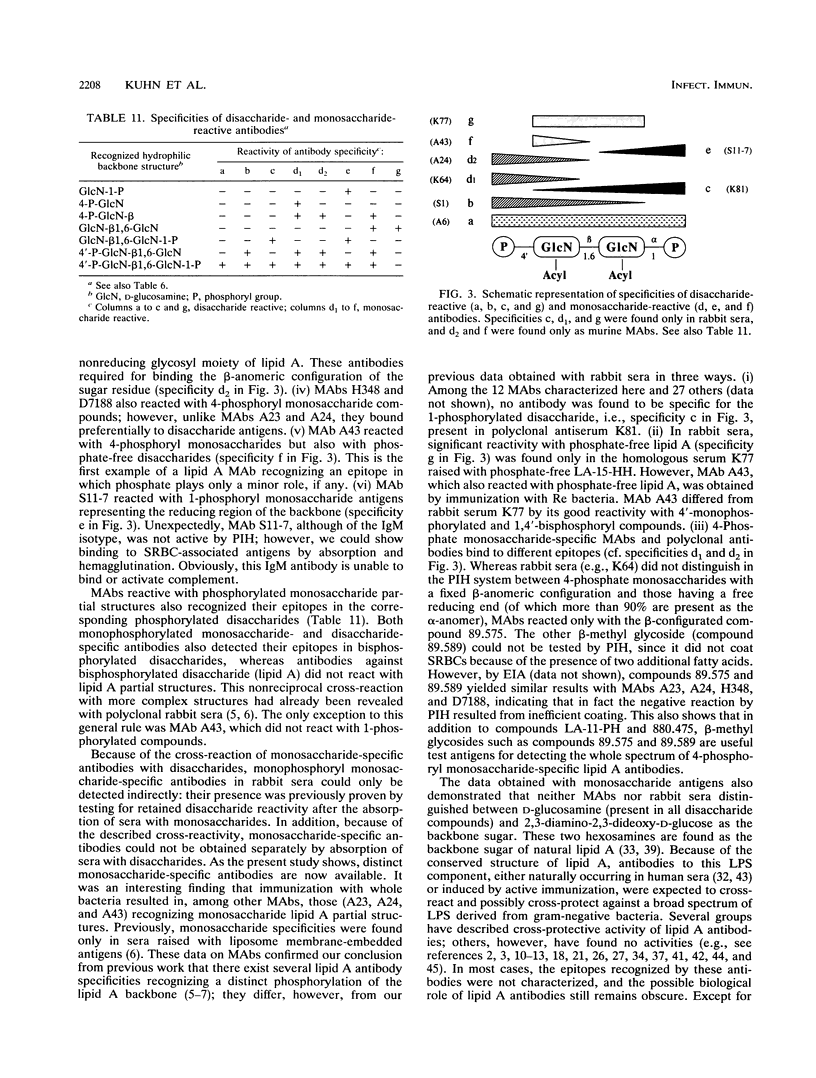
A series of monoclonal antibodies directed against lipid A was characterized by using synthetic lipid A analogs and partial structures. These compounds vary in phosphate substitution, acylation pattern (type, number, and distribution of fatty acids), and, in the case of monosaccharides, in their backbone glycosyl residue. The monoclonal antibodies tested could be subdivided into five groups according to their reactivity patterns. One group reacted exclusively with 1,4'-bisphosphoryl lipid A, and a second also reacted with 4'-monophosphoryl lipid A. Two further groups recognized either 4-phosphoryl or 1-phosphoryl monosaccharide partial structures of lipid A. The fifth group reacted with 4-phosphoryl monosaccharide structures and with phosphate-free compounds. Antibodies reactive with monosaccharide structures also recognized their epitopes in corresponding phosphorylated disaccharide compounds. Both groups of monosaccharide and monophosphoryl lipid A-recognizing antibodies have access to their epitopes in bisphosphoryl compounds as well. Because of this unidirectional reactivity with more complex structures, the various specificities cannot be distinguished by using bisphosphoryl lipid A (e.g., Escherichia coli lipid A) as a test antigen. The epitopes recognized by the various monoclonal antibodies all reside in the hydrophilic backbone of lipid A, and there was no indication that fatty acids were part of the epitopes recognized. Nevertheless, the reactivities of compounds in the different test systems are strongly influenced by their acylation patterns; i.e., acyl groups may modulate the exposure of lipid A epitopes.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Appelmelk B. J., Verweij-van Vught A. M., Maaskant J. J., Schouten W. F., De Jonge A. J., Thijs L. G., Maclaren D. M. Production and characterisation of mouse monoclonal antibodies reacting with the lipopolysaccharide core region of gram-negative bacilli. J Med Microbiol. 1988 Jun;26(2):107–114. doi: 10.1099/00222615-26-2-107. [DOI] [PubMed] [Google Scholar]
- Baumgartner J. D., Heumann D., Gerain J., Weinbreck P., Grau G. E., Glauser M. P. Association between protective efficacy of anti-lipopolysaccharide (LPS) antibodies and suppression of LPS-induced tumor necrosis factor alpha and interleukin 6. Comparison of O side chain-specific antibodies with core LPS antibodies. J Exp Med. 1990 Mar 1;171(3):889–896. doi: 10.1084/jem.171.3.889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogard W. C., Jr, Dunn D. L., Abernethy K., Kilgarriff C., Kung P. C. Isolation and characterization of murine monoclonal antibodies specific for gram-negative bacterial lipopolysaccharide: association of cross-genus reactivity with lipid A specificity. Infect Immun. 1987 Apr;55(4):899–908. doi: 10.1128/iai.55.4.899-908.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brade H., Brade L., Rietschel E. T. Structure-activity relationships of bacterial lipopolysaccharides (endotoxins). Current and future aspects. Zentralbl Bakteriol Mikrobiol Hyg A. 1988 Apr;268(2):151–179. doi: 10.1016/s0176-6724(88)80001-4. [DOI] [PubMed] [Google Scholar]
- Brade L., Brade H. Characterization of two different antibody specificities recognizing distinct antigenic determinants in free lipid A of Escherichia coli. Infect Immun. 1985 Jun;48(3):776–781. doi: 10.1128/iai.48.3.776-781.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brade L., Brandenburg K., Kuhn H. M., Kusumoto S., Macher I., Rietschel E. T., Brade H. The immunogenicity and antigenicity of lipid A are influenced by its physicochemical state and environment. Infect Immun. 1987 Nov;55(11):2636–2644. doi: 10.1128/iai.55.11.2636-2644.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brade L., Rietschel E. T., Kusumoto S., Shiba T., Brade H. Immunogenicity and antigenicity of natural and synthetic Escherichia coli lipid A. Prog Clin Biol Res. 1987;231:75–91. [PubMed] [Google Scholar]
- Brade L., Rietschel E. T., Kusumoto S., Shiba T., Brade H. Immunogenicity and antigenicity of synthetic Escherichia coli lipid A. Infect Immun. 1986 Jan;51(1):110–114. doi: 10.1128/iai.51.1.110-114.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt E., Altman A., Grünefeld M., Ulmer A. J., Flad H. D. Functional and molecular characterization of a monoclonal antibody against human interleukin 2. Immunobiology. 1986 Aug;172(1-2):33–53. doi: 10.1016/S0171-2985(86)80051-1. [DOI] [PubMed] [Google Scholar]
- Bruins S. C., Stumacher R., Johns M. A., McCabe W. R. Immunization with R mutants of Salmonella minnesota. II. Comparison of the protective effect of immunization with lipid A and the Re mutant. Infect Immun. 1977 Jul;17(1):16–20. doi: 10.1128/iai.17.1.16-20.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calandra T., Glauser M. P., Schellekens J., Verhoef J. Treatment of gram-negative septic shock with human IgG antibody to Escherichia coli J5: a prospective, double-blind, randomized trial. J Infect Dis. 1988 Aug;158(2):312–319. doi: 10.1093/infdis/158.2.312. [DOI] [PubMed] [Google Scholar]
- DeMaria A., Jr, Johns M. A., Berberich H., McCabe W. R. Immunization with rough mutants of Salmonella minnesota: initial studies in human subjects. J Infect Dis. 1988 Aug;158(2):301–311. doi: 10.1093/infdis/158.2.301. [DOI] [PubMed] [Google Scholar]
- Dunn D. L., Bogard W. C., Jr, Cerra F. B. Efficacy of type-specific and cross-reactive murine monoclonal antibodies directed against endotoxin during experimental sepsis. Surgery. 1985 Aug;98(2):283–290. [PubMed] [Google Scholar]
- Freudenberg M. A., Fomsgaard A., Mitov I., Galanos C. ELISA for antibodies to lipid A, lipopolysaccharides and other hydrophobic antigens. Infection. 1989 Sep-Oct;17(5):322–328. doi: 10.1007/BF01650719. [DOI] [PubMed] [Google Scholar]
- Galanos C., Freudenberg M. A., Jay F., Nerkar D., Veleva K., Brade H., Strittmatter W. Immunogenic properties of lipid A. Rev Infect Dis. 1984 Jul-Aug;6(4):546–552. doi: 10.1093/clinids/6.4.546. [DOI] [PubMed] [Google Scholar]
- Galanos C., Lüderitz O., Freudenberg M., Brade L., Schade U., Rietschel E. T., Kusumoto S., Shiba T. Biological activity of synthetic heptaacyl lipid A representing a component of Salmonella minnesota R595 lipid A. Eur J Biochem. 1986 Oct 1;160(1):55–59. doi: 10.1111/j.1432-1033.1986.tb09939.x. [DOI] [PubMed] [Google Scholar]
- Galanos C., Lüderitz O., Rietschel E. T., Westphal O., Brade H., Brade L., Freudenberg M., Schade U., Imoto M., Yoshimura H. Synthetic and natural Escherichia coli free lipid A express identical endotoxic activities. Eur J Biochem. 1985 Apr 1;148(1):1–5. doi: 10.1111/j.1432-1033.1985.tb08798.x. [DOI] [PubMed] [Google Scholar]
- Galanos C., Lüderitz O., Westphal O. Preparation and properties of antisera against the lipid-A component of bacterial lipopolysaccharides. Eur J Biochem. 1971 Dec 22;24(1):116–122. doi: 10.1111/j.1432-1033.1971.tb19661.x. [DOI] [PubMed] [Google Scholar]
- Girard R., Chaby R. Inhibition of lipopolysaccharide mitogenicity with characterized anti-lipid A monoclonal antibodies. Immunology. 1985 Nov;56(3):481–487. [PMC free article] [PubMed] [Google Scholar]
- Greisman S. E., Johnston C. A. Failure of antisera to J5 and R595 rough mutants to reduce endotoxemic lethality. J Infect Dis. 1988 Jan;157(1):54–64. doi: 10.1093/infdis/157.1.54. [DOI] [PubMed] [Google Scholar]
- Hansen-Hagge T., Lehmann V., Seydel U., Lindner B., Zähringer U. Isolation and structural analysis of two lipid A precursors from a KDO deficient mutant of Salmonella typhimurium differing in their hexadecanoic acid content. Arch Microbiol. 1985 May;141(4):353–358. doi: 10.1007/BF00428849. [DOI] [PubMed] [Google Scholar]
- Johns M. A., Bruins S. C., McCabe W. R. Immunization with R mutants of Salmonella minnesota. II. Serological response to lipid A and the lipopolysaccharide of Re mutants. Infect Immun. 1977 Jul;17(1):9–15. doi: 10.1128/iai.17.1.9-15.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasai N., Arata S., Mashimo J., Okuda K., Aihara Y., Kotani S., Takada H., Shiba T., Kusumoto S., Imoto M. Synthetic Salmonella-type lipid A antigen with high serological specificity. Infect Immun. 1986 Jan;51(1):43–48. doi: 10.1128/iai.51.1.43-48.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkland T. N., Ziegler E. J. An immunoprotective monoclonal antibody to lipopolysaccharide. J Immunol. 1984 May;132(5):2590–2592. [PubMed] [Google Scholar]
- Mattsby-Baltzer I., Claësson I., Hanson L. A., Jodal U., Kaijser B., Lindberg U., Peterson H. Antibodies to lipid A during urinary tract infection. J Infect Dis. 1981 Oct;144(4):319–328. doi: 10.1093/infdis/144.4.319. [DOI] [PubMed] [Google Scholar]
- Mayer H., Krauss J. H., Yokota A., Weckesser J. Natural variants of lipid A. Adv Exp Med Biol. 1990;256:45–70. doi: 10.1007/978-1-4757-5140-6_3. [DOI] [PubMed] [Google Scholar]
- McCabe W. R., DeMaria A., Jr, Berberich H., Johns M. A. Immunization with rough mutants of Salmonella minnesota: protective activity of IgM and IgG antibody to the R595 (Re chemotype) mutant. J Infect Dis. 1988 Aug;158(2):291–300. doi: 10.1093/infdis/158.2.291. [DOI] [PubMed] [Google Scholar]
- Moran A. P., Rietschel E. T., Kosunen T. U., Zähringer U. Chemical characterization of Campylobacter jejuni lipopolysaccharides containing N-acetylneuraminic acid and 2,3-diamino-2,3-dideoxy-D-glucose. J Bacteriol. 1991 Jan;173(2):618–626. doi: 10.1128/jb.173.2.618-626.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran A. P., Zähringer U., Seydel U., Scholz D., Stütz P., Rietschel E. T. Structural analysis of the lipid A component of Campylobacter jejuni CCUG 10936 (serotype O:2) lipopolysaccharide. Description of a lipid A containing a hybrid backbone of 2-amino-2-deoxy-D-glucose and 2,3-diamino-2,3-dideoxy-D-glucose. Eur J Biochem. 1991 Jun 1;198(2):459–469. doi: 10.1111/j.1432-1033.1991.tb16036.x. [DOI] [PubMed] [Google Scholar]
- Nys M., Cloes J. M., Demonty J., Joassin L. Protective effects of polyclonal sera and of monoclonal antibodies active to Salmonella minnesota Re595 lipopolysaccharide during experimental endotoxemia. J Infect Dis. 1990 Nov;162(5):1087–1095. doi: 10.1093/infdis/162.5.1087. [DOI] [PubMed] [Google Scholar]
- Peters H., Jürs M., Jann B., Jann K., Timmis K. N., Bitter-Suermann D. Monoclonal antibodies to enterobacterial common antigen and to Escherichia coli lipopolysaccharide outer core: demonstration of an antigenic determinant shared by enterobacterial common antigen and E. coli K5 capsular polysaccharide. Infect Immun. 1985 Nov;50(2):459–466. doi: 10.1128/iai.50.2.459-466.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rietschel E. T., Brade L., Schade U., Galanos C., Freudenberg M., Lüderitz O., Kusumoto S., Shiba T. Endotoxic properties of synthetic pentaacyl lipid A precursor Ib and a structural isomer. Eur J Biochem. 1987 Nov 16;169(1):27–31. doi: 10.1111/j.1432-1033.1987.tb13576.x. [DOI] [PubMed] [Google Scholar]
- Rietschel E. T., Galanos C. Lipid A antiserum-mediated protection against lipopolysaccharide- and lipid A-induced fever and skin necrosis. Infect Immun. 1977 Jan;15(1):34–49. doi: 10.1128/iai.15.1.34-49.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salles M. F., Mandine E., Zalisz R., Guenounou M., Smets P. Protective effects of murine monoclonal antibodies in experimental septicemia: E. coli antibodies protect against different serotypes of E. coli. J Infect Dis. 1989 Apr;159(4):641–647. doi: 10.1093/infdis/159.4.641. [DOI] [PubMed] [Google Scholar]
- Teng N. N., Kaplan H. S., Hebert J. M., Moore C., Douglas H., Wunderlich A., Braude A. I. Protection against gram-negative bacteremia and endotoxemia with human monoclonal IgM antibodies. Proc Natl Acad Sci U S A. 1985 Mar;82(6):1790–1794. doi: 10.1073/pnas.82.6.1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler E. J., Fisher C. J., Jr, Sprung C. L., Straube R. C., Sadoff J. C., Foulke G. E., Wortel C. H., Fink M. P., Dellinger R. P., Teng N. N. Treatment of gram-negative bacteremia and septic shock with HA-1A human monoclonal antibody against endotoxin. A randomized, double-blind, placebo-controlled trial. The HA-1A Sepsis Study Group. N Engl J Med. 1991 Feb 14;324(7):429–436. doi: 10.1056/NEJM199102143240701. [DOI] [PubMed] [Google Scholar]


