Abstract
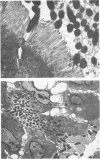
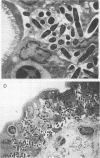
An Escherichia coli K-12 transformant carrying 96.5-kb plasmid pLV501 from enteropathogenic E. coli (EPEC) strain K798 is able to produce the same characteristic attaching-effacing lesions in a rabbit ileal biopsy explant model as its parent strain. Cloned EcoRI-SalI DNA restriction fragments from this plasmid failed to reproduce the attaching-effacing lesions, but one recombinant plasmid, pLV527, containing 4.5 kb of pLV501 DNA, conferred on E. coli DH1 transformants the ability to invade enterocytes in the rabbit explant model. DH1(pLV527) was also able to adhere to and invade HEp-2 cells. The relative invasive ability of DH1(pLV527) was quantified by recovery of internalized bacteria following gentamicin treatment of infected HEp-2 monolayers. DH1(pLV527) was 1,000-fold more invasive than DH1 carrying pBR322 or a recombinant plasmid which had no physiological effect on ileal biopsy explants but was less invasive than an enteroinvasive E. coli strain or a transformant carrying the cloned invasion genes of Shigella flexneri. Invasion by DH1(pLV501) could also be detected but occurred at a level 30 times lower than that by DH1(pLV527). Colony-hybridization of the pLV527 insert against a panel of 49 EPEC and related strains revealed that only 11 contained pLV527-hybridizing sequences; thus, the invasion determinant is not an essential component of the attachment-effacement pathogenic mechanism. One pLV527-hybridizing strain displayed both attachment-effacement and invasiveness in the rabbit ileal biopsy explant model. No significant hybridization was observed to non-EPEC invasive pathogenic enteric bacteria, indicating that the invasion determinant encoded on pLV527 is distinct from those used by these organisms.
Full text
PDF

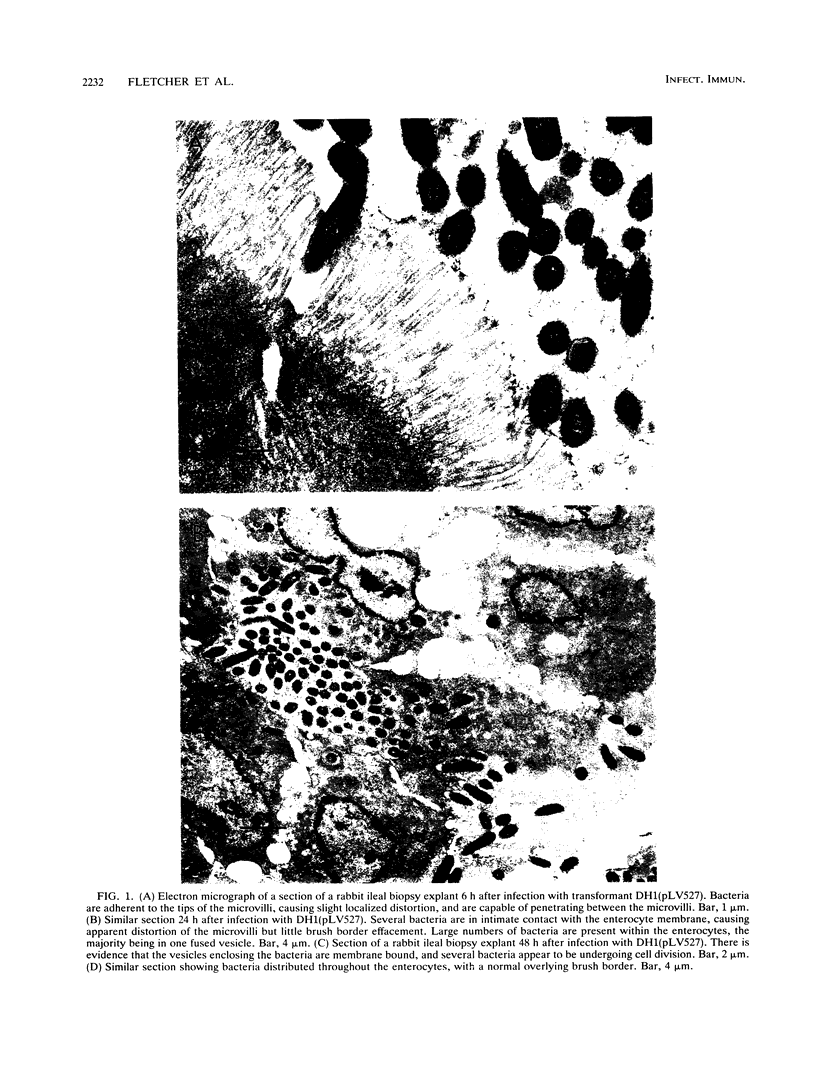
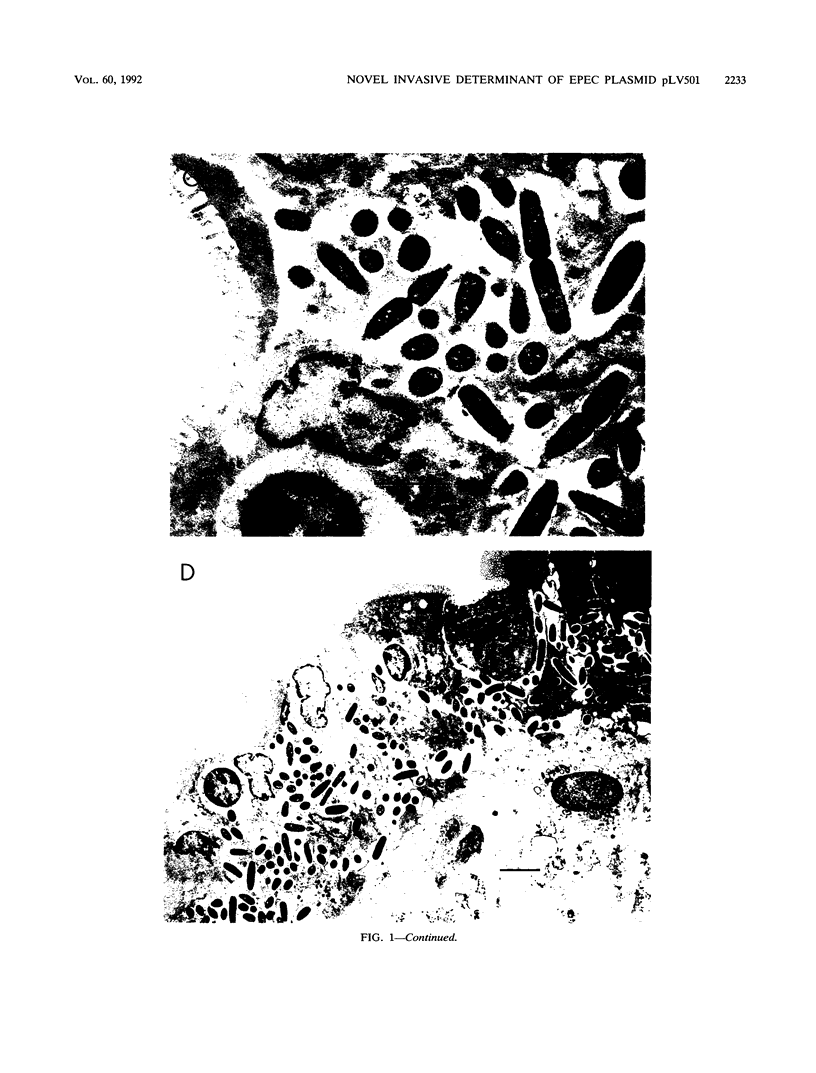



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrade J. R., Da Veiga V. F., De Santa Rosa M. R., Suassuna I. An endocytic process in HEp-2 cells induced by enteropathogenic Escherichia coli. J Med Microbiol. 1989 Jan;28(1):49–57. doi: 10.1099/00222615-28-1-49. [DOI] [PubMed] [Google Scholar]
- Baldini M. M., Kaper J. B., Levine M. M., Candy D. C., Moon H. W. Plasmid-mediated adhesion in enteropathogenic Escherichia coli. J Pediatr Gastroenterol Nutr. 1983;2(3):534–538. doi: 10.1097/00005176-198302030-00023. [DOI] [PubMed] [Google Scholar]
- Baldini M. M., Nataro J. P., Kaper J. B. Localization of a determinant for HEp-2 adherence by enteropathogenic Escherichia coli. Infect Immun. 1986 Apr;52(1):334–336. doi: 10.1128/iai.52.1.334-336.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batt R. M., Hart C. A., McLean L., Saunders J. R. Organ culture of rabbit ileum as a model for the investigation of the mechanism of intestinal damage by enteropathogenic Escherichia coli. Gut. 1987 Oct;28(10):1283–1290. doi: 10.1136/gut.28.10.1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S. N., Chang A. C., Hsu L. Nonchromosomal antibiotic resistance in bacteria: genetic transformation of Escherichia coli by R-factor DNA. Proc Natl Acad Sci U S A. 1972 Aug;69(8):2110–2114. doi: 10.1073/pnas.69.8.2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnenberg M. S., Calderwood S. B., Donohue-Rolfe A., Keusch G. T., Kaper J. B. Construction and analysis of TnphoA mutants of enteropathogenic Escherichia coli unable to invade HEp-2 cells. Infect Immun. 1990 Jun;58(6):1565–1571. doi: 10.1128/iai.58.6.1565-1571.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnenberg M. S., Donohue-Rolfe A., Keusch G. T. Epithelial cell invasion: an overlooked property of enteropathogenic Escherichia coli (EPEC) associated with the EPEC adherence factor. J Infect Dis. 1989 Sep;160(3):452–459. doi: 10.1093/infdis/160.3.452. [DOI] [PubMed] [Google Scholar]
- Embaye H., Batt R. M., Saunders J. R., Getty B., Hart C. A. Interaction of enteropathogenic Escherichia coli 0111 with rabbit intestinal mucosa in vitro. Gastroenterology. 1989 Apr;96(4):1079–1086. doi: 10.1016/0016-5085(89)91626-0. [DOI] [PubMed] [Google Scholar]
- Fletcher J. N., Saunders J. R., Batt R. M., Embaye H., Getty B., Hart C. A. Attaching effacement of the rabbit enterocyte brush border is encoded on a single 96.5-kilobase-pair plasmid in an enteropathogenic Escherichia coli O111 strain. Infect Immun. 1990 May;58(5):1316–1322. doi: 10.1128/iai.58.5.1316-1322.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Formal S. B., Hale T. L., Sansonetti P. J. Invasive enteric pathogens. Rev Infect Dis. 1983 Sep-Oct;5 (Suppl 4):S702–S707. doi: 10.1093/clinids/5.supplement_4.s702. [DOI] [PubMed] [Google Scholar]
- Humphreys G. O., Willshaw G. A., Anderson E. S. A simple method for the preparation of large quantities of pure plasmid DNA. Biochim Biophys Acta. 1975 Apr 2;383(4):457–463. doi: 10.1016/0005-2787(75)90318-4. [DOI] [PubMed] [Google Scholar]
- Janda J. M., Abbott S. L., Oshiro L. S. Penetration and replication of Edwardsiella spp. in HEp-2 cells. Infect Immun. 1991 Jan;59(1):154–161. doi: 10.1128/iai.59.1.154-161.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerse A. E., Yu J., Tall B. D., Kaper J. B. A genetic locus of enteropathogenic Escherichia coli necessary for the production of attaching and effacing lesions on tissue culture cells. Proc Natl Acad Sci U S A. 1990 Oct;87(20):7839–7843. doi: 10.1073/pnas.87.20.7839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutton S., Baldini M. M., Kaper J. B., McNeish A. S. Role of plasmid-encoded adherence factors in adhesion of enteropathogenic Escherichia coli to HEp-2 cells. Infect Immun. 1987 Jan;55(1):78–85. doi: 10.1128/iai.55.1.78-85.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutton S., Baldwin T., Williams P. H., McNeish A. S. Actin accumulation at sites of bacterial adhesion to tissue culture cells: basis of a new diagnostic test for enteropathogenic and enterohemorrhagic Escherichia coli. Infect Immun. 1989 Apr;57(4):1290–1298. doi: 10.1128/iai.57.4.1290-1298.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutton S., Lloyd D. R., McNeish A. S. Adhesion of enteropathogenic Escherichia coli to human intestinal enterocytes and cultured human intestinal mucosa. Infect Immun. 1987 Jan;55(1):69–77. doi: 10.1128/iai.55.1.69-77.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurelli A. T., Baudry B., d'Hauteville H., Hale T. L., Sansonetti P. J. Cloning of plasmid DNA sequences involved in invasion of HeLa cells by Shigella flexneri. Infect Immun. 1985 Jul;49(1):164–171. doi: 10.1128/iai.49.1.164-171.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miliotis M. D., Koornhof H. J., Phillips J. I. Invasive potential of noncytotoxic enteropathogenic Escherichia coli in an in vitro Henle 407 cell model. Infect Immun. 1989 Jul;57(7):1928–1935. doi: 10.1128/iai.57.7.1928-1935.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller V. L., Falkow S. Evidence for two genetic loci in Yersinia enterocolitica that can promote invasion of epithelial cells. Infect Immun. 1988 May;56(5):1242–1248. doi: 10.1128/iai.56.5.1242-1248.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon H. W., Whipp S. C., Argenzio R. A., Levine M. M., Giannella R. A. Attaching and effacing activities of rabbit and human enteropathogenic Escherichia coli in pig and rabbit intestines. Infect Immun. 1983 Sep;41(3):1340–1351. doi: 10.1128/iai.41.3.1340-1351.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pal R., Ghose A. C. Identification of plasmid-encoded mannose-resistant hemagglutinin and HEp-2 and HeLa cell adherence factors of two diarrheagenic Escherichia coli strains belonging to an enteropathogenic serogroup. Infect Immun. 1990 Apr;58(4):1106–1113. doi: 10.1128/iai.58.4.1106-1113.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley L. W., Junio L. N., Libaek L. B., Schoolnik G. K. Plasmid-encoded expression of lipopolysaccharide O-antigenic polysaccharide in enteropathogenic Escherichia coli. Infect Immun. 1987 Sep;55(9):2052–2056. doi: 10.1128/iai.55.9.2052-2056.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley L. W., Junio L. N., Schoolnik G. K. HeLa cell invasion by a strain of enteropathogenic Escherichia coli that lacks the O-antigenic polysaccharide. Mol Microbiol. 1990 Oct;4(10):1661–1666. doi: 10.1111/j.1365-2958.1990.tb00543.x. [DOI] [PubMed] [Google Scholar]
- Robins-Browne R. M., Jacobs M. R., Koornhof H. J. Escherichia coli gastro-enteritis in adults. S Afr Med J. 1978 Jan 21;53(3):93–94. [PubMed] [Google Scholar]
- Rothbaum R., McAdams A. J., Giannella R., Partin J. C. A clinicopathologic study of enterocyte-adherent Escherichia coli: a cause of protracted diarrhea in infants. Gastroenterology. 1982 Aug;83(2):441–454. [PubMed] [Google Scholar]
- Sansonetti P. J., Ryter A., Clerc P., Maurelli A. T., Mounier J. Multiplication of Shigella flexneri within HeLa cells: lysis of the phagocytic vacuole and plasmid-mediated contact hemolysis. Infect Immun. 1986 Feb;51(2):461–469. doi: 10.1128/iai.51.2.461-469.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staley T. E., Jones E. W., Corley L. D. Attachment and penetration of Escherichia coli into intestinal epithelium of the ileum in newborn pigs. Am J Pathol. 1969 Sep;56(3):371–392. [PMC free article] [PubMed] [Google Scholar]
- Taylor C. J., Hart A., Batt R. M., McDougall C., McLean L. Ultrastructural and biochemical changes in human jejunal mucosa associated with enteropathogenic Escherichia coli (0111) infection. J Pediatr Gastroenterol Nutr. 1986 Jan;5(1):70–73. doi: 10.1097/00005176-198601000-00013. [DOI] [PubMed] [Google Scholar]
- Thorén A. The role of enteropathogenic E. coli in infantile diarrhoea. Aspects on bacteriology, epidemiology and therapy. Scand J Infect Dis Suppl. 1983;37:1–51. doi: 10.3109/inf.1982.14.suppl-37.01. [DOI] [PubMed] [Google Scholar]
- Toledo M. R., Alvariza M. do C., Murahovschi J., Ramos S. R., Trabulsi L. R. Enteropathogenic Escherichia coli serotypes and endemic diarrhea in infants. Infect Immun. 1983 Feb;39(2):586–589. doi: 10.1128/iai.39.2.586-589.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzipori S., Gibson R., Montanaro J. Nature and distribution of mucosal lesions associated with enteropathogenic and enterohemorrhagic Escherichia coli in piglets and the role of plasmid-mediated factors. Infect Immun. 1989 Apr;57(4):1142–1150. doi: 10.1128/iai.57.4.1142-1150.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzipori S., Robins-Browne R. M., Gonis G., Hayes J., Withers M., McCartney E. Enteropathogenic Escherichia coli enteritis: evaluation of the gnotobiotic piglet as a model of human infection. Gut. 1985 Jun;26(6):570–578. doi: 10.1136/gut.26.6.570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulshen M. H., Rollo J. L. Pathogenesis of escherichia coli gastroenteritis in man--another mechanism. N Engl J Med. 1980 Jan 10;302(2):99–101. doi: 10.1056/NEJM198001103020207. [DOI] [PubMed] [Google Scholar]