Abstract
Examination of the process of immortal transformation in early passages of two human mammary epithelial cell (HMEC) lines suggests the involvement of an epigenetic step. These lines, 184A1 and 184B5, arose after in vitro exposure of finite lifespan 184 HMEC to a chemical carcinogen, and both are clonally derived. Although early-passage mass cultures of 184A1 and 184B5 maintained continuous slow growth, most individual cells lost proliferative ability. Uniform good growth did not occur until 20–30 passages after the lines first appeared. Early-passage cultures expressed little or no telomerase activity and telomeres continued to shorten with increasing passage. Telomerase activity was first detected when the telomeres became critically short, and activity levels gradually increased thereafter. Early-passage cultures had little or no ability to maintain growth in transforming growth factor-β (TGFβ); however, both mass cultures and clonal isolates showed a very gradual increase in the number of cells displaying progressively increased ability to maintain growth in TGFβ. A strong correlation between capacity to maintain growth in the presence of TGFβ and expression of telomerase activity was observed. We have used the term “conversion” to describe this process of gradual acquisition of increased growth capacity in the absence or presence of TGFβ and reactivation of telomerase. We speculate that the development of extremely short telomeres may result in gradual, epigenetic-based changes in gene expression. Understanding the underlying mechanisms of HMEC conversion in vitro may provide new insight into the process of carcinogenic progression in vivo and offer novel modes for therapeutic intervention.
INTRODUCTION
Immortal transformation is thought to be a critical step in malignant progression of human epithelial cells in vivo. Whereas cells from normal human somatic tissues display a finite lifespan in vitro, cells obtained from human tumor tissues can give rise to cell lines of indefinite lifespan. It is postulated that an immortal lifespan allows cells in tumor tissues to accumulate the multiple errors required for invasion and metastatic growth (Shay et al., 1993). The cellular senescence normally observed in somatic cells from long-lived species such as humans may have developed as a mechanism to prevent carcinogenic progression (Bacchetti, 1996; Smith and Pereira-Smith, 1996).
Much evidence has now accumulated indicating that replicative senescence and immortal transformation may be governed by telomere dynamics (Harley and Villeponteau, 1995; Wright and Shay, 1995). Telomeres form the ends of eukaryotic chromosomes and are composed of long stretches of a short repeat sequence. Due to the inability of DNA polymerases to completely replicate ends of double-stranded DNA, normal human somatic cells lose ∼50–200 nucleotides of telomeric sequence per cell division. Telomere shortening has been observed during both in vivo and in vitro aging of normal human somatic cells (Henderson et al., 1996). The loss of telomeric repeats may signal cells to activate cell cycle checkpoint controls, leading to replicative senescence. Normal cells that have been exposed to viral oncogenes or physical carcinogens may display an extended life (EL); however, EL cells show continued telomere shortening (Counter et al., 1992, 1994) and eventually undergo what has been described as a crisis, i.e., loss of proliferative capacity and death. Depending upon the nature of the carcinogenic exposure, very rare to frequent cells may survive crisis and subsequently display an indefinite lifespan.
It has been suggested that reactivation of telomerase, a ribonucleoprotein enzyme that adds telomeric sequences de novo, can confer an indefinite lifespan. High levels of telomerase activity and stable telomere length are seen in most human tumor-derived immortal cell lines and cancer tissues, whereas most normal human somatic tissues and finite lifespan cells, with the exception of some cells with high self-renewal capacity, do not express detectable telomerase activity (Kim et al., 1994b; Chiu et al., 1996; Härle-Bachor and Boukamp, 1996; Lundblad and Wright, 1996). This differential expression of telomerase activity has generated much interest in telomerase as an avenue for cancer detection and intervention. Clearer understanding of the mechanisms responsible for suppressing telomerase activity in most normal human cells, and reactivating it during carcinogenic progression, would facilitate the possibility of clinical applications.
We have examined the timing of telomerase reactivation during immortal transformation of human epithelial cells in vitro utilizing a model system of HMEC transformation developed in our laboratory. Primary cultures of HMEC from specimen 184 were exposed to the chemical carcinogen benzo(a)pyrene in three separate experiments (Stampfer and Bartley, 1985). Treated cells gave rise to ∼6–10 different EL cultures, which subsequently lost proliferative capacity (Stampfer and Bartley, 1988). Only two cells, from different EL cultures, maintained proliferation, giving rise to the two lines 184A1 and 184B5. Both these lines show a few specific karyotypic abnormalities, indicating their distinct clonal origins. With continued passage, 184A1 and 184B5 displayed a very low level of gross chromosomal instability (Walen and Stampfer, 1989). No defect has been detected in either line in the regulation of retinobastoma (RB) phosphorylation, or in the sequence of the p53 gene (Lehman et al., 1993; Sandhu et al., 1997). Both 184A1 and its EL precursor have homozygous mutations of the p16 gene (Brenner and Aldaz, 1995). Although no mutations in the p16 gene have been detected in 184B5 and its EL precursor, neither has detectable expression of p16 protein. Neither line is tumorigenic in nude mice or displays sustained anchorage-independent growth (Stampfer and Bartley, 1985).
When 184A1 and 184B5 were initially characterized in 1982–1983, we observed two growth patterns with no obvious mechanistic explanations. First, although both immortal lines maintained continuous growth in mass culture after their initial emergence, growth was slow and nonuniform for the first 20–30 passages. Visual observation indicated that many cells lost proliferative capacity. Second, while absolutely no finite lifespan HMEC maintained growth in the continued presence of the pleiotropic cytokine transforming growth factor-β (TGFβ), populations of 184A1 and 184B5 that maintained growth in TGFβ could be isolated. However, the pattern of resistance to TGFβ-induced growth inhibition by these lines was unusual (Hosobuchi and Stampfer, 1989): 184A1 mass cultures exposed to TGFβ at passages (p) 28–35 displayed severe growth inhibition, but a small subpopulation of cells maintained active growth. Assuming these resistant cells represented rare mutations, we attempted to obtain pure populations by clonal isolation. However, like the parental uncloned population, all four clones isolated displayed a small subpopulation of cells capable of continuous growth in TGFβ. 184B5 exposed to TGFβ at p26–40 maintained good growth, but most clones isolated at p13–16 were growth inhibited. One particular severely growth-inhibited clone, B5T1, repeatedly underwent an apparent “crisis” around p30 during which almost all the cells died. The populations derived from the few surviving cells maintained growth in TGFβ. The lack of growth inhibition by TGFβ was not due to loss of the ability to respond to TGFβ. All 184A1 and 184B5 cultures showed morphologic alterations in the presence of TGFβ, and all cells tested displayed TGFβ receptors and induction by TGFβ of extracellular matrix-associated proteins (Stampfer et al., 1993b).
In an effort to understand 1) why so many early passage cells from immortal lines failed to maintain proliferation, and 2) how clonal isolates rapidly produced cell populations heterogeneous for growth in TGFβ, we particularly noted the association of TGFβ resistance with an indefinite lifespan in B5T1. Because the recent literature indicated an association of telomerase activity with an indefinite lifespan, we considered the possibility that expression of TGFβ resistance and telomerase activity might be related. We therefore carefully characterized and correlated morphology, growth capacity in the absence and presence of TGFβ, telomerase activity, and telomere length in 184A1 and 184B5 at different passage levels to ascertain possible associations among these phenotypes. In this paper we describe how early passage cells of these lines are only “conditionally” immortal, i.e., although the mass culture maintains indefinite growth, most individual cells do not remain proliferative and do not express telomerase activity. However, with continued passage, both mass cultures and clonal isolates show a gradual acquisition of increased growth capacity ± TGFβ, reactivation of telomerase, and stabilization of telomere length. This process, which we call conversion, is first detected when the conditionally immortal cells have extremely short telomeres and display slow nonuniform growth. The consistent manifestation of conversion by clonal cell isolates, and the very gradual nature of the conversion process, suggest an epigenetic mechanism. We speculate that the presence of critically short telomeres initiates conversion. Acquisition of a fully immortal phenotype in these HMEC requires overcoming the growth restrictions encountered by conditionally immortal cells and completing the conversion process.
MATERIALS AND METHODS
Cell Culture
Finite lifespan 184 HMEC were obtained from reduction mammoplasty tissue of a 21-y-old individual. They senesce around p22, equivalent to approximately 80 population doublings (Pd), when cultured in serum-free MCDB 170 medium (MEGM, Clonetics Corporation, San Diego, CA) as described (Hammond et al., 1984; Stampfer, 1985). In the serum containing medium MM, they cease growth after approximately 15–25 Pd (Stampfer, 1982, 1985). Extended lifespan 184Aa and 184Be emerged from 184 HMEC grown in MM after exposure of primary cultures to benzo(a)pyrene as described (Stampfer and Bartley, 1985, 1988). 184Aa first appeared as a single colony at p6 and showed complete loss of growth potential by p11 in MM and by p16 when transferred to MCDB 170. 184Be first appeared as two growing areas at p2 and ceased growth by p9 in MM and by p11 in MCDB 170. Indefinite lifespan 184A1 appeared in an MM-grown 184Aa culture at p9, distinguishable from 184Aa by faster growth, greater refractility, smaller size, and growth as single cells versus patches. Indefinite lifespan 184B5 appeared in MM-grown 184Be at p6 as one small tightly packed patch of slowly growing cells. After their initial appearance, 184A1 and 184B5 were maintained in MCDB 170 until p101, with split ratios of approximately 1:8 per passage. Clonal isolates of 184B5 and 184A1 were obtained by seeding cells at low density and using cloning cylinders to select clearly isolated colonies (∼125 cells) with numerous mitotic figures. Cells from each colony were transferred to a 35-mm dish and grown to subconfluence (∼2 × 105 cells). Unless otherwise indicated, cells were grown in MCDB 170 and routinely subcultured at split ratios of 1:8. Where colony-forming efficiency was low, this means that individual cells underwent many more than 3 Pd per passage.
Growth Assays
Human recombinant TGFβ1 was purchased from R&D Systems (Minneapolis, MN) or provided by Genentech Inc. (South San Francisco, CA.) and used at 5 ng/ml in the presence of 0.1% bovine serum albumin (Sigma Chemical, St. Louis, MO). The ability to maintain growth in the absence or presence of TGFβ was assayed by three methods: 1) To detect growth capacity and heterogeneity of single cell-derived colonies, 200-2000 cells were seeded per 100-mm dish. Cultures were maintained for 15–21 d after seeding. [3H]Thymidine (0.5–1.0 μCi/ml) was then added 4–7 h after refeeding for 24 h, and labeled cells were visualized by autoradiography as described (Stampfer et al., 1993a). Growth capacity was determined by counting the percentage of labeled nuclei in colonies of >50 cells, with uniform good growth defined as a labeling index (LI) of >50%. Colony-forming efficiency (CFE) was determined by counting the number of colonies of >50 cells. To determine growth capacity in TGFβ, TGFβ was added to some cultures for 10–15 d once the largest colonies contained 100–250 cells. Growth capacity per colony was determined as above. 2) To detect very rare TGFβ-resistant cells in mass cultures, 184A1 cells were seeded at 1–2 × 105/100-mm dish, and 184B5 at 1 × 105/60-mm dish or 0.2–0.5 × 105/35-mm dish. TGFβ was added 24–48 h later and cultures maintained for 10–18 d in TGFβ. Control cells received bovine serum albumin alone. Cultures were then labeled and prepared for autoradiography as above. 3) Cultures ± TGFβ were visually monitored at least twice weekly for growth, mitotic activity, and morphology. These observations were recorded, and representative photographs were taken.
It is important to note that we defined the HMEC as TGFβ resistant if they could sustain growth in the presence of TGFβ for at least 10 d, even if TGFβ led to some growth inhibition, because this definition completely distinguishes between finite lifespan and immortal HMEC. Some finite lifespan and conditionally immortal cells can undergo 5–10 Pd in TGFβ before complete cessation of growth; therefore, short-term assays of growth inhibition would not demonstrate the capacity of TGFβ to fully inhibit the growth of these cells. Conversely, HMEC undergoing conversion are still growth inhibited by TGFβ, but some growth is maintained indefinitely. Our definition differs from that used for breast tumor-derived cell lines, which are called TGFβ sensitive if TGFβ leads to any significant reduction in growth rate over time.
Viability was assayed by a 3-h exposure of cultured cells to 5 mg/ml 3-(4, 5 dimethylthiazol-2yl-2, 5-dipehenyltetrazolium bromide (MTT, Sigma). Cells were considered positive that converted the soluble yellow dye to an insoluble purple precipitate.
Telomerase Assays
Cell extracts were prepared by a modification of the detergent lysis method (Kim et al., 1994), and protein concentrations were determined using the Coomassie protein assay reagent (Pierce, Rockford, IL). Telomerase activity in the cell extracts was determined by a modified polymerase chain reaction-based telomeric repeat amplification protocol (TRAP) assay (Kim et al., 1994; Wright et al., 1995; Bodnar et al., 1996) using 2 μg protein for a routine assay. Cell extracts with no detectable telomerase activity at this level were assayed at higher protein concentrations. Because telomerase is a ribonucleoprotein, the specificity of the telomerase products was determined by their sensitivity to RNase added to the reaction mix before the TRAP assay. The 32P-labeled telomerase products were detected using the PhosphorImager system (Molecular Dynamics, Sunnyvale, CA), and semiquantitation was performed by comparing PCR signals from the HMEC extracts to signals from cell extracts of 293 cells (an adenovirus-transformed human kidney cell line). Cell extracts that exhibited low or no telomerase activity were examined for possible diffusible telomerase inhibitors by mixing extracts with 293 cell extracts. No diffusible inhibitors were detected.
Analysis of Terminal Restriction Fragments
DNA isolation and mean telomere restriction fragment (TRF) analysis were performed as previously described (Bodnar et al., 1996). Briefly, genomic DNA was isolated from cells, and 3 μg were digested with RsaI and HinfI and resolved on 0.5% agarose gels. The dried gels were hybridized with a 32P-labeled telomere-specific probe (CCCTAA)3, washed, and exposed to PhosphorImager screens. The mean TRF length was calculated based on the intensities and size distribution of the hybridization signals.
RESULTS
Gradual Acquisition of Good Growth in the Absence and Presence of TGFβ in Early Passage 184A1
When 184A1 was first established, a complex growth pattern was noted. Early passages grew rapidly, followed by an extended period of slow nonuniform growth, followed by a gradual reacquisition of rapid uniform growth. 184A1 cells at the earliest available frozen passage were now placed into culture to carefully characterize their pattern of growth and to look for correlations among growth pattern, capacity to grow in TGFβ, telomerase activity, and telomere length (Figure 1). 184A1 p9–15 mass cultures grew rapidly in MCDB 170, and almost all individual colonies showed good growth (>50% LI after a 24-h exposure to [3H]thymidine). However, the CFE steadily decreased with passage. Around p16, there was an abrupt decrease in growth. Mass cultures now required 3–5 wk to reach subconfluence compared with ∼1 wk at p13. By p18 the CFE had decreased to less than 1%, and many large, vacuolated cells were visible. Between passages 18–30 the CFE remained low. Most cells did not grow or gave rise to small patches that did not maintain proliferation (Figure 2a). Although nonproliferative, the cells still attached to the culture dishes were almost all viable as determined by their ability to metabolize MTT and exclude trypan blue. Most growing colonies contained a mixture of growing and nonproliferative cells (Figure 2b and Table 1). Only the largest colonies contained few nongrowing cells (Figure 2c). Therefore, most individual cells at these passage levels were either nonproliferative or within a few population doublings of giving rise to almost all nonproliferative cells. However, because some cells continued to proliferate at each passage, growth in mass culture was maintained. After p30, fewer large vacuolated cells were visible, the CFE increased, and the growth displayed by individual colonies gradually became uniform (Table 1 and Figure 1).
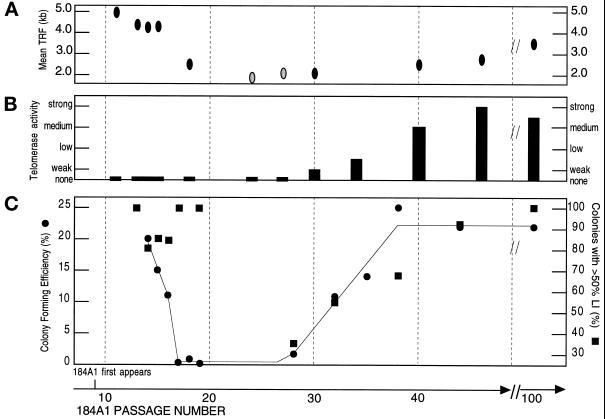
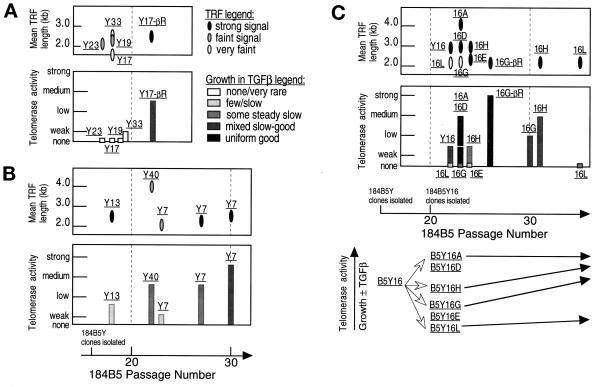
Figure 1.
Comparison of mean TRF length, telomerase activity, and growth in 184A1 at different passage levels. (A) TRF length: lighter shaded ovals indicate a faint signal. (B) Telomerase activity, determined semiquantitatively by comparing the levels of HMEC telomerase products generated to those generated for a constant number of 293 cells (1,000 cell equivalents). The following categories were used to designate semiquantitative values. Note that the points are presented in a semilog form: none, no detectable telomerase products by PhosphorImager analysis; weak, ∼ 5% of telomerase activity of 293 cell control; low, ∼10% of 293 control; medium, 25–50% of 293 control; strong, 75–100% of 293 control. (C) Colony-forming efficiency and labeling index (LI) in colonies. TRF length, telomerase activity, CFE, and labeling index were determined as described in MATERIALS AND METHODS. See Figure 4 for examples of telomerase activity assay semiquantitation.
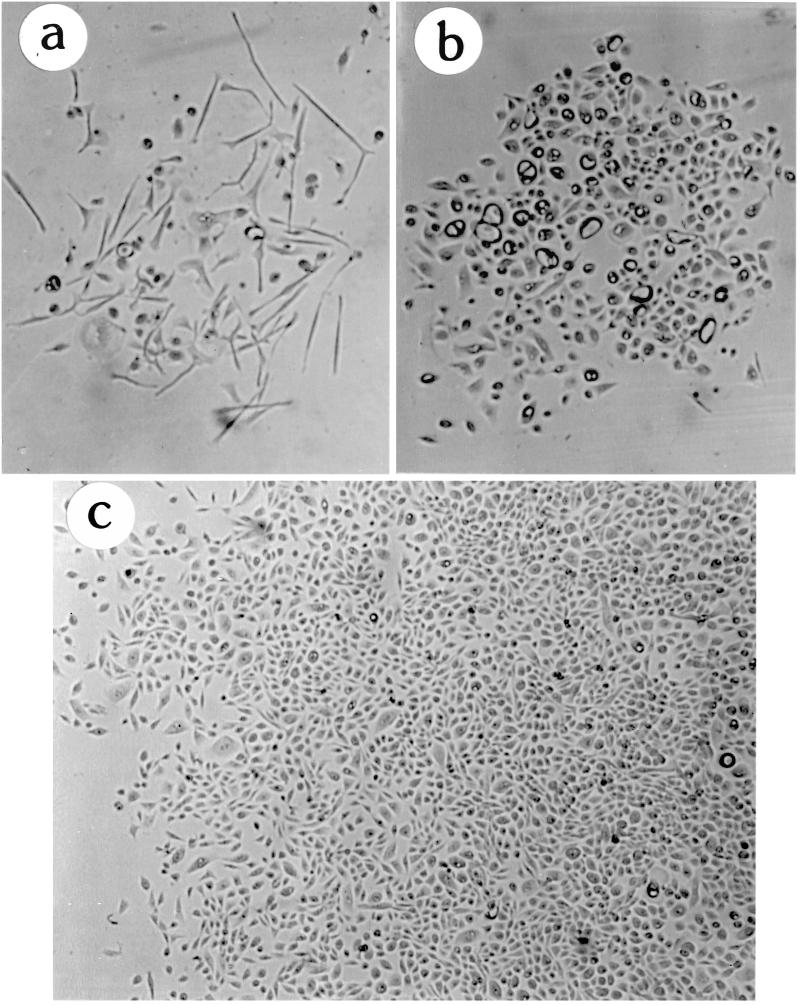
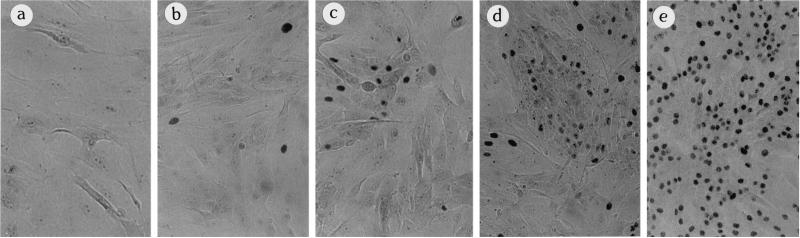
Figure 2.
Live cultures of 184A1 colonies at p28 showing morphologic heterogeneity. (a) Small colony that did not maintain growth; (b) colony with mixed growing and nongrowing, large vacuolated cells; (c) large colony with mostly growing cells. Magnification, 32×.
Table 1.
Growth of 184A1 and 184B5 colonies at different passage levels in the absence or presence of TGFβ
| Cell type | Passage no. | TGFβ | Labeling index (%)
|
No. of colonies | |||
|---|---|---|---|---|---|---|---|
| <10 | 10-25 | 26-50 | >50 | ||||
| 184A1 | 28 | − | 0 | 12 | 53 | 35 | 47 |
| 32 | − | 12 | 16 | 17 | 55 | 95 | |
| 38 | − | 10 | 10 | 12 | 68 | 389 | |
| 44 | − | 0 | 2 | 7 | 91 | 272 | |
| 184A1 | 28 | + | 100 | 0 | 0 | 0 | 34 |
| 35 | + | 59 | 37 | 4 | 0 | 83 | |
| 44 | + | 3 | 11 | 11 | 75 | 85 | |
| 184A1-TP | 28 | − | 0 | 0 | 0 | 100 | 17 |
| 28 | + | 12 | 11 | 22 | 55 | 42 | |
| B5Y16G | 25 | − | 6 | 14 | 44 | 34 | 50 |
| 31 | − | 0 | 6 | 0 | 94 | 51 | |
| 38 | − | 0 | 0 | 0 | 100 | 91 | |
| B5Y16G | 25 | + | 71 | 8 | 13 | 8 | 189 |
| 31 | + | 20 | 28 | 21 | 31 | 102 | |
| 38 | + | 0 | 0 | 0 | 100 | 135 | |
| B5Y16G-βR | 26 | − | 0 | 0 | 0 | 100 | 14 |
| 26 | + | 0 | 10 | 9 | 81 | 17 | |
Single cells were seeded and the labeling index ± TGFβ in colonies containing >50 cells was determined as described in MATERIALS AND METHODS. No. of colonies refers to the number of colonies counted to determine percentage labeling index. For 184A1-TP and B5Y16G-βR, cells were exposed to TGFβ for only 3 d.
184A1 populations at different passage levels were assayed for ability to maintain growth in TGFβ as described in MATERIALS AND METHODS. Using the mass culture assay, at p14 and p18, none of 106 cells were capable of generating focal areas of proliferation. At p24 one small, very slow growing area was generated from 4.5 × 105 cells seeded (≈2/106 cells). At p28, four small growing areas were generated from 8 × 105 cells seeded (≈5/106 cells); these areas contained flat cells and had a LI of 15–25% (Figure 3a). At p30, ∼39 growing areas were generated from 4.5 × 105 cells seeded (≈87/106 cells). Several areas were larger than the p28 patches, and 23% of these growing areas had a LI >50%. By p35 there was too much growth in the mass culture assay to permit quantitation of individual areas. Analysis of colonies in the single-cell assay showed considerable heterogeneity of growth in TGFβ (Table 1). Approximately 50% of the 83 single-cell-derived colonies examined showed some growth in TGFβ, but none of these colonies had a LI >50% and only four had a LI of 25–50%. Some individual colonies were morphologically heterogeneous, containing flat cells with low LI in the center and areas of smaller cells with a higher LI at the edges. By p44, 75% of single-cell-derived colonies had a LI of >50% in TGFβ (Table 1 and Figure 3b). These data indicate a very gradual acquisition of increasing capacity to maintain growth in the presence of TGFβ; cells capable of good growth in TGFβ were not observed until many passages after the detection of cells capable of poor growth in TGFβ. These results suggest a nonmutational origin of this phenotype because a single mutation would be expected to confer a one time quantal change rather than the observed gradual incremental changes.
Figure 3.
184A1 growth in TGFβ at different passages. Giemsa-stained cells labeled with [3H]thymidine for 24 h as described in MATERIALS AND METHODS. (a) Small slowly growing area at p28, part of a slightly larger area, labeled after 14 d exposure to TGFβ; this represents the best growth seen at p28. (b) Representative area of a typical p44 colony with good growth (LI >50%) labeled after 13 d exposure to TGFβ. Magnification, 125×.
Correlation of Telomere Length, Telomerase Activity, and Growth ± TGFβ in Early-Passage 184A1
The 184A1 populations assayed for growth capacity ± TGFβ were tested for mean TRF length and telomerase activity (Figures 1 and 4). When first assayed at p11, 184A1 mean TRF was 4.8 kb. This compared with a mean TRF of 5.5 kb in near-senescent p20 finite lifespan 184 HMEC and 5.2 kb in p13 184Aa, the EL precursor of 184A1 (data not shown). The 184A1 mean TRF decreased to a faint signal of <2.0 kb by p24. Mean TRF increased slightly thereafter to 2.3 kb at p31 and 3.5 kb at p100. No telomerase activity was detected in any finite-lifespan 184 HMEC or 184Aa populations grown in MCDB 170. In 184A1, no telomerase activity was detectable through p18. At p24–27, very weak activity could be detected only by using higher protein amounts per assay (>6 μg, data not shown). Weak activity was detected at p30 and the activity level increased thereafter. Thus, the first detection of telomerase activity and the gradual increase in this activity with continued passage paralleled the passage levels in which the mean TRF level stabilized and then increased slightly, and the capacity to maintain growth in TGFβ was first detected and then gradually increased.
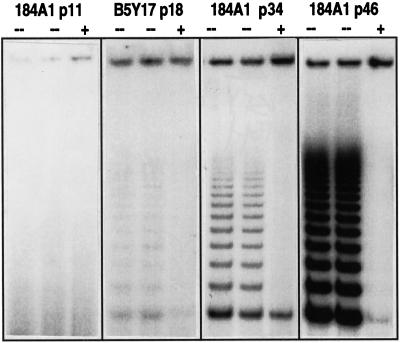
Figure 4.
Telomerase activity in 184A1 and 184B5 at different passages. Telomerase activity was determined by the TRAP assay as described in MATERIALS AND METHODS. Each sample was run in duplicate or with RNase added to the reaction mix in the indicated lanes (+) as a negative control. Each lane shows signals generated from 1 μg of cell extract. (a) 184A1 at p11 with no visible telomerase products. (b) B5Y17 at p18 shows a very weak level of telomerase activity (below the limits of detection of the PhosphorImager quantitation system). Similar results were seen with B5Y16G (p23), B5Y16L (p22, p35), B5Y16E (p24), and B5Y23 (p17). (c) 184A1 at p34 showing low levels of telomerase products. (d) 184A1 at p46 showing strong telomerase activity.
Early Conversion in 184A1
During our initial characterization of 184A1, we noted that cells at <p30 grew as single cells at low density, whereas higher passage cells grew in patches. In one instance, we noticed that a p23 dish contained proliferative cells growing in patches. By p25, the progeny cultures from this dish, unlike other 184A1 p25 cultures, contained primarily good growing cells. These were named 184A1-TP (tight patch) and stored frozen. To test whether 184A1-TP might represent a rare instance of early conversion in 184A1, p26 184A1-TP were replaced into culture ± TGFβ. Steady growth was maintained in the presence of TGFβ, although much slower and with flatter cells than the parallel cultures without TGFβ. At p28, 184A1-TP was assayed for growth ± TGFβ (Table 1) and telomerase activity and showed values similar to those seen at ∼p40 in the unselected 184A1 mass population. However, the 184A1-TP short mean TRF of 2.0 kb was similar to p28 184A1. These results suggest that 184A1-TP was generated by a rare cell, with a critically short mean TRF, that had undergone early conversion. They also support an association between the capacity to maintain growth ± TGFβ and expression of telomerase activity. 184A1-TP is the only example of such early conversion in 184A1 growing in MCDB 170 that we have noticed. It illustrates that a rare converted cell will rapidly take over a very slow growing nonconverted population and indicates that the gradual phenotypic changes usually observed are not due to a rare mutated cell.
Correlation of Growth ± TGFβ, Telomere Length, and Telomerase Activity in Early Passage 184B5
184B5 grows as tightly packed colonies derived from single cells, allowing the fate of individual cells to be readily followed. Unlike very early 184A1 (p9–15), early passages of 184B5 (p6–20) grew very slowly, with many large flat cells that did not produce colonies and many colonies that did not remain proliferative. At the earliest available freezedown (p9), 184B5 showed weak telomerase activity and a mean TRF of 3.3 kb. Because early 184B5 was heterogeneous in morphology and growth, and previous studies had also shown heterogeneity with respect to growth inhibition by TGFβ, we used clonal isolates of early 184B5 for our studies characterizing the correlation among growth patterns ± TGFβ, telomerase activity, and telomere length. Nineteen growing clones were isolated at p15, two of which did not maintain growth beyond p17. Eight of the remaining 17 clones have been carefully examined and illustrate the consistent and gradual emergence of fully converted cells from clonal populations.
Three examined clones did not maintain growth beyond p18 or p19 (Figure 5A, B5Y19, B5Y23, and B5Y33) and also displayed no growth in TGFβ, no or weak telomerase activity, and short mean TRFs of <2.0–2.5 kb with faint or very faint signals. A fourth clone B5Y17 also had no or very weak telomerase activity, no detectable TRF signal, and no growth in TGFβ at p18. By p19 almost all B5Y17 cells appeared flat and nonproliferative, but a few areas of morphologically similar proliferative tight patches were noted. When assayed at p22, progeny of these proliferative cells (B5Y17-βR) maintained slow to moderate growth in TGFβ, displayed low to medium telomerase activity, and had a mean TRF of 2.4–2.7 kb. Like 184A1-TP, the short mean TRF of the recently converted B5Y17-βR suggests that these cells emerged from a cell with very short telomeres. Similar to B5Y17, the previously described TGFβ-sensitive B5T1 clone (Hosobuchi and Stampfer, 1989) had no or weak telomerase activity before p30, whereas the progeny of the rare, TGFβ-resistant, proliferative cells were telomerase positive (data not shown). B5Y17 and B5T1 illustrate the emergence of rare telomerase-positive, TGFβ-resistant cells from largely telomerase-negative, TGFβ-sensitive clones and the association of telomerase activity with TGFβ resistance.
Figure 5.
Comparison of mean TRF length, telomerase activity, and growth in TGFβ in early-passage 184B5 and subclones. (A) Subclones that did not maintain growth ± TGFβ or had only rare cells maintaining growth. Since most of the cells generated from these clones were used for assays, only 2–5 × 105 cells/clone were available for observation of ability to maintain growth in mass culture. Consequently, a rare cell capable of maintaining growth may not have been detected. Y17-βR represents the progeny of rare B5Y17 cells that maintained proliferation. (B) Subclones that maintained growth and showed a gradual increase in the ability of cells to maintain growth in the presence of TGFβ. (C) The heterogeneous B5Y16 clone and B5Y16 subclones. 16G-βR represents the progeny of a rare p24 colony that grew well in TGFβ and was clonally isolated. Assays were performed as described in MATERIALS AND METHODS and the Figure 1 legend.
Three clones displayed a pattern of conversion similar to 184A1 (Figure 5B, clones B5Y13, B5Y40, B5Y7). On repeated examination from p17–30, cells with initial slow, nonuniform growth, rare poor growth in TGFβ, and weak-to-low initial telomerase activity gradually converted to populations with good uniform growth ± TGFβ and increasing levels of telomerase activity. Mean TRF initially ranged from 2.1 to 4.0 kb and was initially faint in two of these three clones. As illustrated for B5Y7, with continued passage, telomere length stabilized. The initially mixed morphology of tight cobblestone and spindle- shaped cells gradually became homogeneously cobblestone. The few small growing areas present in TGFβ at p20–23 contained both proliferative cobblestone cells and less proliferative spindle-shaped or flat cells (e.g., see Figure 6 below). With increasing passage, more and larger colonies with a higher LI and greater areas of cobblestone cells were seen in mass cultures exposed to TGFβ. These three clones illustrate that even within recently cloned populations there is a heterogeneous growth response to TGFβ and, like 184A1, a very gradual, reproducible acquisition of the ability to maintain good growth in the presence of TGFβ, associated with increasing levels of telomerase activity.
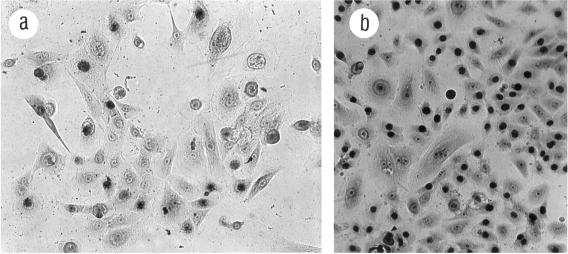
Figure 6.
Heterogeneity of subclone B5Y16G p25 colony growth in TGFβ. One thousand cells were seeded into 100-mm dishes and exposed to TGFβ 15 d after seeding. Cells remained in TGFβ an additional 18 d and were labeled with [3H]thymidine for the last 24 h. The Giemsa stained, single-cell–derived colonies shown are from the same dish. (a) Colony with no growth in TGFβ. (b) Mostly flat colony with rare scattered labeled cells. (c) Colony with growing small cobblestone cells amid flatter cells with little growth. (d) Colony with larger growing areas of small cobblestone cells amid flat cells. (e) Rare large colony with uniform good growth in TGFβ. These pictures are also illustrative of B5Y7, B5Y13, and B5Y40. In general, growth of these clones in TGFβ produced colonies: mostly like panel a and a few like panel b at ∼p17–20, ranging from panels a-c at p20–24; ranging from panels a-d at p23–27. By ∼ p27 and higher, colonies like panel e could be seen. Magnification, 125×
One clone, B5Y16, was already heterogeneous for growth when first observed after transfer to a 35-mm dish at p16. A few uniformly cobblestone colonies, with refractile cells and many mitoses, were present in addition to the colonies with tight cobblestone and spindle-shaped cells. When tested at p17, the refractile cobblestone colonies maintained good growth in the presence of TGFβ. This extremely rapid generation of heterogeneity in a clonal isolate was further investigated by isolating 14 growing subclones of B5Y16 at p20 and carefully characterizing six representative subclones (Figure 5C). One of the 14 isolated subclones did not maintain growth beyond p22. Two examined subclones, with the refractile cobblestone morphology, showed good uniform growth ± TGFβ, moderate telomerase activity, and mean TRF of 4.1 and 3.0 kb, respectively, when first assayed at p23. The other four subclones (B5Y16E, B5Y16L, B5Y16G, B5Y16H) showed a range of behavior similar to the three clones (B5Y13, B5Y40, B5Y7) described above, with the B5Y16H and B5Y16G subclones progressing to cells with good growth ± TGFβ, and increasing levels of telomerase activity, sooner than the B5Y16E and B5Y16L subclones. B5Y16 and its subclones demonstrate that a cell population obtained after <10 Pd from one conditionally immortal cell may be widely heterogeneous, containing cells ranging from no proliferative capacity to fully immortal.
The B5Y16G cells, which are a subclone of a clone of a clonal cell line, are a good illustration of the inherent heterogeneity in growth response to TGFβ and the gradual nature of conversion. At p21, growth was slow and nonuniform. At p23 in the presence of TGFβ, most cells were growth inhibited but some heterogeneous colonies were present. At p24, rare colonies that showed abundant mitotic figures and a more uniform refractile cobblestone morphology were noted in the absence of TGFβ, while in TGFβ-exposed cultures, three colonies with good growth were present. One of these colonies was isolated with a cloning cylinder and named B5Y16G-βR. It maintained good growth ± TGFβ, and when assayed at p26, it showed strong telomerase activity, while the mean TRF, at 2.2 kb, was still short (Figure 5C). B5Y16G-βR provides another example, along with B5Y17-βR and 184A1-TP, of the short mean TRF of recently converted cells, and of the correlation between telomerase activity and capacity to maintain growth in TGFβ.
B5Y16G was further examined for heterogeneity by seeding p25 cells at clonal densities for the single-cell assay for growth ± TGFβ (Table 1 and Figure 6). In the presence of TGFβ, cells gave rise to colonies with no growth (71%), a few proliferating cells (8%), variably sized areas of growing tight cobblestone cells and less proliferative spindle cells (13%), and nearly uniformly proliferative refractile cobblestone cells (8%). In the absence of TGFβ, approximately one-third of the colonies showed uniform good growth. When assayed again at p31, almost all cells gave rise to good growing colonies in the absence of TGFβ, while approximately half the cells generated colonies with moderate to good growth in TGFβ. By p38, all cells gave rise to good growing colonies even in the presence of TGFβ. Thus, individual cells from cell populations that had recently undergone repeated clonal isolation show an inherently heterogeneous growth response to TGFβ. The data in Table 1 also indicate that the phenotype of uniform good growth minus TGFβ is acquired before that for good growth in the presence of TGFβ.
Of the 15 184B5 clones and subclones we have studied, the 12 that maintained growth for more than three passages ultimately and reproducibly gave rise to fully converted cells. This result in clonal and subclonal populations is inconsistent with a rare mutational origin of the converted phenotype.
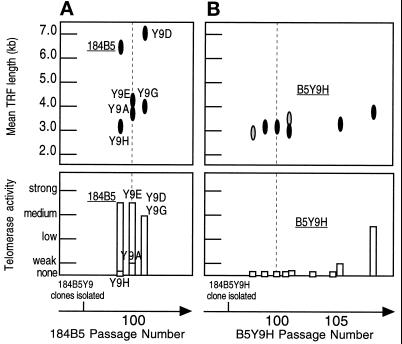
Variability in Growth, Telomere Length, and Telomerase Activity in Late Passage 184B5
To test whether all cells in fully converted HMEC populations had unlimited growth potential and telomerase activity, we examined uncloned 184B5 at p99 and five clones isolated at p96 (Figure 7A). The range of mean TRF lengths for these clones was 2.9–7.0 kb. To our surprise, B5Y9H, the clone with the shortest mean TRF, showed no detectable telomerase activity when first assayed at p99, although all these clones exhibited good growth ± TGFβ at that passage. TGFβ did induce morphologic changes in all five subclones. With continued passage, B5Y9H, but not the other four clones, showed a slowdown in growth around p103 and an initial total loss of proliferation at p105. However, after a few weeks, some B5Y9H p105 cells began to give rise to large outgrowths. These cells were subcultured and have maintained good growth until at least p116. This growth pattern ± TGFβ was reproduced when B5Y9H cells were again placed in culture at p98. Assay for telomerase activity indicated no or very weak activity up to and including the nonproliferative p105 population (Figure 7B). After the p105 dishes displayed the large outgrowths, telomerase activity was detectable. The mean TRF length of B5Y9H hovered around 3.0 kb before p105 and increased slightly thereafter. These data indicate that telomerase activity may cycle off and on even in converted cells. Unlike conditionally immortal cells, reactivation of telomerase in B5Y9H occurred relatively rapidly, within one passage. Additionally, these converted cells differed from the conditionally immortal in their ability to exhibit TGFβ resistance in the absence of telomerase activity. Their mean TRFs at the point of telomerase reactivation were also longer (∼3 vs. ∼2 kb).
Figure 7.
Mean TRF length and telomerase activity in late passage 184B5 and subclones at different passages. (A) 184B5 and subclones. (B) B5Y9H cells at different passage levels from two separate freezedowns. The first telomerase assay at p105 was from dishes containing vacuolated, nongrowing cells. The second assay for telomerase at p105 and TRF values were obtained from sister cultures that contained good growing patches. All B5Y9 clones assayed showed good growth in TGFβ. Assays were performed as described in MATERIALS AND METHODS and the Figure 1 legend. For TRF length, lighter shaded ovals indicate a faint signal.
DISCUSSION
This paper describes a new, apparently epigenetic step in immortal transformation of two different clonally derived HMEC lines, 184A1 and 184B5. The data suggest that the discrete event(s) that permitted growth beyond replicative senescence produced “conditionally” immortal cells, most of which did not maintain proliferative potential or express telomerase activity. Full immortality required an additional conversion step, characterized by gradual acquisition of uniform good growth in the absence and presence of TGFβ, and expression of telomerase activity. We speculate that the presence of extremely short telomeres is by itself sufficient to trigger the conversion process.
Based upon 184A1 and 184B5, as well as one new line (184AA4, unpublished), cells converting from conditional to full immortality exhibit the following phenotype. Conditionally immortal cells with a mean TRF >3 kb may initially show rapid uniform growth, but display no sustained growth in TGFβ, no telomerase activity, and continued telomere shortening with passage. When the cells reach, or first emerge with, a mean TRF of ∼3 kb, a severe growth constraint becomes prominent, as indicated by a very low CFE and nonuniform growth. There is still no sustained growth in TGFβ and no detectable telomerase activity. By the time the mean TRF has declined to ∼2 kb, most conditionally immortal cells have lost proliferative potential, but some cells have initiated a gradual conversion process. Rare cells showing poor but sustained growth in the presence of TGFβ, and weak telomerase activity, can now be detected. Subsequently, the cell population gradually and coordinately shows 1) more uniform and rapid growth, 2) increasing numbers of cells with progressively better growth in TGFβ, and 3) increasing levels of telomerase activity. Acquisition of these properties, considered characteristic of immortal epithelial cell lines, occurred within 10–30 passages under the conditions described here. The existence of continued telomere shortening for around 30 passages postestablishment before stabilization of telomere length has also been reported for two immortally transformed cell lines derived from breast cancer pleural effusions (Rogalla et al., 1994), suggesting that a conversion process might be occurring in this situation, as well.
Based largely on viral oncogene-mediated models of human cell transformation, previous investigators have suggested that immortal transformation involves overcoming at least two blocks, M1 and M2 (Shay et al., 1993). At M1, shortened telomeres signal activation of cell cycle checkpoint controls that cause a viable G1 arrest. Overcoming this block, ascribed to loss of normal pRB and p53 functions, yields EL cultures. Most EL cells cease growth or die when they encounter the block at M2. Overcoming this block has been considered to involve a rare mutation that occurs during crisis. Since the telomeres of EL cultures continue to shorten with passage, while most immortally transformed cell lines show telomerase activity and stabilized telomere length, it has been postulated that this mutation may involve reactivation of telomerase activity (Shay et al., 1993).
In the chemically transformed HMEC described in this paper, we observe a process of immortal transformation that differs from the viral-based model. Previous studies have not detected any defect in expression or regulation of p53 or RB in either the EL or immortal cell cultures (Lehman et al., 1993; Sandhu et al., 1997). However, these cultures, like postselection finite lifespan HMEC, show a relatively stable p53 protein and loss of p16 protein expression, either via mutation (184Aa, 184A1) or methylation of the p16 promoter region (184, 184Be, 184B5) (Brenner and Aldaz, 1995; Brenner, Stampfer, Aldaz, unpublished data). Although we do not know the nature of the event(s) that gave rise to the conditionally immortal cells, the data presented in this paper indicate that it was not immediate reactivation of telomerase activity. Instead, we postulate that there is an inherent epigenetic mechanism to reactivate telomerase when telomere length becomes critically short. This program is not normally encountered due to the multiple checkpoints preventing proliferation of cells with shortened telomeres. Our data indicate the existence of a novel checkpoint that must be overcome for conditionally immortal cells to convert to full immortality. Recent results suggest that this growth constraint may be mediated by the cyclin-dependent kinase (cdk) inhibitor p57KIP2 (Yaswen, Wigington, Garbe, Wong, and Stampfer, unpublished data). Unlike either finite lifespan or fully immortal HMEC, conditionally immortal cells accumulate high levels of p57 during G0 arrest. Good growing conditional immortal cells (mean TRF >3 kb) down-regulate p57 when released into the cycle, whereas in the nonproliferative or slowly growing cells (mean TRF ≤3 kb), p57 levels remain high.
Another novel aspect of our data is the association of TGFβ resistance and telomerase activity in early-passage cultures undergoing conversion, although these two phenotypes were separable in the fully immortal late-passage 184B5 clone B5Y9H. We do not yet know how these two phenotypes are linked during conversion; a single gene could be affecting multiple, independent pathways, or multiple independent genes could be coordinately affected by epigenetic changes. Our previous studies have shown that TGFβ growth inhibition in these HMEC is mediated by the cdk inhibitor p27KIP1 (Slingerland et al., 1994; Sandhu et al., 1997), which accumulates during G0 arrest and, in the absence of TGFβ, is down-regulated after release into the cycle. Current studies indicate that TGFβ-exposed, TGFβ-sensitive, telomerase-negative conditionally immortal cells maintain high levels of p27 in G1, whereas the fully immortal, TGFβ-resistant cells do not (Yaswen, Wigington, Garbe, Wong, and Stampfer, unpublished data). The relationship between this change in p27 regulation and the other changes occurring during conversion is presently under investigation.
Our presumption that conversion is an epigenetic mechanism is based upon its gradual incremental nature and reproducible manifestation in clonal populations. This is most clearly demonstrated in the acquisition of TGFβ resistance, in that the rare cells initially capable of proliferation in TGFβ show only poor growth, with an additional five to 20 passages required before cells capable of good growth in TGFβ can be detected. This gradual increase in both the number of cells capable of growing in TGFβ and the extent of growth exhibited by these cells, as well as the heterogeneity seen in single-cell outgrowths, is not consistent with either a mutational origin of TGFβ resistance or the takeover of the population by a rare cell capable of good growth in TGFβ. Similarly, there is a gradual increase in the capacity of individual cells to display good growth in the absence of TGFβ. Unlike most virally transformed cell lines, there is no evidence to support a phenotype of general genomic instability in 184A1 or 184B5. The karyotype does not develop gross aneuploidy (Walen and Stampfer, 1989) and normal p53 continues to be expressed (Lehman et al., 1993). Additionally, the lines do not spontaneously display other phenotypic changes associated with malignant progression, e.g., anchorage-independent growth or loss of growth factor requirements (Stampfer and Bartley, 1988; Stampfer et al., 1993a).
We postulate that critically short telomeres trigger the conversion process based on the following observations that: 1) conversion in 184A1 and 184B5 clones was first detected only after the mean TRF had declined to <2–2.5 kb; 2) the mean TRF in recently converted cells was 2.0–2.7 kb. Two possible mechanisms whereby short telomeres could initiate conversion are suggested by studies on gene silencing in yeast (Aparicio and Gottschling, 1994; Moretti et al., 1994; Hecht et al., 1996; Kim et al., 1996; Maillet et al., 1996). In one model, telomeres and their associated proteins create an area of heterochromatin extending beyond the telomeric and subtelomeric regions, leading to silencing of nearby genes. As telomeres shorten, the region of heterochromatin propagated down the end of the chromosome decreases, and previously silenced areas are gradually derepressed. Differences in telomere length of specific chromosomes could result in different gene expression among cells. In a second model, proteins associated with telomeric repeats may also serve as positive or negative regulators of gene transcription. The release of these proteins with progressive loss of telomere regions could lead to gradual alterations in gene expression elsewhere. In this model, differences in the overall level of remaining telomeric repeats could result in different gene expression among cells. Heterogeneity in telomere length of individual chromosomes of individual cells can be generated at each population doubling. Thus, it is theoretically possible to rapidly generate a multitude of branching lineages based upon varying telomere length. Such heterogeneity could explain one of the most unusual aspects of our data, the short time required to generate extensive heterogeneity from repeatedly cloned populations. Currently, there are no data to support the existence of gene silencing in human cells; however, this possibility has not been explored in nonimmortal cells.
Although unicellular organisms such as yeast express telomerase activity, this activity is regulated to maintain control of telomere length within a set range (Krauskopf and Blackburn, 1996; Cooper et al., 1997; Marcand et al., 1997). Mechanisms that measure the number of telomere-binding proteins enable telomerase to access and extend short telomeric ends, while preventing access/activity to telomeres beyond a set length. Human cells may likewise retain an epigenetic mechanism to ensure telomerase activity when telomeres shorten beyond a set length. However, normal finite lifespan human somatic cells, unlike yeast, possess mechanisms to halt proliferation before critically short telomeres are attained. In contrast, immortal human cells, like yeast, may maintain telomere length within a given range through regulation of telomerase access/activity (van Steensel and de Lange, 1997). This capacity is also suggested by the absence, and then subsequent reactivation, of telomerase activity in the late-passage 184B5 clone B5Y9H and the limited range of mean TRFs (3–7 kb) in fully converted late-passage 184A1 and 184B5 populations.
Immortal transformation of cultured human epithelial cells without the use of viral oncogenes, and with retention of a normal p53 gene, is an extremely rare occurrence, not reproducibly achieved. Consequently, most in vitro transformed human epithelial cell lines have been derived by exposure to viral oncogenes, particularly HPV E6 and/or HPV E7, or SV40 T, or show loss of p53 function. The viral oncogenes have multiple effects, and data from our laboratory (Garbe, Wong, Wigington, Yaswen, and Stampfer, unpublished data) indicate that they can circumvent the growth constraint encountered by conditionally immortal cells. 184A1 p12 cells exposed to the HPV 16 E6, HPV 16 E7, or SV40 T oncogenes showed immediate or rapid conversion to the fully immortal phenotype. Thus, examination of the conversion process may not be possible in cell lines immortalized by these oncogenes. Preliminary results from our laboratory also indicate that loss of p53 function may accelerate the conversion process. We have established two new cell lines, from the 184Aa EL culture, that show loss of p53 expression (Garbe, Wong, Wigington, Yaswen, and Stampfer, unpublished data). Both of these lines demonstrated accelerated conversion compared with the three p53-positive lines. Additionally, introduction of p53 dominant negative mutants into early- passage 184A1 leads to a more rapid conversion process. Thus, cells immortalized via total loss of p53 function may still proceed through a conversion step, but more rapidly.
Based on the HMEC lines we have developed, we propose the following model for their immortal transformation. Escape from replicative senescence requires loss of several distinct pathways of negative growth restraints. One pathway involves regulation of RB, but loss of RB is not required. In our HMEC, loss of p16 protein expression is sufficient. Another pathway may involve maintenance of a normal function regulated by p53, but total loss of p53 is not required. It is also possible that very short telomeres may induce structural-tensile growth constraints (Maniotis et al., 1997) that can be relieved via a loss of function change. Alleviation of all of these growth restraint pathways produces conditionally immortal cells that initially lack telomerase activity. Continued telomere shortening leads to gradual changes in gene expression due to decreased propagation of heterochromatic structure and/or redistribution of telomere-associated proteins. As a consequence of altered gene expression, a p57-mediated growth constraint is encountered, and/or telomerase expression and activity at telomeric ends is altered. Heterogeneity in the length of critically short telomeres may produce a stochastic heterogeneity in the ability of individual cells to maintain proliferation. In addition to this inherent heterogeneity, external conditions may also influence an individual cell’s ability to maintain growth. Ongoing studies indicate that culture conditions (e.g., the presence of serum) can influence the efficacy with which conditionally immortal cells overcome this growth constraint. We theorize that the relative levels of expression of several interacting molecules will determine the fate of individual conditional immortal cells undergoing conversion. This model proposes that the minimal requirements for immortal transformation involve only loss of the negative growth restraints imposed on long-lived multicellular organisms to prevent continued growth with shortened telomeres, and that human epithelial cells possess an inherent epigenetic mechanism to reactivate telomerase when telomeres decrease below a set length. We postulate that malignant transformation requires additional errors providing positive growth advantages and invasive capacity.
The molecular mechanism(s) that mediate the conversion process are presently unknown. Such mechanisms are likely to depend upon quantitative interactions of multiple cellular components, each of whose levels may vary over a continuous range, in addition to the all-or-none effects exerted by somatic mutations. Such complex interactions may be difficult to precisely determine in a system with multiple undefined variables. The existence of the conversion process in these HMEC highlights the importance of considering epigenetic bases for biological processes.
We consider the most interesting question to be whether a conversion process occurs during carcinogenic progression in vivo. Several features of tumor development could be related to the existence of conversion:
1. Many primary carcinomas, including breast, exhibit an extended period of slow, heterogeneous growth before the appearance of more aggressive, invasive tumors (Fujii et al., 1996). An extended period of conversion in vivo could provide a continuous pool of slowly dividing cells able to accumulate errors that promote malignant behavior. Our data indicate both that conditionally immortal cells can undergo a very large number of population doublings before becoming fully converted, and that there can be stochastic emergence of rare, more fully converted cells.
2. Human carcinomas commonly display resistance to growth inhibition by TGFβ (Fynan and Reiss, 1993; Arteaga et al., 1996). In some cases, resistance can be attributed to loss of functional TGFβ receptors; however, in most instances, normal receptors are still present. Although some receptor-positive breast tumor cell lines show reduced growth rates in TGFβ, growth is maintained. An obligate gain of TGFβ resistance with conversion could explain why this phenotype is common to tumor cells. However, the gradual nature of conversion could still provide a selective advantage for cells that gain TGFβ resistance via receptor loss. Gradual conversion in vivo might account for the large variability in growth inhibition observed when tumor cells and cell lines from a variety of organ systems are exposed to TGFβ in vitro (Hurteau et al., 1994; Blaydes et al., 1995; Havrilesky et al., 1995).
3. Most primary breast carcinomas display telomerase activity (Hiyama et al., 1996) but very rarely give rise to immortal cell lines. We have seen that conditionally immortal cells that have begun the conversion process may express detectable telomerase activity, while still exhibiting slow nonuniform growth. The immortal potential of these slowly growing populations could be easily missed. In addition to their poor growth, we have observed that failure to subculture conditionally immortal cell populations well before confluence can result in loss of viability of the entire culture.
Understanding the minimal steps required to attain immortality will provide a clearer picture of 1) the obligate differences between finite lifespan and immortal cells; 2) the extent to which existing in vitro-transformed or tumor-derived cells lines harbor derangements unrelated to immortalization; 3) the role of immortal transformation in carcinogenic progression in vivo. For example, minimal immortal transformation may require obligate changes in cdk inhibitor regulation but may not require genomic instability. In vivo tumor development requires accumulation of aberrations in addition to immortality, a process that genomic instability would facilitate. Acquisition of full immortality may not be necessary for these other growth control errors to occur; an extended period of conditional immortality could be sufficient. Regardless of the origin of an immortal cell line, normal human somatic cells are not immortal, and therefore immortal cell lines are not normal. Our studies suggest that all immortal lines will have some obligate differences in cell cycle regulation compared with finite lifespan cells. Consequently, indiscriminate use of immortal cell lines, most of which also have the tumor-associated properties of loss of p53 function and aneuploidy, as “normal” controls or to model cell cycle control mechanisms, may seriously obscure our understanding of both normal cellular physiology and the growth control derangements occurring during malignant progression.
In summary, we have uncovered a novel, apparently epigenetic step involved in the immortal transformation of HMEC in culture. This step, which we have called conversion, occurs in cells that have overcome replicative senescence, but have not obtained uniform indefinite proliferative potential. These conditionally immortal cells show a gradual reactivation of telomerase expression along with increasing capacity for uniform good growth in the absence or presence of TGFβ. Conversion from conditional to full immortality is a reproducible program manifested in both mass cultures and clonal isolates. It is possible that the slow heterogeneous growth we observe in conditionally immortal HMEC models the slow heterogeneous growth observed during development of many primary carcinomas in vivo. If so, determining whether the process of conversion can be prevented, halted, or slowed may open the door to novel methods for clinical intervention in cancer progression. Our work describing the phenotypic changes manifested by HMEC during conversion provides the information essential for future studies exploring the as-yet-unknown underlying molecular mechanisms.
ACKNOWLEDGMENTS
This work was supported by National Institutes of Health grant CA-24844 (M.R.S.) and the Office of Energy Research, Office of Health and Environmental Research, U.S. Department of Energy under Contract No. DE-AC03–76SF00098 (M.R.S., P.Y.).
Footnotes
Abbreviations used: CFE, colony-forming efficiency; EL, extended life; HMEC, human mammary epithelial cells; LI, labeling index; TRF, telomere restriction fragment.
REFERENCES
- Aparicio OM, Gottschling DE. Overcoming telomeric silencing: a trans-activator competes to establish gene expression in a cell-cycle dependent way. Genes & Dev. 1994;8:1133–1146. doi: 10.1101/gad.8.10.1133. [DOI] [PubMed] [Google Scholar]
- Arteaga CL, Dugger TC, Hurd SD. The mulltifunctional role of TGF-beta on mammary epithelial cell biology. Breast Cancer Res Treat. 1996;38:49–56. doi: 10.1007/BF01803783. [DOI] [PubMed] [Google Scholar]
- Bacchetti S. Telomere dynamics and telomerase activity in cell senescence and cancer. Cell Dev Biol. 1996;7:31–39. [Google Scholar]
- Blaydes JP, Schlumberger M, Wynford-Thomas D, Wyllie F. Interaction between p53 and TGFβ1 in control of epithelial cell proliferation. Oncogene. 1995;10:307–317. [PubMed] [Google Scholar]
- Bodnar AG, Kim NW, Effros RB, Chiu C-P. Mechanism of telomerase induction during T cell activation. Exp Cell Res. 1996;228:58–64. doi: 10.1006/excr.1996.0299. [DOI] [PubMed] [Google Scholar]
- Brenner AJ, Aldaz CM. Chromosome 9p allelic loss and p16/CDKN2 in breast cancer and evidence of p16 inactivation in immortal breast epithelial cells. Cancer Res. 1995;55:2892–2895. [PubMed] [Google Scholar]
- Chiu C-P, Dragowska W, Kim NW, Vuziri H, Yui J, Thomas TE, Harley CB, Lansdorp PM. Differential expression of telomerase activity in hematopoietic progenitors from adult human bone marrow. Stem Cells. 1996;14:239–248. doi: 10.1002/stem.140239. [DOI] [PubMed] [Google Scholar]
- Cooper JP, Nimmo ER, Allshire RC, Cech TR. Regulation of telomere length and function by a myb-domain protein in fission yeast. Nature. 1997;385:744–747. doi: 10.1038/385744a0. [DOI] [PubMed] [Google Scholar]
- Counter CM, Avilion AA, LeFeuvre CE, Stewart NG, Greider CW, Harley CB, Bacchetti S. Telomere shortening associated with chromosome instability is arrested in immortal cells which express telomerase activity. EMBO J. 1992;11:1921–1929. doi: 10.1002/j.1460-2075.1992.tb05245.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Counter CM, Botelho FM, Wang P, Harley CB, Bacchetti S. Stabilization of short telomeres and telomerase activity accompany immortalization of Epstein-Barr virus-transformed human B lymphocytes. J Virol. 1994;68:3410–3414. doi: 10.1128/jvi.68.5.3410-3414.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujii H, Marsh C, Cairns P, Sidransky D, Gabrielson E. Genetic divergence in the clonal evolution of breast cancer. Cancer Res. 1996;56:1493–1497. [PubMed] [Google Scholar]
- Fynan TM, Reiss M. Resistance to inhibition of cell growth by transforming growth factor-beta and its role in oncogenesis. Crit Rev Oncog. 1993;4:493–540. [PubMed] [Google Scholar]
- Hammond SL, Ham RG, Stampfer MR. Serum-free growth of human mammary epithelial cells: rapid clonal growth in defined medium and extended serial passage with pituitary extract. Proc Natl Acad Sci USA. 1984;81:5435–5439. doi: 10.1073/pnas.81.17.5435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Härle-Bachor C, Boukamp P. Telomerase activity in the regenerative basal layer of the epidermis in human skin and in immortal and carcinoma-derived skin keratinocytes. Proc Natl Acad Sci USA. 1996;93:6476–6481. doi: 10.1073/pnas.93.13.6476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harley C, Villeponteau B. Telomeres and telomerase in aging and cancer. Curr Opin Genet Devel. 1995;5:249–255. doi: 10.1016/0959-437x(95)80016-6. [DOI] [PubMed] [Google Scholar]
- Havrilesky LJ, Hurteau JA, Whitaker RS, Elbendary A, Wu S, Rodriguez GC, Bast RC, Jr, Berchuck A. Regulation of apoptosis in normal and malignant ovarian epithelial cells by transforming growth factor β1. Cancer Res. 1995;55:944–948. [PubMed] [Google Scholar]
- Hecht A, Strahl-Bolsinger S, Grunstein M. Spreading of transcriptional repressor SIR3 from telomeric heterochromatin. Nature. 1996;383:92–95. doi: 10.1038/383092a0. [DOI] [PubMed] [Google Scholar]
- Henderson S, Allsopp R, Spector D, Wang S-S, Harley C. In situ analysis of changes in telomere size during replicative aging and cell transformation. J Cell Biol. 1996;134:1–12. doi: 10.1083/jcb.134.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiyama E, Gollahon L, Kataoka T, Kruoi K, Yokoyama T, Gazdar AF, Hiyama K, Piatyszek MA, Shay JW. Telomerase activity in human breast tumors. J Natl Cancer Inst. 1996;88:116–122. doi: 10.1093/jnci/88.2.116. [DOI] [PubMed] [Google Scholar]
- Hosobuchi M, Stampfer MR. Effects of transforming growth factor-β on growth of human mammary epithelial cells in culture. In Vitro. 1989;25:705–712. doi: 10.1007/BF02623723. [DOI] [PubMed] [Google Scholar]
- Hurteau J, Rodriguez GC, Whitaker RS, Shah S, Mills G, Bast RC, Berchuck A. Transforming growth factor-beta inhibits proliferation of human ovarian cancer cells obtained from ascites. Cancer. 1994;74:93–99. doi: 10.1002/1097-0142(19940701)74:1<93::aid-cncr2820740117>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- Kim NW, Piatyszek MA, Prowse KR, Harley CB, West MD, Ho PL C, Coviello GM, Wright WE, Weinrich SL, Shay JW. Specific association of human telomerase activity with immortal cells and cancer. Science. 1994;266:2011–2015. doi: 10.1126/science.7605428. [DOI] [PubMed] [Google Scholar]
- Kim S, Villeponteau B, Jazwinski SM. Effect of replicative age on transcriptional silencing near telomeres in Saccharomyces cervisiae. Biochem Biophys Res Commun. 1996;219:370–376. doi: 10.1006/bbrc.1996.0240. [DOI] [PubMed] [Google Scholar]
- Krauskopf A, Blackburn EH. Control of telomere growth by interactions with the most distal telomeric repeats. Nature. 1996;383:354–357. doi: 10.1038/383354a0. [DOI] [PubMed] [Google Scholar]
- Lehman T, Modali R, Boukamp P, Stanek J, Bennett W, Welsh J, Metcalf R, Stampfer M, Fusenig N, Rogan E, Reddel R, Harris C. p53 mutations in human immortalized epithelial cell lines. Carcinogenesis. 1993;14:833–839. doi: 10.1093/carcin/14.5.833. [DOI] [PubMed] [Google Scholar]
- Lundblad V, Wright WE. Telomeres and telomerase: a simple picture becomes complex. Cell. 1996;87:369–375. doi: 10.1016/s0092-8674(00)81358-6. [DOI] [PubMed] [Google Scholar]
- Maillet L, Boscheron C, Gotta M, Marcand S, E, G, Gasser SM. Evidence for silencing compartments within the yeast nucleus: a role for telomere proximity and Sir protein concentration in silencer-mediated repression. Genes & Dev. 1996;10:1796–1811. doi: 10.1101/gad.10.14.1796. [DOI] [PubMed] [Google Scholar]
- Maniotis AJ, Chen CS, Ingber DE. Demonstration of mechanical connections between integrins, cytoskeletal filaments, and nucleoplasm that stabilize nuclear structure. Proc Natl Acad Sci USA. 1997;94:849–854. doi: 10.1073/pnas.94.3.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcand S, Gilson E, Shore D. A protein counting mechanism for telomere length regulation in yeast. Science. 1997;275:986–990. doi: 10.1126/science.275.5302.986. [DOI] [PubMed] [Google Scholar]
- Moretti P, Freeman K, Coodly L, Shore D. Evidence that a complex of SIR proteins interacts with the silencer and telomere-binding protein RAP1. Genes & Dev. 1994;8:2257–2269. doi: 10.1101/gad.8.19.2257. [DOI] [PubMed] [Google Scholar]
- Rogalla P, Kazmierczak B, Rohen C, Trams G, Bartnitzke S, Bullerdiek J. Two human breast cancer cell lines showing decreasing telomeric repeat length during early in vitro passaging. Cancer Genet Cytogenet. 1994;77:19–25. doi: 10.1016/0165-4608(94)90143-0. [DOI] [PubMed] [Google Scholar]
- Sandhu C, Garbe J, Bhattacharya N, Daksis JI, Pan C-H, Yaswen P, Koh J, Slingerland JM, Stampfer MR. TGF-β stabilizes p15INK4B protein, increases p15INK4B/cdk4 complexes and inhibits cyclin D1/cdk4 association in human mammary epithelial cells. Mol Cell Biol. 1997;17:2458–2467. doi: 10.1128/mcb.17.5.2458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shay JW, Wright WE, Werbin H. Toward a molecular understanding of human breast cancer: a hypothesis. Breast Cancer Res Treat. 1993;25:83–94. doi: 10.1007/BF00662404. [DOI] [PubMed] [Google Scholar]
- Slingerland JM, Hengst L, Pan C-H, Alexander D, Stampfer MR, Reed SI. A novel inhibitor of cyclin/cdk activity detected in TGF-β arrested cells. Mol Cell Biol. 1994;14:3683–3694. doi: 10.1128/mcb.14.6.3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JR, Pereira-Smith OM. Replicative senescence: implications for in vivo aging and tumor suppression. Science. 1996;273:63–67. doi: 10.1126/science.273.5271.63. [DOI] [PubMed] [Google Scholar]
- Stampfer MR. Cholera toxin stimulation of human mammary epithelial cells in culture. In Vitro. 1982;18:531–537. doi: 10.1007/BF02810076. [DOI] [PubMed] [Google Scholar]
- Stampfer MR. Isolation and growth of human mammary epithelial cells. J Tissue Culture Methods. 1985;9:107–116. [Google Scholar]
- Stampfer MR, Bartley JC. Induction of transformation and continuous cell lines from normal human mammary epithelial cells after exposure to benzo(a)pyrene. Proc Natl Acad Sci USA. 1985;82:2394–2398. doi: 10.1073/pnas.82.8.2394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stampfer MR, Bartley JC. Human mammary epithelial cells in culture: differentiation and transformation. In: Dickson R, Lippman M, editors. Breast Cancer: Cellular and Molecular Biology. Norwall, MA: Kluwer Academic Publishers; 1988. pp. 1–24. [DOI] [PubMed] [Google Scholar]
- Stampfer MR, Pan CH, Hosoda J, Bartholomew J, Mendelsohn J, Yaswen P. Blockage of EGF receptor signal transduction causes reversible arrest of normal and transformed human mammary epithelial cells with synchronous reentry into the cell cycle. Exp Cell Res. 1993a;208:175–188. doi: 10.1006/excr.1993.1236. [DOI] [PubMed] [Google Scholar]
- Stampfer MR, Yaswen P, Alhadeff M, Hosoda J. TGFβ induction of extracellular matrix associated proteins in normal and transformed human mammary epithelial cells in culture is independent of growth effects. J Cell Physiol. 1993b;155:210–221. doi: 10.1002/jcp.1041550127. [DOI] [PubMed] [Google Scholar]
- van Steensel B, de Lange T. Control of telomere length by the human telomeric protein TRF1. Nature. 1997;385:740–743. doi: 10.1038/385740a0. [DOI] [PubMed] [Google Scholar]
- Walen K, Stampfer MR. Chromosome analyses of human mammary epithelial cells at stages of chemically-induced transformation progression to immortality. Cancer Genet Cytogenet. 1989;37:249–261. doi: 10.1016/0165-4608(89)90056-3. [DOI] [PubMed] [Google Scholar]
- Wright WE, Shay JW. Time, telomeres and tumors: is cellular senescence more than an anticancer mechanism. Trends Cell Biol. 1995;5:293–297. doi: 10.1016/s0962-8924(00)89044-3. [DOI] [PubMed] [Google Scholar]
- Wright WE, Shay JW, Piatyszek MA. Modifications of a telomeric repeat amplification protocol (TRAP) result in increased reliability, linearity and sensitivity. Nucleic Acids Res. 1995;23:3794–3795. doi: 10.1093/nar/23.18.3794. [DOI] [PMC free article] [PubMed] [Google Scholar]