Abstract
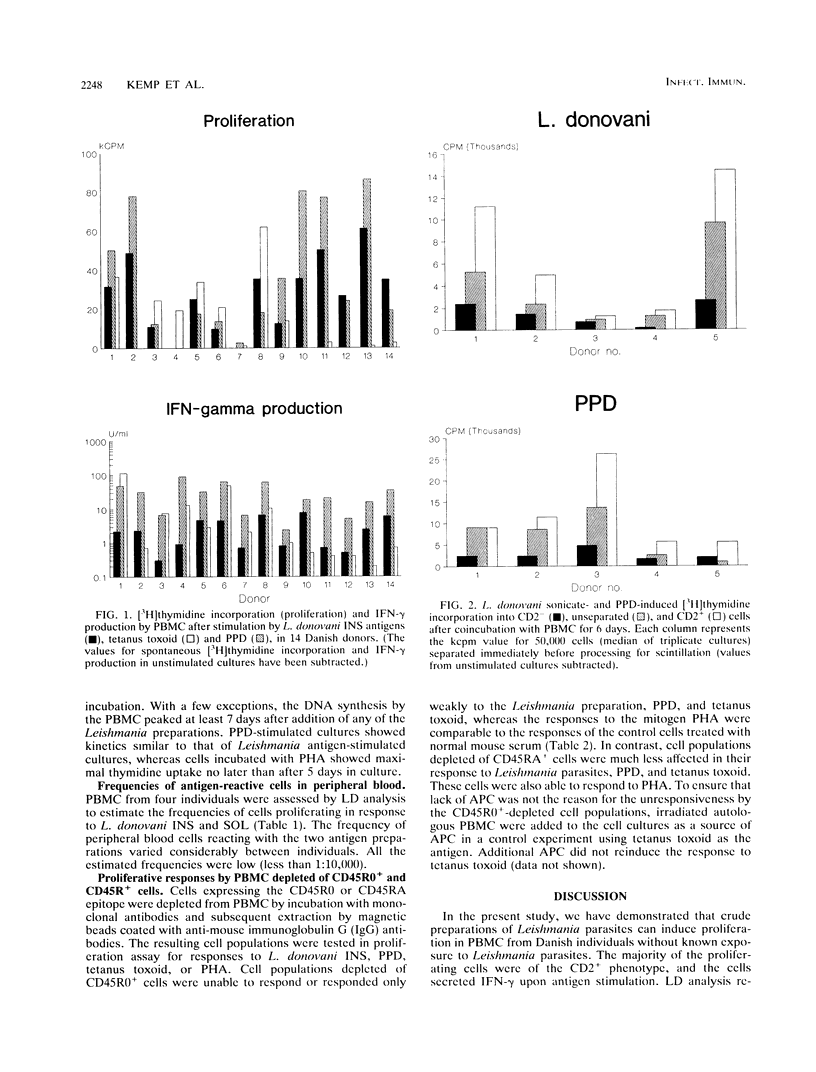
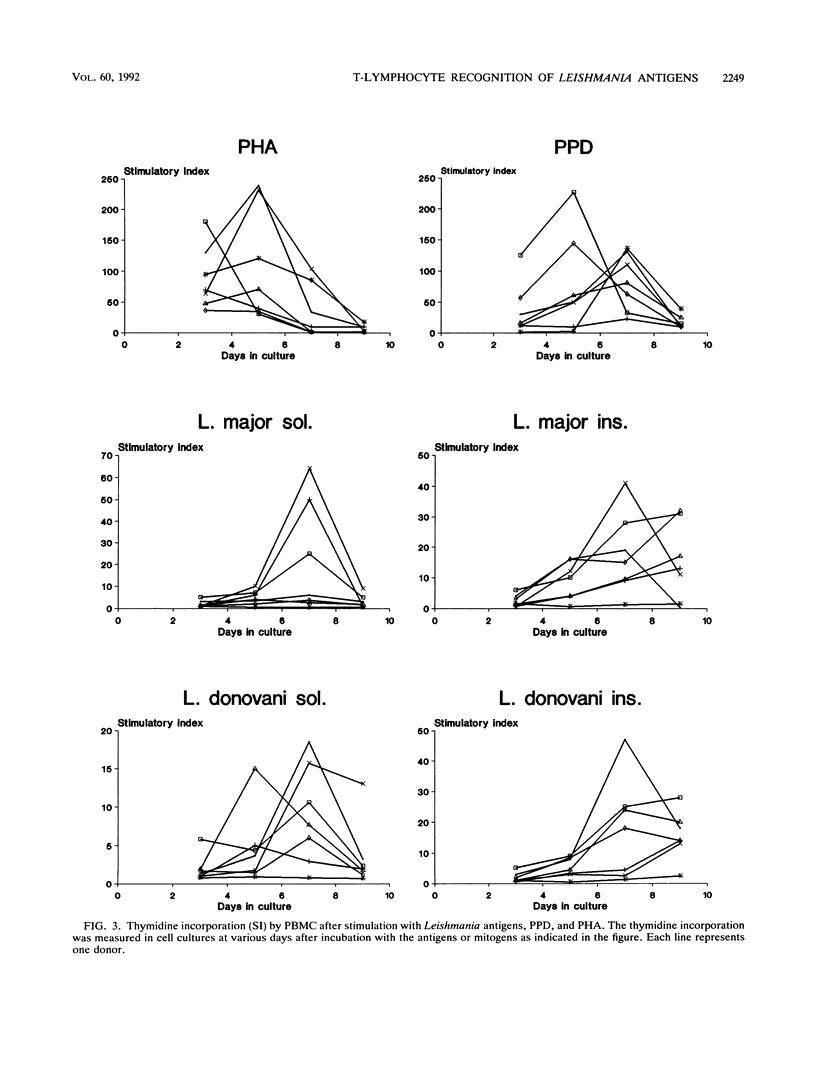
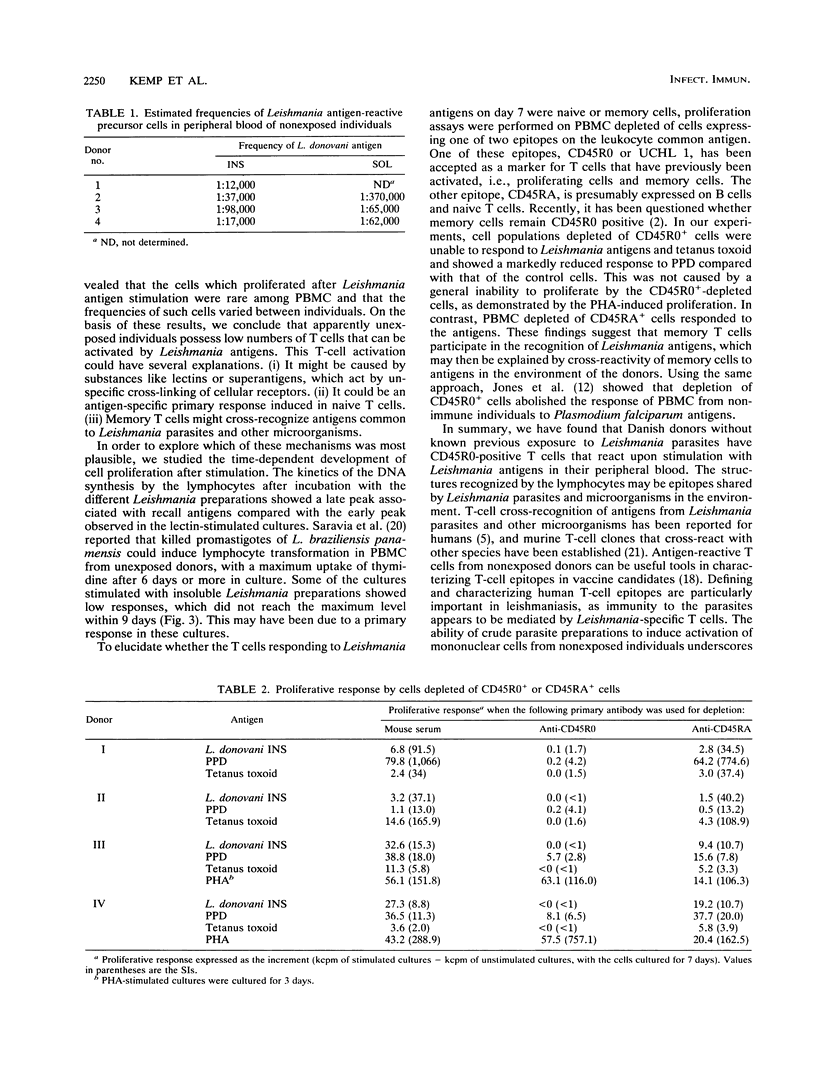
Crude antigen preparations of Leishmania promastigote sonicates were found to induce in vitro proliferation and gamma interferon production in peripheral blood mononuclear cells (PBMC) from individuals without known exposure to the parasite. The proliferating cells were mainly CD2-positive T cells. The proliferative response was maximal after more than 6 days of incubation with the antigens in contrast to the proliferation induced by the mitogenic lectin phytohemagglutinin (PHA), which peaked after 3 to 5 days. Judged by limiting dilution analysis, the frequencies of antigen-reactive precursor cells were less than 1:10,000 and varied considerably between individuals. Depletion of CD45R0-positive (memory) cells from the PBMC abolished proliferative responses induced by Leishmania antigen and by tetanus toxoid. In cell populations depleted of CD45RA-positive (naive) cells, only a small reduction in response was observed. Cell populations depleted of either CD45R0-positive cells or CD45RA-positive cells both responded to PHA. We conclude that presumably unexposed individuals have a low number of Leishmania-reactive T cells in their circulatory systems. The Leishmania-reactive T cells in these individuals are most likely memory cells recognizing antigens present both in the Leishmania preparations and in the environment.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Badaro R., Falcoff E., Badaro F. S., Carvalho E. M., Pedral-Sampaio D., Barral A., Carvalho J. S., Barral-Netto M., Brandely M., Silva L. Treatment of visceral leishmaniasis with pentavalent antimony and interferon gamma. N Engl J Med. 1990 Jan 4;322(1):16–21. doi: 10.1056/NEJM199001043220104. [DOI] [PubMed] [Google Scholar]
- Bell E. B., Sparshott S. M. Interconversion of CD45R subsets of CD4 T cells in vivo. Nature. 1990 Nov 8;348(6297):163–166. doi: 10.1038/348163a0. [DOI] [PubMed] [Google Scholar]
- Berenguer J., Moreno S., Cercenado E., Bernaldo de Quirós J. C., García de la Fuente A., Bouza E. Visceral leishmaniasis in patients infected with human immunodeficiency virus (HIV). Ann Intern Med. 1989 Jul 15;111(2):129–132. doi: 10.7326/0003-4819-111-2-129. [DOI] [PubMed] [Google Scholar]
- Brett S. J., Kingston A. E., Colston M. J. Limiting dilution analysis of the human T cell response to mycobacterial antigens from BCG vaccinated individuals and leprosy patients. Clin Exp Immunol. 1987 Jun;68(3):510–520. [PMC free article] [PubMed] [Google Scholar]
- Carvalho E. M., Reed S. G., Johnson W. D., Jr Cross reactivity between Trypanosoma cruzi and Leishmania antigens in the lymphocyte blastogenesis assay. Trans R Soc Trop Med Hyg. 1987;81(1):82–84. doi: 10.1016/0035-9203(87)90291-4. [DOI] [PubMed] [Google Scholar]
- Douvas G. S., Looker D. L., Vatter A. E., Crowle A. J. Gamma interferon activates human macrophages to become tumoricidal and leishmanicidal but enhances replication of macrophage-associated mycobacteria. Infect Immun. 1985 Oct;50(1):1–8. doi: 10.1128/iai.50.1.1-8.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández-Guerrero M. L., Aguado J. M., Buzón L., Barros C., Montalbán C., Martín T., Bouza E. Visceral leishmaniasis in immunocompromised hosts. Am J Med. 1987 Dec;83(6):1098–1102. doi: 10.1016/0002-9343(87)90948-x. [DOI] [PubMed] [Google Scholar]
- Harms G., Zwingenberger K., Chéhadé A. K., Talhari S., Racz P., Mouakeh A., Douba M., Näkel L., Naiff R. D., Kremsner P. G. Effects of intradermal gamma-interferon in cutaneous leishmaniasis. Lancet. 1989 Jun 10;1(8650):1287–1292. doi: 10.1016/s0140-6736(89)92686-x. [DOI] [PubMed] [Google Scholar]
- Ho M., Koech D. K., Iha D. W., Bryceson A. D. Immunosuppression in Kenyan visceral leishmaniasis. Clin Exp Immunol. 1983 Feb;51(2):207–214. [PMC free article] [PubMed] [Google Scholar]
- Hoover D. L., Nacy C. A., Meltzer M. S. Human monocyte activation for cytotoxicity against intracellular Leishmania donovani amastigotes: induction of microbicidal activity by interferon-gamma. Cell Immunol. 1985 Sep;94(2):500–511. doi: 10.1016/0008-8749(85)90274-6. [DOI] [PubMed] [Google Scholar]
- Hviid L., Sørensen A. L., Kharazmi A., Theander T. G. Functional and phenotypic changes in human lymphocytes after coincubation with Leishmania donovani in vitro. Infect Immun. 1990 Oct;58(10):3163–3167. doi: 10.1128/iai.58.10.3163-3167.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones K. R., Hickling J. K., Targett G. A., Playfair J. H. Polyclonal in vitro proliferative responses from nonimmune donors to Plasmodium falciparum malaria antigens require UCHL1+ (memory) T cells. Eur J Immunol. 1990 Feb;20(2):307–315. doi: 10.1002/eji.1830200212. [DOI] [PubMed] [Google Scholar]
- Kemp M., Theander T. G., Handman E., Hey A. S., Kurtzhals J. A., Hviid L., Sørensen A. L., Were J. O., Koech D. K., Kharazmi A. Activation of human T lymphocytes by Leishmania lipophosphoglycan. Scand J Immunol. 1991 Feb;33(2):219–224. doi: 10.1111/j.1365-3083.1991.tb03752.x. [DOI] [PubMed] [Google Scholar]
- Kurtzhals J. A., Hey A. S., Theander T. G., Odera E., Christensen C. B., Githure J. I., Koech D. K., Schaefer K. U., Handman E., Kharazmi A. Cellular and humoral immune responses in a population from the Baringo District, Kenya to Leishmania promastigote lipophosphoglycan. Am J Trop Med Hyg. 1992 Apr;46(4):480–488. doi: 10.4269/ajtmh.1992.46.480. [DOI] [PubMed] [Google Scholar]
- Mendonça S. C., Russell D. G., Coutinho S. G. Analysis of the human T cell responsiveness to purified antigens of Leishmania: lipophosphoglycan (LPG) and glycoprotein 63 (gp 63). Clin Exp Immunol. 1991 Mar;83(3):472–478. doi: 10.1111/j.1365-2249.1991.tb05663.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray H. W., Oca M. J., Granger A. M., Schreiber R. D. Requirement for T cells and effect of lymphokines in successful chemotherapy for an intracellular infection. Experimental visceral leishmaniasis. J Clin Invest. 1989 Apr;83(4):1253–1257. doi: 10.1172/JCI114009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray H. W., Rubin B. Y., Rothermel C. D. Killing of intracellular Leishmania donovani by lymphokine-stimulated human mononuclear phagocytes. Evidence that interferon-gamma is the activating lymphokine. J Clin Invest. 1983 Oct;72(4):1506–1510. doi: 10.1172/JCI111107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pink J. R., Sinigaglia F. Characterizing T-cell epitopes in vaccine candidates. Immunol Today. 1989 Dec;10(12):408–409. doi: 10.1016/0167-5699(89)90037-6. [DOI] [PubMed] [Google Scholar]
- Sacks D. L., Lal S. L., Shrivastava S. N., Blackwell J., Neva F. A. An analysis of T cell responsiveness in Indian kala-azar. J Immunol. 1987 Feb 1;138(3):908–913. [PubMed] [Google Scholar]
- Saravia N. G., Valderrama L., Labrada M., Holguín A. F., Navas C., Palma G., Weigle K. A. The relationship of Leishmania braziliensis subspecies and immune response to disease expression in New World leishmaniasis. J Infect Dis. 1989 Apr;159(4):725–735. doi: 10.1093/infdis/159.4.725. [DOI] [PubMed] [Google Scholar]
- Sheppard H. W., Scott P. A., Dwyer D. M. Recognition of Leishmania donovani antigens by murine T lymphocyte lines and clones. Species cross-reactivity, functional correlates of cell-mediated immunity, and antigen characterization. J Immunol. 1983 Sep;131(3):1496–1503. [PubMed] [Google Scholar]
- Sørensen A. L., Kharazmi A., Nielsen H. Leishmania interaction with human monocytes and neutrophils: modulation of the chemotactic response. APMIS. 1989 Aug;97(8):754–760. doi: 10.1111/j.1699-0463.1989.tb00474.x. [DOI] [PubMed] [Google Scholar]
- van Oers M. H., Pinkster J., Zeijlemaker W. P. Quantification of antigen-reactive cells among human T lymphocytes. Eur J Immunol. 1978 Jul;8(7):477–484. doi: 10.1002/eji.1830080706. [DOI] [PubMed] [Google Scholar]



