Abstract
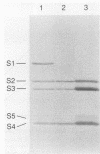
The subunits that make up the pentameric B oligomer of pertussis toxin (S2, S3, S4, and S5) were individually synthesized as recombinant polypeptides in Escherichia coli, isolated as insoluble inclusion bodies, and assembled into a multimeric form in vitro by spontaneous association following treatment with a chaotropic agent, reduction, and reoxidation. The recombinant B multimer, purified by fetuin-Sepharose affinity chromatography, contained all four of the individual subunits and possessed the mitogenic and hemagglutinating activities characteristic of the native B oligomer. Immunization of mice with the recombinant B oligomer elicited antibodies that neutralized pertussis toxin in vitro and, moreover, provided protection in vivo against the leukocytosis-promoting activity of the toxin. These results demonstrate the potential for assembly of complex multimeric proteins from recombinant DNA-derived polypeptides and provide a novel means for production of an acellular pertussis vaccine component.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arciniega J. L., Burns D. L., Garcia-Ortigoza E., Manclark C. R. Immune response to the B oligomer of pertussis toxin. Infect Immun. 1987 May;55(5):1132–1136. doi: 10.1128/iai.55.5.1132-1136.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arciniega J. L., Shahin R. D., Burnette W. N., Bartley T. D., Whiteley D. W., Mar V. L., Burns D. L. Contribution of the B oligomer to the protective activity of genetically attenuated pertussis toxin. Infect Immun. 1991 Oct;59(10):3407–3410. doi: 10.1128/iai.59.10.3407-3410.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong G. D., Howard L. A., Peppler M. S. Use of glycosyltransferases to restore pertussis toxin receptor activity to asialoagalactofetuin. J Biol Chem. 1988 Jun 25;263(18):8677–8684. [PubMed] [Google Scholar]
- Bartley T. D., Whiteley D. W., Mar V. L., Burns D. L., Burnette W. N. Pertussis holotoxoid formed in vitro with a genetically deactivated S1 subunit. Proc Natl Acad Sci U S A. 1989 Nov;86(21):8353–8357. doi: 10.1073/pnas.86.21.8353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackwelder W. C., Storsaeter J., Olin P., Hallander H. O. Acellular pertussis vaccines. Efficacy and evaluation of clinical case definitions. Am J Dis Child. 1991 Nov;145(11):1285–1289. doi: 10.1001/archpedi.1991.02160110077024. [DOI] [PubMed] [Google Scholar]
- Bokoch G. M., Katada T., Northup J. K., Hewlett E. L., Gilman A. G. Identification of the predominant substrate for ADP-ribosylation by islet activating protein. J Biol Chem. 1983 Feb 25;258(4):2072–2075. [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brennan M. J., David J. L., Kenimer J. G., Manclark C. R. Lectin-like binding of pertussis toxin to a 165-kilodalton Chinese hamster ovary cell glycoprotein. J Biol Chem. 1988 Apr 5;263(10):4895–4899. [PubMed] [Google Scholar]
- Burnette W. N., Cieplak W., Mar V. L., Kaljot K. T., Sato H., Keith J. M. Pertussis toxin S1 mutant with reduced enzyme activity and a conserved protective epitope. Science. 1988 Oct 7;242(4875):72–74. doi: 10.1126/science.2459776. [DOI] [PubMed] [Google Scholar]
- Burnette W. N. The advent of recombinant pertussis vaccines. Biotechnology (N Y) 1990 Nov;8(11):1002–1005. doi: 10.1038/nbt1190-1002. [DOI] [PubMed] [Google Scholar]
- Burns D. L., Kenimer J. G., Manclark C. R. Role of the A subunit of pertussis toxin in alteration of Chinese hamster ovary cell morphology. Infect Immun. 1987 Jan;55(1):24–28. doi: 10.1128/iai.55.1.24-28.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hausman S. Z., Burns D. L., Sickler V. C., Manclark C. R. Immune response to dimeric subunits of the pertussis toxin B oligomer. Infect Immun. 1989 Jun;57(6):1760–1764. doi: 10.1128/iai.57.6.1760-1764.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katada T., Ui M. Direct modification of the membrane adenylate cyclase system by islet-activating protein due to ADP-ribosylation of a membrane protein. Proc Natl Acad Sci U S A. 1982 May;79(10):3129–3133. doi: 10.1073/pnas.79.10.3129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Munoz J. J., Arai H., Bergman R. K., Sadowski P. L. Biological activities of crystalline pertussigen from Bordetella pertussis. Infect Immun. 1981 Sep;33(3):820–826. doi: 10.1128/iai.33.3.820-826.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicosia A., Bartoloni A., Perugini M., Rappuoli R. Expression and immunological properties of the five subunits of pertussis toxin. Infect Immun. 1987 Apr;55(4):963–967. doi: 10.1128/iai.55.4.963-967.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nogimori K., Tamura M., Yajima M., Ito K., Nakamura T., Kajikawa N., Maruyama Y., Ui M. Dual mechanisms involved in development of diverse biological activities of islet-activating protein, pertussis toxin, as revealed by chemical modification of lysine residues in the toxin molecule. Biochim Biophys Acta. 1984 Sep 28;801(2):232–243. doi: 10.1016/0304-4165(84)90072-2. [DOI] [PubMed] [Google Scholar]
- Oda M., Cowell J. L., Burstyn D. G., Manclark C. R. Protective activities of the filamentous hemagglutinin and the lymphocytosis-promoting factor of Bordetella pertussis in mice. J Infect Dis. 1984 Dec;150(6):823–833. doi: 10.1093/infdis/150.6.823. [DOI] [PubMed] [Google Scholar]
- Olin P. Comparing the efficacy of pertussis vaccines. Lancet. 1989 Jan 14;1(8629):95–96. doi: 10.1016/s0140-6736(89)91445-1. [DOI] [PubMed] [Google Scholar]
- Olin P., Storsaeter J., Romanus V. The efficacy of acellular pertussis vaccine. JAMA. 1989 Jan 27;261(4):560–560. [PubMed] [Google Scholar]
- Pizza M., Bugnoli M., Manetti R., Covacci A., Rappuoli R. The subunit S1 is important for pertussis toxin secretion. J Biol Chem. 1990 Oct 15;265(29):17759–17763. [PubMed] [Google Scholar]
- Pizza M., Covacci A., Bartoloni A., Perugini M., Nencioni L., De Magistris M. T., Villa L., Nucci D., Manetti R., Bugnoli M. Mutants of pertussis toxin suitable for vaccine development. Science. 1989 Oct 27;246(4929):497–500. doi: 10.1126/science.2683073. [DOI] [PubMed] [Google Scholar]
- Podda A., Nencioni L., De Magistris M. T., Di Tommaso A., Bossù P., Nuti S., Pileri P., Peppoloni S., Bugnoli M., Ruggiero P. Metabolic, humoral, and cellular responses in adult volunteers immunized with the genetically inactivated pertussis toxin mutant PT-9K/129G. J Exp Med. 1990 Sep 1;172(3):861–868. doi: 10.1084/jem.172.3.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato H., Sato Y. Bordetella pertussis infection in mice: correlation of specific antibodies against two antigens, pertussis toxin, and filamentous hemagglutinin with mouse protectivity in an intracerebral or aerosol challenge system. Infect Immun. 1984 Nov;46(2):415–421. doi: 10.1128/iai.46.2.415-421.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato Y., Kimura M., Fukumi H. Development of a pertussis component vaccine in Japan. Lancet. 1984 Jan 21;1(8369):122–126. doi: 10.1016/s0140-6736(84)90061-8. [DOI] [PubMed] [Google Scholar]
- Saukkonen K., Burnette W. N., Mar V. L., Masure H. R., Tuomanen E. I. Pertussis toxin has eukaryotic-like carbohydrate recognition domains. Proc Natl Acad Sci U S A. 1992 Jan 1;89(1):118–122. doi: 10.1073/pnas.89.1.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekura R. D., Fish F., Manclark C. R., Meade B., Zhang Y. L. Pertussis toxin. Affinity purification of a new ADP-ribosyltransferase. J Biol Chem. 1983 Dec 10;258(23):14647–14651. [PubMed] [Google Scholar]
- Shahin R. D., Witvliet M. H., Manclark C. R. Mechanism of pertussis toxin B oligomer-mediated protection against Bordetella pertussis respiratory infection. Infect Immun. 1990 Dec;58(12):4063–4068. doi: 10.1128/iai.58.12.4063-4068.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storsaeter J., Hallander H., Farrington C. P., Olin P., Möllby R., Miller E. Secondary analyses of the efficacy of two acellular pertussis vaccines evaluated in a Swedish phase III trial. Vaccine. 1990 Oct;8(5):457–461. doi: 10.1016/0264-410x(90)90246-i. [DOI] [PubMed] [Google Scholar]
- Tamura M., Nogimori K., Murai S., Yajima M., Ito K., Katada T., Ui M., Ishii S. Subunit structure of islet-activating protein, pertussis toxin, in conformity with the A-B model. Biochemistry. 1982 Oct 26;21(22):5516–5522. doi: 10.1021/bi00265a021. [DOI] [PubMed] [Google Scholar]
- Tamura M., Nogimori K., Yajima M., Ase K., Ui M. A role of the B-oligomer moiety of islet-activating protein, pertussis toxin, in development of the biological effects on intact cells. J Biol Chem. 1983 Jun 10;258(11):6756–6761. [PubMed] [Google Scholar]
- Tuomanen E., Towbin H., Rosenfelder G., Braun D., Larson G., Hansson G. C., Hill R. Receptor analogs and monoclonal antibodies that inhibit adherence of Bordetella pertussis to human ciliated respiratory epithelial cells. J Exp Med. 1988 Jul 1;168(1):267–277. doi: 10.1084/jem.168.1.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss A. A., Hewlett E. L. Virulence factors of Bordetella pertussis. Annu Rev Microbiol. 1986;40:661–686. doi: 10.1146/annurev.mi.40.100186.003305. [DOI] [PubMed] [Google Scholar]
- Witvliet M. H., Burns D. L., Brennan M. J., Poolman J. T., Manclark C. R. Binding of pertussis toxin to eucaryotic cells and glycoproteins. Infect Immun. 1989 Nov;57(11):3324–3330. doi: 10.1128/iai.57.11.3324-3330.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]