Abstract
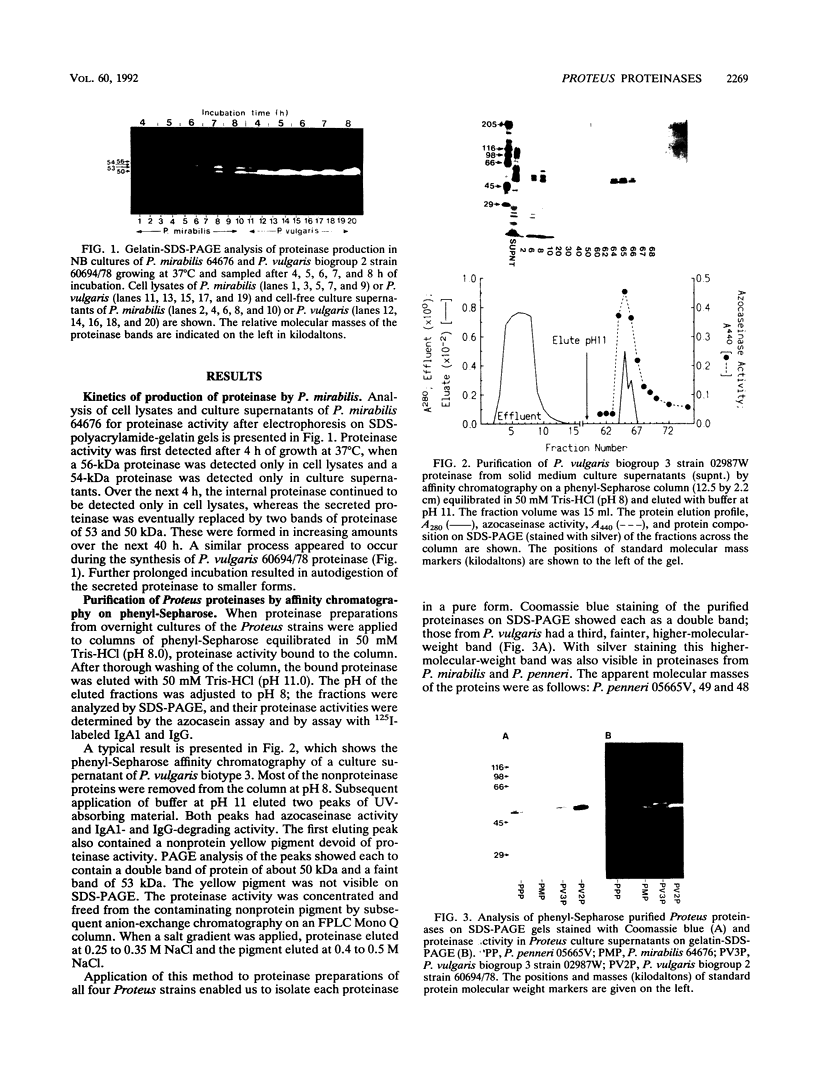
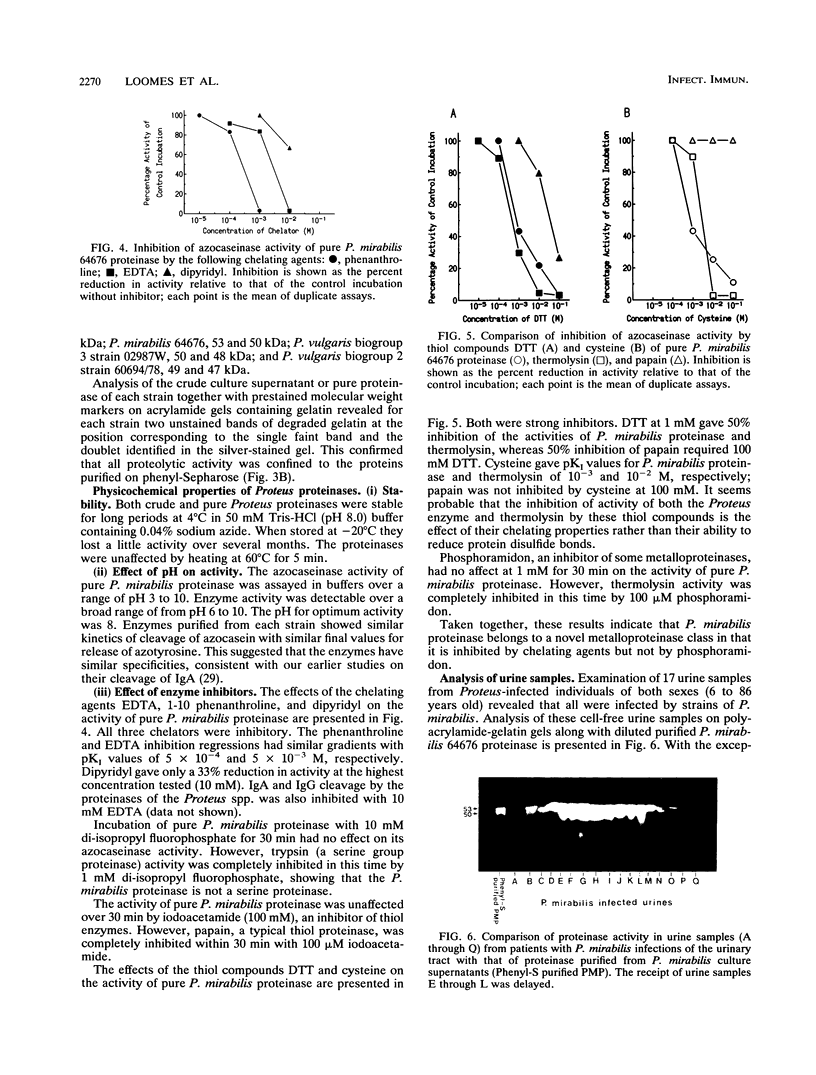
The proteinases secreted by pathogenic strains of Proteus mirabilis, P. vulgaris biotype 2, P. vulgaris biotype 3, and P. penneri were purified with almost 100% recovery by affinity chromatography on phenyl-Sepharose followed by anion-exchange chromatography. The proteinase purified from the urinary tract pathogen P. mirabilis, which we had previously shown to degrade immunoglobulins A and G, appeared as a composite of a single band and a double band (53 and 50 kDa, respectively) on sodium dodecyl sulfate-polyacrylamide gel electrophoresis. The other Proteus proteinases had similar patterns but slightly different mobilities. In each case all proteinase activity in culture supernatants was demonstrated by gelatin-sodium dodecyl sulfate-polyacrylamide gel electrophoresis to be associated with only the triple-band complex; all three bands were proteolytically active. The P. mirabilis proteinase was resistant to inhibitors of both serine and thiol proteinases but strongly inhibited by metal chelators, although it was not affected by phosphoramidon, an inhibitor of the thermolysin group of bacterial metalloproteinases. Active proteinase was detected in urine samples from P. mirabilis-infected patients; this is consistent with our detection of immunoglobulin A fragments of a size suggestive of P. mirabilis proteinase activity.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albrechtsen M., Yeaman G. R., Kerr M. A. Characterization of the IgA receptor from human polymorphonuclear leucocytes. Immunology. 1988 Jun;64(2):201–205. [PMC free article] [PubMed] [Google Scholar]
- BEESON P. B., ROWLEY D. The anticomplementary effect of kidney tissue; its association with ammonia production. J Exp Med. 1959 Nov 1;110:685–697. doi: 10.1084/jem.110.5.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergström T. Sex differences in childhood urinary tract infection. Arch Dis Child. 1972 Apr;47(252):227–232. doi: 10.1136/adc.47.252.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bienenstock J., Tomasi T. B., Jr Secretory gamma-A in normal urine. J Clin Invest. 1968 May;47(5):1162–1171. doi: 10.1172/JCI105805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Döring G., Obernesser H. J., Botzenhart K. Extrazelluläre Toxine von Pseudomonas aeruginosa. II. Einwirkung zweier gereinigter Proteasen auf die menschlichen Immunoglobuline IgG, IgA und sekretorisches IgA. Zentralbl Bakteriol A. 1981 Mar;249(1):89–98. [PubMed] [Google Scholar]
- GREENWOOD F. C., HUNTER W. M., GLOVER J. S. THE PREPARATION OF I-131-LABELLED HUMAN GROWTH HORMONE OF HIGH SPECIFIC RADIOACTIVITY. Biochem J. 1963 Oct;89:114–123. doi: 10.1042/bj0890114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith D. P., Musher D. M., Campbell J. W. Inhibition of bacterial urease. Invest Urol. 1973 Nov;11(3):234–238. [PubMed] [Google Scholar]
- HAMPSON S. E., MILLS G. L., SPENCER T. OBSERVATIONS ON A PROTEASE FROM PROTEUS MIRABILIS. Biochim Biophys Acta. 1963 Jul 9;73:476–487. doi: 10.1016/0006-3002(63)90449-9. [DOI] [PubMed] [Google Scholar]
- Hallett R. J., Pead L., Maskell R. Urinary infection in boys.A three-year prospective study. Lancet. 1976 Nov 20;2(7995):1107–1110. doi: 10.1016/s0140-6736(76)91087-4. [DOI] [PubMed] [Google Scholar]
- Higerd T. B., Virella G., Cardenas R., Koistinen J., Fett J. W. New method for obtaining IgA-specific protease. J Immunol Methods. 1977;18(3-4):245–249. doi: 10.1016/0022-1759(77)90178-8. [DOI] [PubMed] [Google Scholar]
- Khan A. J., Ubriani R. S., Bombach E., Agbayani M. M., Ratner H., Evans H. E. Initial urinary tract infection caused by Proteus mirabilis in infancy and childhood. J Pediatr. 1978 Nov;93(5):791–793. doi: 10.1016/s0022-3476(78)81079-8. [DOI] [PubMed] [Google Scholar]
- Kilian M., Thomsen B., Petersen T. E., Bleeg H. S. Occurrence and nature of bacterial IgA proteases. Ann N Y Acad Sci. 1983 Jun 30;409:612–624. doi: 10.1111/j.1749-6632.1983.tb26903.x. [DOI] [PubMed] [Google Scholar]
- Koronakis V., Cross M., Senior B., Koronakis E., Hughes C. The secreted hemolysins of Proteus mirabilis, Proteus vulgaris, and Morganella morganii are genetically related to each other and to the alpha-hemolysin of Escherichia coli. J Bacteriol. 1987 Apr;169(4):1509–1515. doi: 10.1128/jb.169.4.1509-1515.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krajden S., Fuksa M., Lizewski W., Barton L., Lee A. Proteus penneri and urinary calculi formation. J Clin Microbiol. 1984 Apr;19(4):541–542. doi: 10.1128/jcm.19.4.541-542.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Loomes L. M., Senior B. W., Kerr M. A. A proteolytic enzyme secreted by Proteus mirabilis degrades immunoglobulins of the immunoglobulin A1 (IgA1), IgA2, and IgG isotypes. Infect Immun. 1990 Jun;58(6):1979–1985. doi: 10.1128/iai.58.6.1979-1985.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazengera R. L., Kerr M. A. The specificity of the IgA receptor purified from human neutrophils. Biochem J. 1990 Nov 15;272(1):159–165. doi: 10.1042/bj2720159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molla A., Kagimoto T., Maeda H. Cleavage of immunoglobulin G (IgG) and IgA around the hinge region by proteases from Serratia marcescens. Infect Immun. 1988 Apr;56(4):916–920. doi: 10.1128/iai.56.4.916-920.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morihara K., Tsuzuki H. Phosphoramidon as an inhibitor of elastase from Pseudomonas aeruginosa. Jpn J Exp Med. 1978 Feb;48(1):81–84. [PubMed] [Google Scholar]
- Morrissey J. H. Silver stain for proteins in polyacrylamide gels: a modified procedure with enhanced uniform sensitivity. Anal Biochem. 1981 Nov 1;117(2):307–310. doi: 10.1016/0003-2697(81)90783-1. [DOI] [PubMed] [Google Scholar]
- Pazin G. J., Braude A. I. Immobilizing antibodies in urine. II. Prevention of ascending spread of Proteus mirabilis. Invest Urol. 1974 Sep;12(2):129–133. [PubMed] [Google Scholar]
- Peerbooms P. G., Verweij A. M., MacLaren D. M. Vero cell invasiveness of Proteus mirabilis. Infect Immun. 1984 Mar;43(3):1068–1071. doi: 10.1128/iai.43.3.1068-1071.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sareneva T., Holthöfer H., Korhonen T. K. Tissue-binding affinity of Proteus mirabilis fimbriae in the human urinary tract. Infect Immun. 1990 Oct;58(10):3330–3336. doi: 10.1128/iai.58.10.3330-3336.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senior B. W., Albrechtsen M., Kerr M. A. A survey of IgA protease production among clinical isolates of Proteeae. J Med Microbiol. 1988 Jan;25(1):27–31. doi: 10.1099/00222615-25-1-27. [DOI] [PubMed] [Google Scholar]
- Senior B. W., Albrechtsen M., Kerr M. A. Proteus mirabilis strains of diverse type have IgA protease activity. J Med Microbiol. 1987 Sep;24(2):175–180. doi: 10.1099/00222615-24-2-175. [DOI] [PubMed] [Google Scholar]
- Senior B. W., Bradford N. C., Simpson D. S. The ureases of Proteus strains in relation to virulence for the urinary tract. J Med Microbiol. 1980 Nov;13(4):507–512. doi: 10.1099/00222615-13-4-507. [DOI] [PubMed] [Google Scholar]
- Senior B. W., Leslie D. L. Rare occurrence of Proteus vulgaris in faeces: a reason for its rare association with urinary tract infections. J Med Microbiol. 1986 Mar;21(2):139–144. doi: 10.1099/00222615-21-2-139. [DOI] [PubMed] [Google Scholar]
- Senior B. W., Loomes L. M., Kerr M. A. The production and activity in vivo of Proteus mirabilis IgA protease in infections of the urinary tract. J Med Microbiol. 1991 Oct;35(4):203–207. doi: 10.1099/00222615-35-4-203. [DOI] [PubMed] [Google Scholar]
- Senior B. W. Proteus morgani is less frequently associated with urinary tract infections than Proteus mirabilis--an explanation. J Med Microbiol. 1983 Aug;16(3):317–322. doi: 10.1099/00222615-16-3-317. [DOI] [PubMed] [Google Scholar]
- Senior B. W. The special affinity of particular types of Proteus mirabilis for the urinary tract. J Med Microbiol. 1979 Feb;12(1):1–8. doi: 10.1099/00222615-12-1-1. [DOI] [PubMed] [Google Scholar]
- Silverblatt F. J. Host-parasite interaction in the rat renal pelvis: a possible role for pili in the pathogenesis of pyelonephritis. J Exp Med. 1974 Dec 1;140(6):1696–1711. doi: 10.1084/jem.140.6.1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverblatt F. J., Ofek I. Influence of pili on the virulence of Proteus mirabilis in experimental hematogenous pyelonephritis. J Infect Dis. 1978 Nov;138(5):664–667. doi: 10.1093/infdis/138.5.664. [DOI] [PubMed] [Google Scholar]
- Stewart W. W., Kerr M. A. The specificity of the human neutrophil IgA receptor (Fc alpha R) determined by measurement of chemiluminescence induced by serum or secretory IgA1 or IgA2. Immunology. 1990 Nov;71(3):328–334. [PMC free article] [PubMed] [Google Scholar]