Abstract
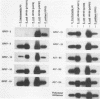
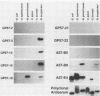
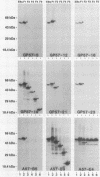
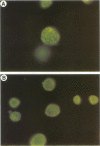
Ocular and urogenital tract infections with Chlamydia trachomatis can progress to chronic inflammatory diseases that produce blindness and tubal infertility. The pathophysiology of these chronic disease conditions is thought to be immunologically mediated, and the chlamydial 60-kDa heat shock protein (hsp60) has been implicated as a major target antigen that stimulates the immunopathological response. The lack of chlamydial hsp60 antibodies and purified hsp60 has severely restricted studies to define more thoroughly the role of this protein in the immunopathogenesis of chlamydial disease. We produced a panel of antichlamydial hsp60 monoclonal antibodies (MAbs) and defined their specificities by immunoblotting against lysates of C. trachomatis, C. psittaci, and six other genera of bacteria. Three patterns of anti-hsp60 immunoreactivity were observed: chlamydial species specific, chlamydial genus specific, and cross-reactive. The epitopes recognized by these MAbs were localized within the primary amino acid sequence of hsp60 by immunoblotting against recombinant amino-terminal truncated hsp60 fusion polypeptides and then precisely mapped by use of overlapping synthetic peptides. The majority of the MAbs mapped to either the amino or the carboxyl termini of hsp60. Epitopes defining all three MAb reactivities mapped within amino-terminal residues 6 to 16. Genus-specific hsp60 MAbs mapped to epitopes located within this region and to residues 17 to 28 and 177 to 189. Antichlamydial hsp60 MAbs stained inclusions as effectively as MAbs specific for the major outer membrane protein. Homogeneous preparations of full-length recombinant chlamydial hsp60 and amino-terminal truncated recombinant hsp60 polypeptides were obtained by immunoabsorption chromatography with an hsp60 MAb reactive to the carboxyl terminus of the protein. Thus, the antichlamydial MAbs described here should be extremely useful for the specific immunodetection of hsp60 in tissues from individuals having different disease manifestations and for the purification of hsp60 or truncated hsp60 polypeptides for use in serologic and lymphocyte proliferation assays. The availability of these MAbs will facilitate studies to define more precisely the role of hsp60 in the immunopathogenesis of chlamydial disease.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arno J. N., Ricker V. A., Batteiger B. E., Katz B. P., Caine V. A., Jones R. B. Interferon-gamma in endocervical secretions of women infected with Chlamydia trachomatis. J Infect Dis. 1990 Dec;162(6):1385–1389. doi: 10.1093/infdis/162.6.1385. [DOI] [PubMed] [Google Scholar]
- Brunham R. C., Maclean I. W., Binns B., Peeling R. W. Chlamydia trachomatis: its role in tubal infertility. J Infect Dis. 1985 Dec;152(6):1275–1282. doi: 10.1093/infdis/152.6.1275. [DOI] [PubMed] [Google Scholar]
- Burd E. M., Tabbara K. F., Nasr A. M., Taylor P. B. Conjunctival lymphocyte subsets in trachoma. Int Ophthalmol. 1988;12(1):53–57. doi: 10.1007/BF00133782. [DOI] [PubMed] [Google Scholar]
- Byrne G. I., Faubion C. L. Lymphokine-mediated microbistatic mechanisms restrict Chlamydia psittaci growth in macrophages. J Immunol. 1982 Jan;128(1):469–474. [PubMed] [Google Scholar]
- Caldwell H. D., Kromhout J., Schachter J. Purification and partial characterization of the major outer membrane protein of Chlamydia trachomatis. Infect Immun. 1981 Mar;31(3):1161–1176. doi: 10.1128/iai.31.3.1161-1176.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell H. D., Schachter J. Antigenic analysis of the major outer membrane protein of Chlamydia spp. Infect Immun. 1982 Mar;35(3):1024–1031. doi: 10.1128/iai.35.3.1024-1031.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerrone M. C., Ma J. J., Stephens R. S. Cloning and sequence of the gene for heat shock protein 60 from Chlamydia trachomatis and immunological reactivity of the protein. Infect Immun. 1991 Jan;59(1):79–90. doi: 10.1128/iai.59.1.79-90.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung C. T., Niemela S. L., Miller R. H. One-step preparation of competent Escherichia coli: transformation and storage of bacterial cells in the same solution. Proc Natl Acad Sci U S A. 1989 Apr;86(7):2172–2175. doi: 10.1073/pnas.86.7.2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collier L. H. The immunopathology of trachoma: some facts and fancies. Arch Gesamte Virusforsch. 1967;22(1):280–293. doi: 10.1007/BF01240523. [DOI] [PubMed] [Google Scholar]
- Dreyfuss G., Adam S. A., Choi Y. D. Physical change in cytoplasmic messenger ribonucleoproteins in cells treated with inhibitors of mRNA transcription. Mol Cell Biol. 1984 Mar;4(3):415–423. doi: 10.1128/mcb.4.3.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRAYSTON J. T., WOOLRIDGE R. L., WANG S. Trachoma vaccine studies on Taiwan. Ann N Y Acad Sci. 1962 Mar 5;98:352–367. doi: 10.1111/j.1749-6632.1962.tb30558.x. [DOI] [PubMed] [Google Scholar]
- Grayston J. T., Wang S. P., Yeh L. J., Kuo C. C. Importance of reinfection in the pathogenesis of trachoma. Rev Infect Dis. 1985 Nov-Dec;7(6):717–725. doi: 10.1093/clinids/7.6.717. [DOI] [PubMed] [Google Scholar]
- Hemmingsen S. M., Woolford C., van der Vies S. M., Tilly K., Dennis D. T., Georgopoulos C. P., Hendrix R. W., Ellis R. J. Homologous plant and bacterial proteins chaperone oligomeric protein assembly. Nature. 1988 May 26;333(6171):330–334. doi: 10.1038/333330a0. [DOI] [PubMed] [Google Scholar]
- Inman R. D., Johnston M. E., Chiu B., Falk J., Petric M. Immunochemical analysis of immune response to Chlamydia trachomatis in Reiter's syndrome and nonspecific urethritis. Clin Exp Immunol. 1987 Aug;69(2):246–254. [PMC free article] [PubMed] [Google Scholar]
- Jindal S., Dudani A. K., Singh B., Harley C. B., Gupta R. S. Primary structure of a human mitochondrial protein homologous to the bacterial and plant chaperonins and to the 65-kilodalton mycobacterial antigen. Mol Cell Biol. 1989 May;9(5):2279–2283. doi: 10.1128/mcb.9.5.2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones B. R. The prevention of blindness from trachoma. Trans Ophthalmol Soc U K. 1975 Apr;95(1):16–33. [PubMed] [Google Scholar]
- Kennett R. H. Cell fusion. Methods Enzymol. 1979;58:345–359. doi: 10.1016/s0076-6879(79)58149-x. [DOI] [PubMed] [Google Scholar]
- Kihlström E., Grönberg A., Bengtsson A. Immunoblot analysis of antibody response to Chlamydia trachomatis in patients with reactive arthritis and ankylosing spondylitis. Scand J Rheumatol. 1989;18(6):377–383. doi: 10.3109/03009748909102099. [DOI] [PubMed] [Google Scholar]
- Kramer M. J., Gordon F. B. Ultrastructural analysis of the effects of penicillin and chlortetracycline on the development of a genital tract Chlamydia. Infect Immun. 1971 Feb;3(2):333–341. doi: 10.1128/iai.3.2.333-341.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Matsumoto A., Manire G. P. Electron Microscopic Observations on the Fine Structure of Cell Walls of Chlamydia psittaci. J Bacteriol. 1970 Dec;104(3):1332–1337. doi: 10.1128/jb.104.3.1332-1337.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
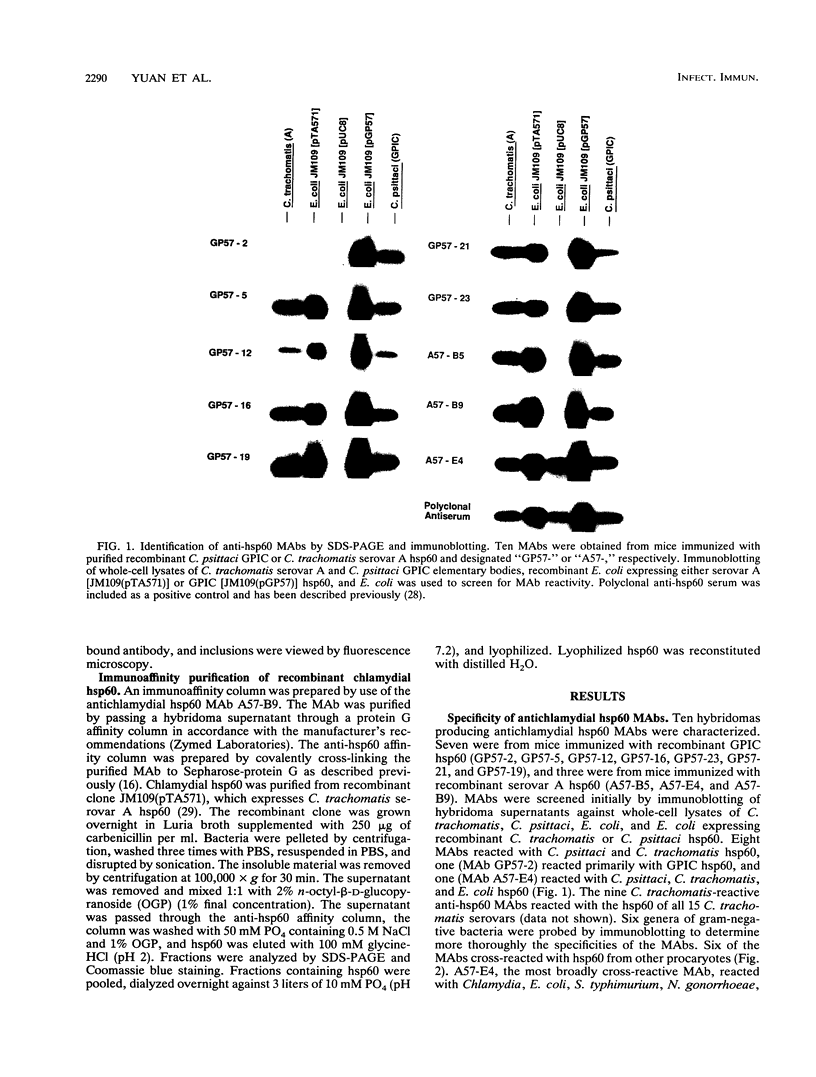
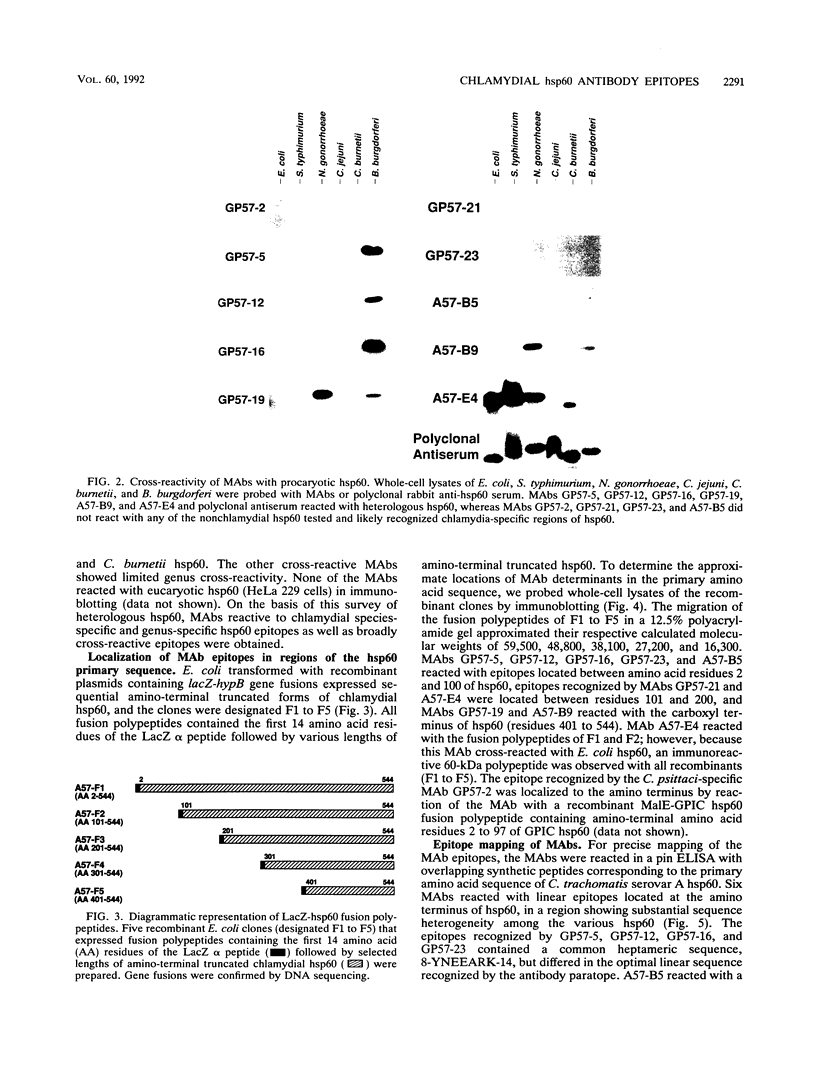
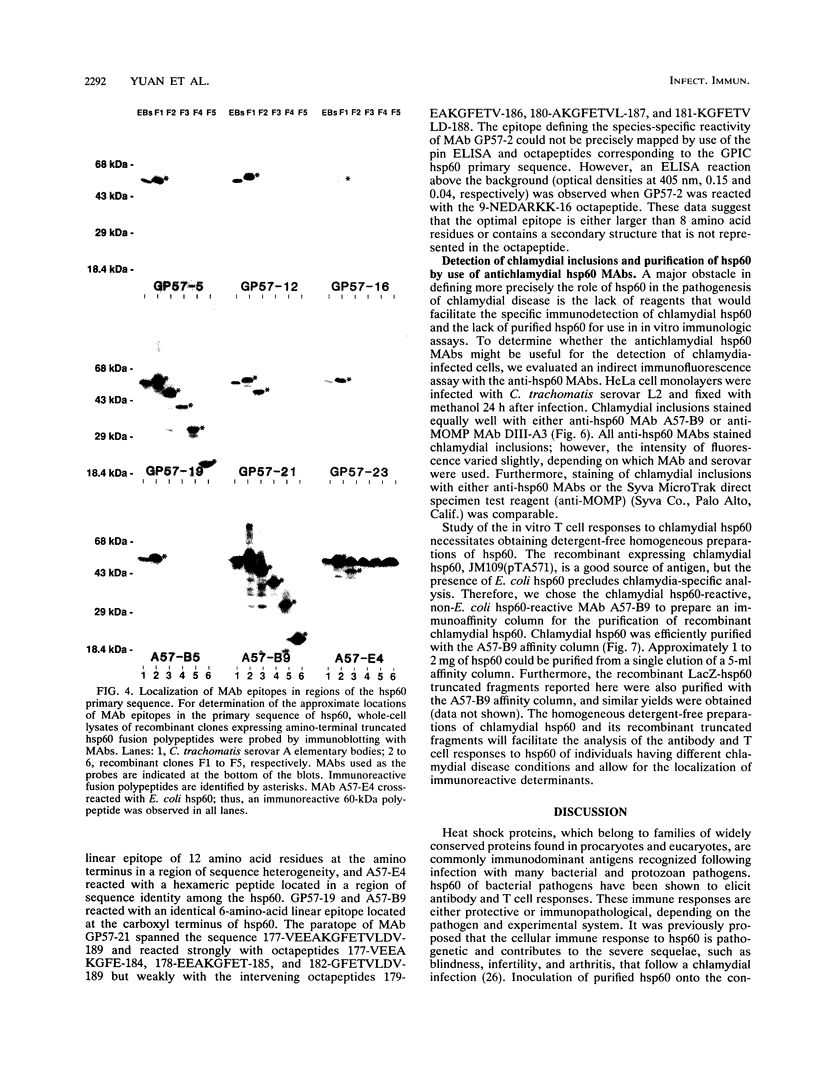
- Morrison R. P., Belland R. J., Lyng K., Caldwell H. D. Chlamydial disease pathogenesis. The 57-kD chlamydial hypersensitivity antigen is a stress response protein. J Exp Med. 1989 Oct 1;170(4):1271–1283. doi: 10.1084/jem.170.4.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison R. P. Chlamydial hsp60 and the immunopathogenesis of chlamydial disease. Semin Immunol. 1991 Jan;3(1):25–33. [PubMed] [Google Scholar]
- Morrison R. P., Lyng K., Caldwell H. D. Chlamydial disease pathogenesis. Ocular hypersensitivity elicited by a genus-specific 57-kD protein. J Exp Med. 1989 Mar 1;169(3):663–675. doi: 10.1084/jem.169.3.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison R. P., Su H., Lyng K., Yuan Y. The Chlamydia trachomatis hyp operon is homologous to the groE stress response operon of Escherichia coli. Infect Immun. 1990 Aug;58(8):2701–2705. doi: 10.1128/iai.58.8.2701-2705.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patton D. L. Immunopathology and histopathology of experimental chlamydial salpingitis. Rev Infect Dis. 1985 Nov-Dec;7(6):746–753. doi: 10.1093/clinids/7.6.746. [DOI] [PubMed] [Google Scholar]
- Reacher M. H., Pe'er J., Rapoza P. A., Whittum-Hudson J. A., Taylor H. R. T cells and trachoma. Their role in cicatricial disease. Ophthalmology. 1991 Mar;98(3):334–341. doi: 10.1016/s0161-6420(91)32290-5. [DOI] [PubMed] [Google Scholar]
- Schachter J., Moncada J., Dawson C. R., Sheppard J., Courtright P., Said M. E., Zaki S., Hafez S. F., Lorincz A. Nonculture methods for diagnosing chlamydial infection in patients with trachoma: a clue to the pathogenesis of the disease? J Infect Dis. 1988 Dec;158(6):1347–1352. doi: 10.1093/infdis/158.6.1347. [DOI] [PubMed] [Google Scholar]
- Shanafelt M. C., Hindersson P., Soderberg C., Mensi N., Turck C. W., Webb D., Yssel H., Peltz G. T cell and antibody reactivity with the Borrelia burgdorferi 60-kDa heat shock protein in Lyme arthritis. J Immunol. 1991 Jun 1;146(11):3985–3992. [PubMed] [Google Scholar]
- Shemer Y., Sarov I. Inhibition of growth of Chlamydia trachomatis by human gamma interferon. Infect Immun. 1985 May;48(2):592–596. doi: 10.1128/iai.48.2.592-596.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinnick T. M. The 65-kilodalton antigen of Mycobacterium tuberculosis. J Bacteriol. 1987 Mar;169(3):1080–1088. doi: 10.1128/jb.169.3.1080-1088.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vodkin M. H., Williams J. C. A heat shock operon in Coxiella burnetti produces a major antigen homologous to a protein in both mycobacteria and Escherichia coli. J Bacteriol. 1988 Mar;170(3):1227–1234. doi: 10.1128/jb.170.3.1227-1234.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagar E. A., Schachter J., Bavoil P., Stephens R. S. Differential human serologic response to two 60,000 molecular weight Chlamydia trachomatis antigens. J Infect Dis. 1990 Oct;162(4):922–927. doi: 10.1093/infdis/162.4.922. [DOI] [PubMed] [Google Scholar]
- Winkler B., Crum C. P. Chlamydia trachomatis infection of the female genital tract. Pathogenetic and clinicopathologic correlations. Pathol Annu. 1987;22(Pt 1):193–223. [PubMed] [Google Scholar]
- Woolridge R. L., Grayston J. T., Chang I. H., Cheng K. H., Yang C. Y., Neave C. Field trial of a monovalent and of a bivalent mineral oil adjuvant trachoma vaccine in Taiwan school children. Am J Ophthalmol. 1967 May;63(5 Suppl):1645–1650. doi: 10.1016/0002-9394(67)94158-x. [DOI] [PubMed] [Google Scholar]
- Zhang Y. X., Stewart S. J., Caldwell H. D. Protective monoclonal antibodies to Chlamydia trachomatis serovar- and serogroup-specific major outer membrane protein determinants. Infect Immun. 1989 Feb;57(2):636–638. doi: 10.1128/iai.57.2.636-638.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y. X., Stewart S., Joseph T., Taylor H. R., Caldwell H. D. Protective monoclonal antibodies recognize epitopes located on the major outer membrane protein of Chlamydia trachomatis. J Immunol. 1987 Jan 15;138(2):575–581. [PubMed] [Google Scholar]
- de la Maza L. M., Plunkett M. J., Carlson E. J., Peterson E. M., Czarniecki C. W. Ultrastructural analysis of the anti-chlamydial activity of recombinant murine interferon-gamma. Exp Mol Pathol. 1987 Aug;47(1):13–25. doi: 10.1016/0014-4800(87)90003-7. [DOI] [PubMed] [Google Scholar]
- el-Asrar A. M., Van den Oord J. J., Geboes K., Missotten L., Emarah M. H., Desmet V. Immunopathology of trachomatous conjunctivitis. Br J Ophthalmol. 1989 Apr;73(4):276–282. doi: 10.1136/bjo.73.4.276. [DOI] [PMC free article] [PubMed] [Google Scholar]