Abstract
Swainsonine, an indolizidine alkaloid with immunomodulatory activity, has been found to be effective in inhibiting metastatic dissemination and growth of primary tumors of both murine and human origins. The unique ability of swainsonine to exhibit antimetastatic, anti-proliferative, and immunomodulatory activity imparts this drug a promising future in cancer therapy.
Full text
PDF
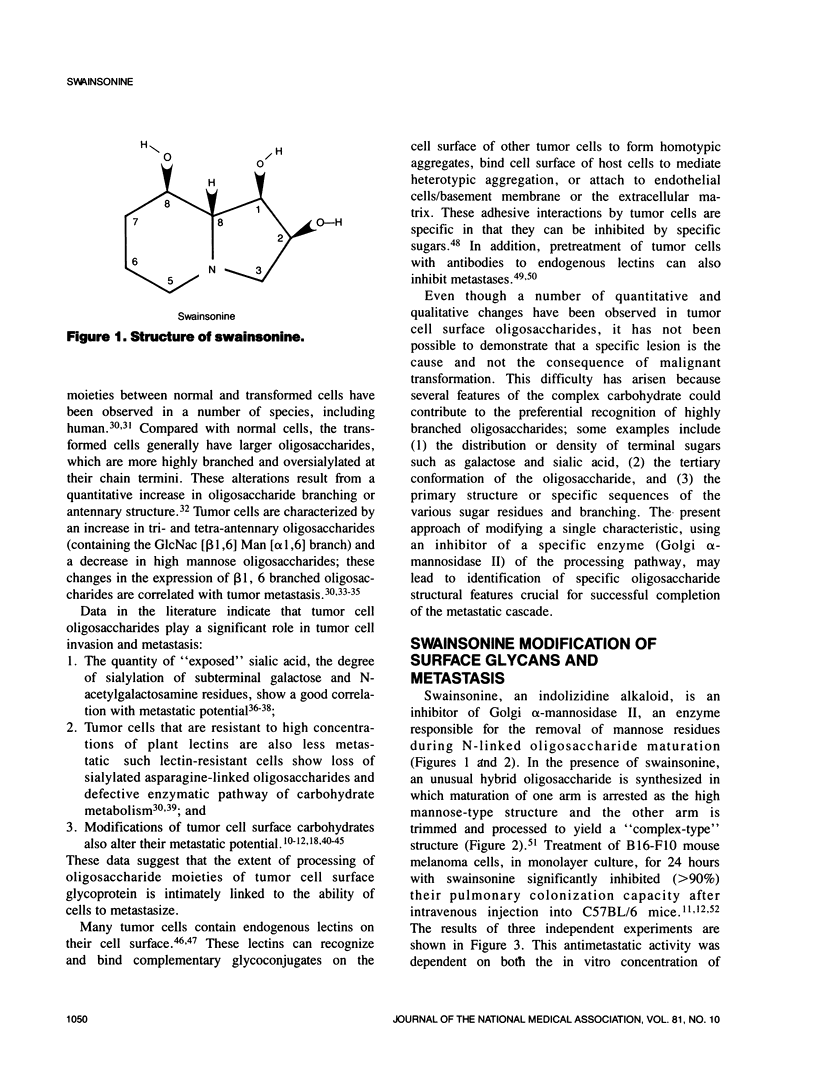
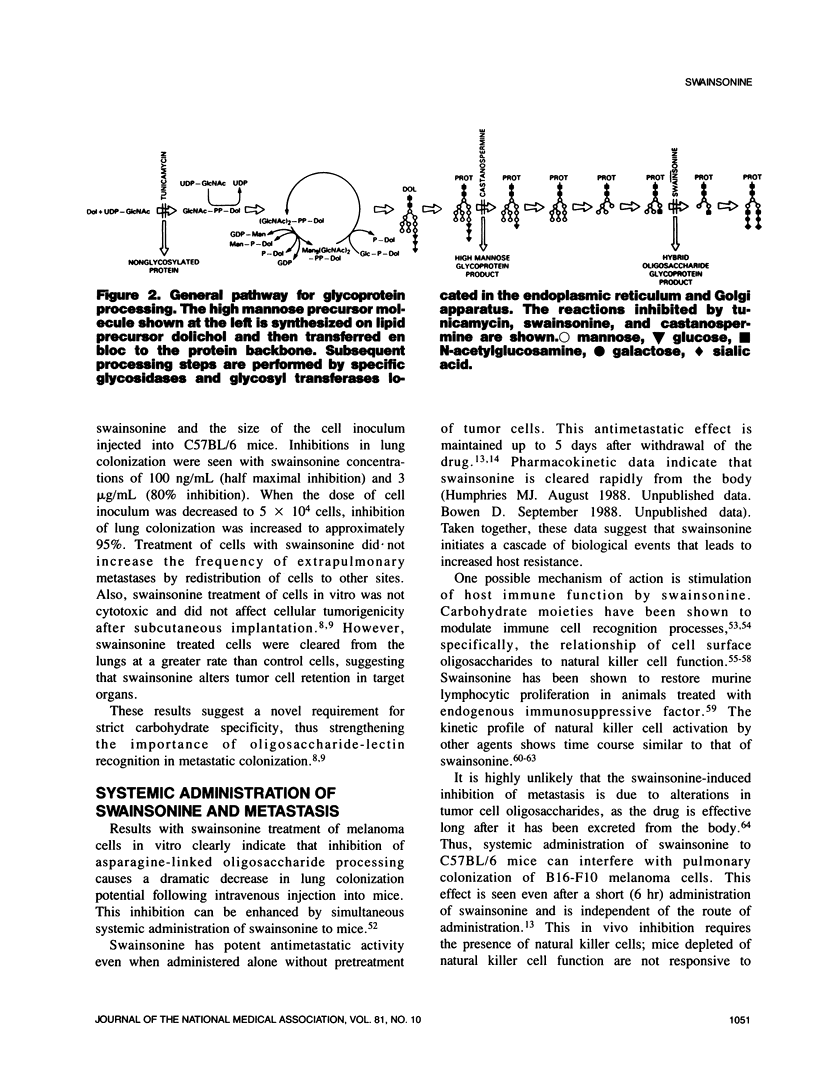
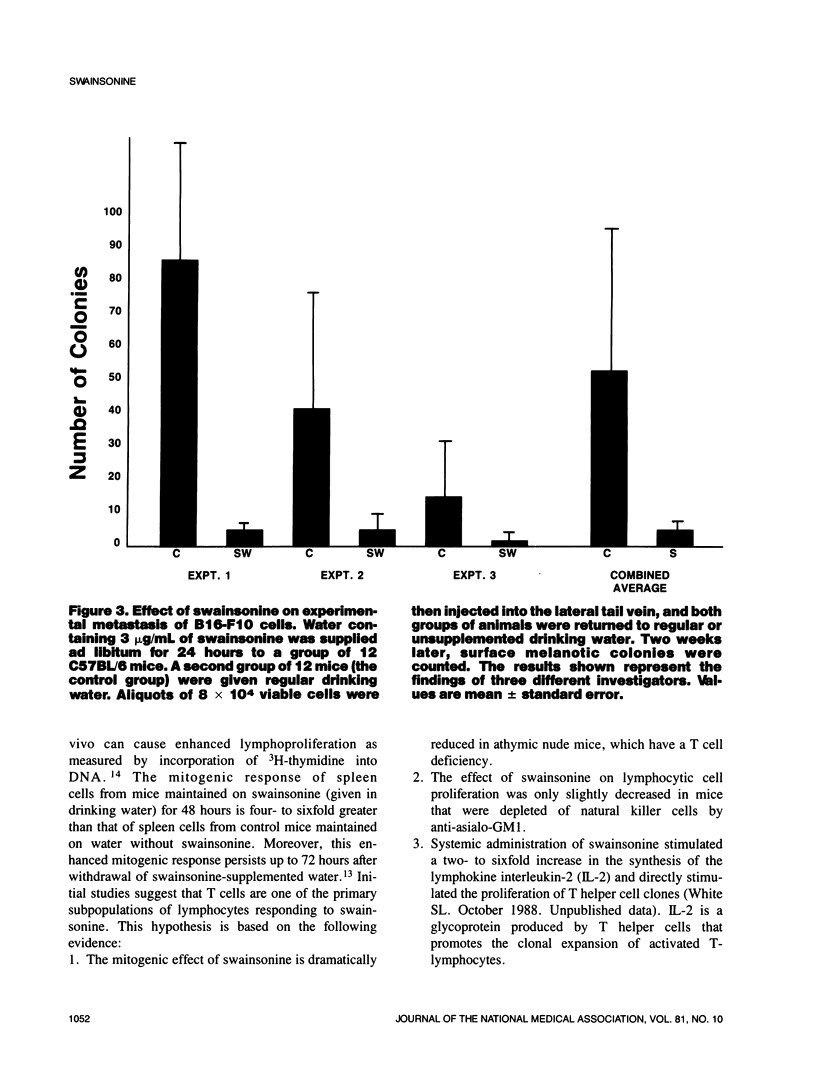




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altevogt P., Fogel M., Cheingsong-Popov R., Dennis J., Robinson P., Schirrmacher V. Different patterns of lectin binding and cell surface sialylation detected on related high- and low-metastatic tumor lines. Cancer Res. 1983 Nov;43(11):5138–5144. [PubMed] [Google Scholar]
- Bosmann H. B., Bieber G. F., Brown A. E., Case K. R., Gersten D. M., Kimmerer T. W., Lione A. Biochemical parameters correlated with tumour cell implantation. Nature. 1973 Dec 21;246(5434):487–489. doi: 10.1038/246487a0. [DOI] [PubMed] [Google Scholar]
- Burke D. C. The flowering of interferon. Biochim Biophys Acta. 1982 Sep 30;695(1):1–4. doi: 10.1016/0304-419x(82)90002-6. [DOI] [PubMed] [Google Scholar]
- DeSantis R., Santer U. V., Glick M. C. NIH 3T3 cells transfected with human tumor DNA lose the transformed phenotype when treated with swainsonine. Biochem Biophys Res Commun. 1987 Jan 30;142(2):348–353. doi: 10.1016/0006-291x(87)90280-4. [DOI] [PubMed] [Google Scholar]
- Dennis J. W. Effects of swainsonine and polyinosinic:polycytidylic acid on murine tumor cell growth and metastasis. Cancer Res. 1986 Oct;46(10):5131–5136. [PubMed] [Google Scholar]
- Dennis J. W. Effects of swainsonine and polyinosinic:polycytidylic acid on murine tumor cell growth and metastasis. Cancer Res. 1986 Oct;46(10):5131–5136. [PubMed] [Google Scholar]
- Dennis J. W., Laferté S. Recognition of asparagine-linked oligosaccharides on murine tumor cells by natural killer cells. Cancer Res. 1985 Dec;45(12 Pt 1):6034–6040. [PubMed] [Google Scholar]
- Dennis J. W., Laferté S., Waghorne C., Breitman M. L., Kerbel R. S. Beta 1-6 branching of Asn-linked oligosaccharides is directly associated with metastasis. Science. 1987 May 1;236(4801):582–585. doi: 10.1126/science.2953071. [DOI] [PubMed] [Google Scholar]
- Djeu J. Y., Heinbaugh J. A., Holden H. T., Herberman R. B. Augmentation of mouse natural killer cell activity by interferon and interferon inducers. J Immunol. 1979 Jan;122(1):175–181. [PubMed] [Google Scholar]
- Elbein A. D., Solf R., Dorling P. R., Vosbeck K. Swainsonine: an inhibitor of glycoprotein processing. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7393–7397. doi: 10.1073/pnas.78.12.7393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fidler I. J., Gersten D. M., Hart I. R. The biology of cancer invasion and metastasis. Adv Cancer Res. 1978;28:149–250. doi: 10.1016/s0065-230x(08)60648-x. [DOI] [PubMed] [Google Scholar]
- Fidler I. J. The relationship of embolic homogeneity, number, size and viability to the incidence of experimental metastasis. Eur J Cancer. 1973 Mar;9(3):223–227. doi: 10.1016/s0014-2964(73)80022-2. [DOI] [PubMed] [Google Scholar]
- Forbes J. T., Bretthauer R. K., Oeltmann T. N. Mannose 6-, fructose 1-, and fructose 6-phosphates inhibit human natural cell-mediated cytotoxicity. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5797–5801. doi: 10.1073/pnas.78.9.5797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasic G. J., Gasic T. B., Stewart C. C. Antimetastatic effects associated with platelet reduction. Proc Natl Acad Sci U S A. 1968 Sep;61(1):46–52. doi: 10.1073/pnas.61.1.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasic G. J. Role of plasma, platelets, and endothelial cells in tumor metastasis. Cancer Metastasis Rev. 1984;3(2):99–114. doi: 10.1007/BF00047657. [DOI] [PubMed] [Google Scholar]
- Gasic G. J., Tuszynski G. P., Gorelik E. Interaction of the hemostatic and immune systems in the metastatic spread of tumor cells. Int Rev Exp Pathol. 1986;29:173–212. [PubMed] [Google Scholar]
- Gilbert J. M., Hellmann K., Evans M., Cassell P. G., Stoodley B., Ellis H., Wastell C. Adjuvant oral razoxane (ICRF-159) in resectable colorectal cancer. Cancer Chemother Pharmacol. 1982;8(3):293–299. doi: 10.1007/BF00254053. [DOI] [PubMed] [Google Scholar]
- Hagmar B., Norrby K. Influence of cultivation, trypsinization and aggregation on the transplantability of melanoma B16 cells. Int J Cancer. 1973 May;11(3):663–675. doi: 10.1002/ijc.2910110317. [DOI] [PubMed] [Google Scholar]
- Harrison F. L., Chesterton C. J. Factors mediating cell--cell recognition and adhesion. Galaptins, a recently discovered class of bridging molecules. FEBS Lett. 1980 Dec 29;122(2):157–165. doi: 10.1016/0014-5793(80)80428-5. [DOI] [PubMed] [Google Scholar]
- Hart I. R. The selection and characterization of an invasive variant of the B16 melanoma. Am J Pathol. 1979 Dec;97(3):587–600. [PMC free article] [PubMed] [Google Scholar]
- Hellmann K. Antimetastatic drugs: laboratory to clinic. Clin Exp Metastasis. 1984 Jan-Mar;2(1):1–4. doi: 10.1007/BF00132302. [DOI] [PubMed] [Google Scholar]
- Herberman R. B., Djeu J. Y., Ortaldo J. R., Holden H. T., West W. H., Bonnard G. D. Role of interferon in augmentation of natural and antibody-dependent cell-mediated cytotoxicity. Cancer Treat Rep. 1978 Nov;62(11):1893–1896. [PubMed] [Google Scholar]
- Herberman R. B., Nunn M. E., Holden H. T., Staal S., Djeu J. Y. Augmentation of natural cytotoxic reactivity of mouse lymphoid cells against syngeneic and allogeneic target cells. Int J Cancer. 1977 Apr 15;19(4):555–564. doi: 10.1002/ijc.2910190417. [DOI] [PubMed] [Google Scholar]
- Hino M., Nakayama O., Tsurumi Y., Adachi K., Shibata T., Terano H., Kohsaka M., Aoki H., Imanaka H. Studies of an immunomodulator, swainsonine. I. Enhancement of immune response by swainsonine in vitro. J Antibiot (Tokyo) 1985 Jul;38(7):926–935. doi: 10.7164/antibiotics.38.926. [DOI] [PubMed] [Google Scholar]
- Humphries M. J., Matsumoto K., White S. L., Molyneux R. J., Olden K. Augmentation of murine natural killer cell activity by swainsonine, a new antimetastatic immunomodulator. Cancer Res. 1988 Mar 15;48(6):1410–1415. [PubMed] [Google Scholar]
- Humphries M. J., Matsumoto K., White S. L., Olden K. Inhibition of experimental metastasis by castanospermine in mice: blockage of two distinct stages of tumor colonization by oligosaccharide processing inhibitors. Cancer Res. 1986 Oct;46(10):5215–5222. [PubMed] [Google Scholar]
- Humphries M. J., Olden K., Yamada K. M. A synthetic peptide from fibronectin inhibits experimental metastasis of murine melanoma cells. Science. 1986 Jul 25;233(4762):467–470. doi: 10.1126/science.3726541. [DOI] [PubMed] [Google Scholar]
- Humphries M. J., Yamada K. M., Olden K. Investigation of the biological effects of anti-cell adhesive synthetic peptides that inhibit experimental metastasis of B16-F10 murine melanoma cells. J Clin Invest. 1988 Mar;81(3):782–790. doi: 10.1172/JCI113384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irimura T., Gonzalez R., Nicolson G. L. Effects of tunicamycin on B16 metastatic melanoma cell surface glycoproteins and blood-borne arrest and survival properties. Cancer Res. 1981 Sep;41(9 Pt 1):3411–3418. [PubMed] [Google Scholar]
- Kerbel R. S., Dennis J. W., Largarde A. E., Frost P. Tumor progression in metastasis: an experimental approach using lectin resistant tumor variants. Cancer Metastasis Rev. 1982;1(2):99–140. doi: 10.1007/BF00048223. [DOI] [PubMed] [Google Scholar]
- Kijima-Suda I., Miyamoto Y., Toyoshima S., Itoh M., Osawa T. Inhibition of experimental pulmonary metastasis of mouse colon adenocarcinoma 26 sublines by a sialic acid:nucleoside conjugate having sialyltransferase inhibiting activity. Cancer Res. 1986 Feb;46(2):858–862. [PubMed] [Google Scholar]
- Liotta L. A., Rao C. N., Wewer U. M. Biochemical interactions of tumor cells with the basement membrane. Annu Rev Biochem. 1986;55:1037–1057. doi: 10.1146/annurev.bi.55.070186.005133. [DOI] [PubMed] [Google Scholar]
- Liotta L. A., Saidel M. G., Kleinerman J. The significance of hematogenous tumor cell clumps in the metastatic process. Cancer Res. 1976 Mar;36(3):889–894. [PubMed] [Google Scholar]
- Mareel M. M., Dragonetti C. H., Hooghe R. J., Bruyneel E. A. Effect of inhibitors of glycosylation and carbohydrate processing on invasion of malignant mouse MO4 cells in organ culture. Clin Exp Metastasis. 1985 Jul-Sep;3(3):197–207. doi: 10.1007/BF01786763. [DOI] [PubMed] [Google Scholar]
- McCarthy J. B., Basara M. L., Palm S. L., Sas D. F., Furcht L. T. The role of cell adhesion proteins--laminin and fibronectin--in the movement of malignant and metastatic cells. Cancer Metastasis Rev. 1985;4(2):125–152. doi: 10.1007/BF00050692. [DOI] [PubMed] [Google Scholar]
- Meromsky L., Lotan R., Raz A. Implications of endogenous tumor cell surface lectins as mediators of cellular interactions and lung colonization. Cancer Res. 1986 Oct;46(10):5270–5275. [PubMed] [Google Scholar]
- Morgan D. A., Ruscetti F. W., Gallo R. Selective in vitro growth of T lymphocytes from normal human bone marrows. Science. 1976 Sep 10;193(4257):1007–1008. doi: 10.1126/science.181845. [DOI] [PubMed] [Google Scholar]
- Newton S. A., White S. L., Humphries M. J., Olden K. Swainsonine inhibition of spontaneous metastasis. J Natl Cancer Inst. 1989 Jul 5;81(13):1024–1028. doi: 10.1093/jnci/81.13.1024. [DOI] [PubMed] [Google Scholar]
- Nicolson G. L. Tumor cell instability, diversification, and progression to the metastatic phenotype: from oncogene to oncofetal expression. Cancer Res. 1987 Mar 15;47(6):1473–1487. [PubMed] [Google Scholar]
- Ogata S. I., Muramatsu T., Kobata A. New structural characteristic of the large glycopeptides from transformed cells. Nature. 1976 Feb 19;259(5544):580–582. doi: 10.1038/259580a0. [DOI] [PubMed] [Google Scholar]
- Olden K., Mohla S., Newton S. A., White S. L., Humphries M. J. Use of antiadhesive peptide and swainsonine to inhibit metastasis. Ann N Y Acad Sci. 1988;551:421–442. doi: 10.1111/j.1749-6632.1988.tb22375.x. [DOI] [PubMed] [Google Scholar]
- Olden K., Parent J. B., White S. L. Carbohydrate moieties of glycoproteins. A re-evaluation of their function. Biochim Biophys Acta. 1982 May 12;650(4):209–232. doi: 10.1016/0304-4157(82)90017-x. [DOI] [PubMed] [Google Scholar]
- Poste G., Doll J., Brown A. E., Tzeng J., Zeidman I. Comparison of the metastatic properties of B16 melanoma clones isolated from cultured cell lines, subcutaneous tumors, and individual lung metastases. Cancer Res. 1982 Jul;42(7):2770–2778. [PubMed] [Google Scholar]
- Poste G. Pathogenesis of metastatic disease: implications for current therapy and for the development of new therapeutic strategies. Cancer Treat Rep. 1986 Jan;70(1):183–199. [PubMed] [Google Scholar]
- Rapin A. M., Burger M. M. Tumor cell surfaces: general alterations detected by agglutinins. Adv Cancer Res. 1974;20:1–91. doi: 10.1016/s0065-230x(08)60108-6. [DOI] [PubMed] [Google Scholar]
- Raz A., Lotan R. Endogenous galactoside-binding lectins: a new class of functional tumor cell surface molecules related to metastasis. Cancer Metastasis Rev. 1987;6(3):433–452. doi: 10.1007/BF00144274. [DOI] [PubMed] [Google Scholar]
- Reading C. L., Hutchins J. T. Carbohydrate structure in tumor immunity. Cancer Metastasis Rev. 1985;4(3):221–260. doi: 10.1007/BF00048097. [DOI] [PubMed] [Google Scholar]
- Schirrmacher V. Cancer metastasis: experimental approaches, theoretical concepts, and impacts for treatment strategies. Adv Cancer Res. 1985;43:1–73. doi: 10.1016/s0065-230x(08)60942-2. [DOI] [PubMed] [Google Scholar]
- Sinha B. K., Goldenberg G. J. The effect of trypsin and neuraminidase on the circulation and organ distribution of tumor cells. Cancer. 1974 Dec;34(6):1956–1961. doi: 10.1002/1097-0142(197412)34:6<1956::aid-cncr2820340614>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- Stackpole C. W. Distinct lung-colonizing and lung-metastasizing cell populations in B16 mouse melanoma. Nature. 1981 Feb 26;289(5800):798–800. doi: 10.1038/289798a0. [DOI] [PubMed] [Google Scholar]
- Stackpole C. W., Fornabaio D. M., Alterman A. L. Phenotypic interconversion of B16 melanoma clonal cell populations: relationship between metastasis and tumor growth rate. Int J Cancer. 1985 May 15;35(5):667–674. doi: 10.1002/ijc.2910350516. [DOI] [PubMed] [Google Scholar]
- Stojanovic D., Hughes R. C. An endogenous carbohydrate-binding agglutinin of BHK cells. Purification, specificity and interaction with normal and ricin-resistant cell lines. Biol Cell. 1984;51(2):197–205. doi: 10.1111/j.1768-322x.1984.tb00299.x. [DOI] [PubMed] [Google Scholar]
- Stutman O., Dien P., Wisun R. E., Lattime E. C. Natural cytotoxic cells against solid tumors in mice: blocking of cytotoxicity by D-mannose. Proc Natl Acad Sci U S A. 1980 May;77(5):2895–2898. doi: 10.1073/pnas.77.5.2895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren L., Fuhrer J. P., Buck C. A. Surface glycoproteins of cells before and after transformation by oncogenic viruses. Fed Proc. 1973 Jan;32(1):80–85. [PubMed] [Google Scholar]
- Welsh R. M., Jr, Zinkernagel R. M. Heterospecific cytotoxic cell activity induced during the first three days of acute lymphocytic choriomeningitis virus infection in mice. Nature. 1977 Aug 18;268(5621):646–648. doi: 10.1038/268646a0. [DOI] [PubMed] [Google Scholar]
- Werkmeister J. A., Pross H. F., Roder J. C. Modulation of K562 cells with sodium butyrate. Association of impaired NK susceptibility with sialic acid and analysis of other parameters. Int J Cancer. 1983 Jul 15;32(1):71–78. doi: 10.1002/ijc.2910320112. [DOI] [PubMed] [Google Scholar]
- White S. L., Schweitzer K., Humphries M. J., Olden K. Stimulation of DNA synthesis in murine lymphocytes by the drug swainsonine: immunomodulatory properties. Biochem Biophys Res Commun. 1988 Jan 29;150(2):615–625. doi: 10.1016/0006-291x(88)90437-8. [DOI] [PubMed] [Google Scholar]
- Yogeeswaran G. Cell surface glycolipids and glycoproteins in malignant transformation. Adv Cancer Res. 1983;38:289–350. doi: 10.1016/s0065-230x(08)60191-8. [DOI] [PubMed] [Google Scholar]
- Yogeeswaran G., Salk P. L. Metastatic potential is positively correlated with cell surface sialylation of cultured murine tumor cell lines. Science. 1981 Jun 26;212(4502):1514–1516. doi: 10.1126/science.7233237. [DOI] [PubMed] [Google Scholar]


