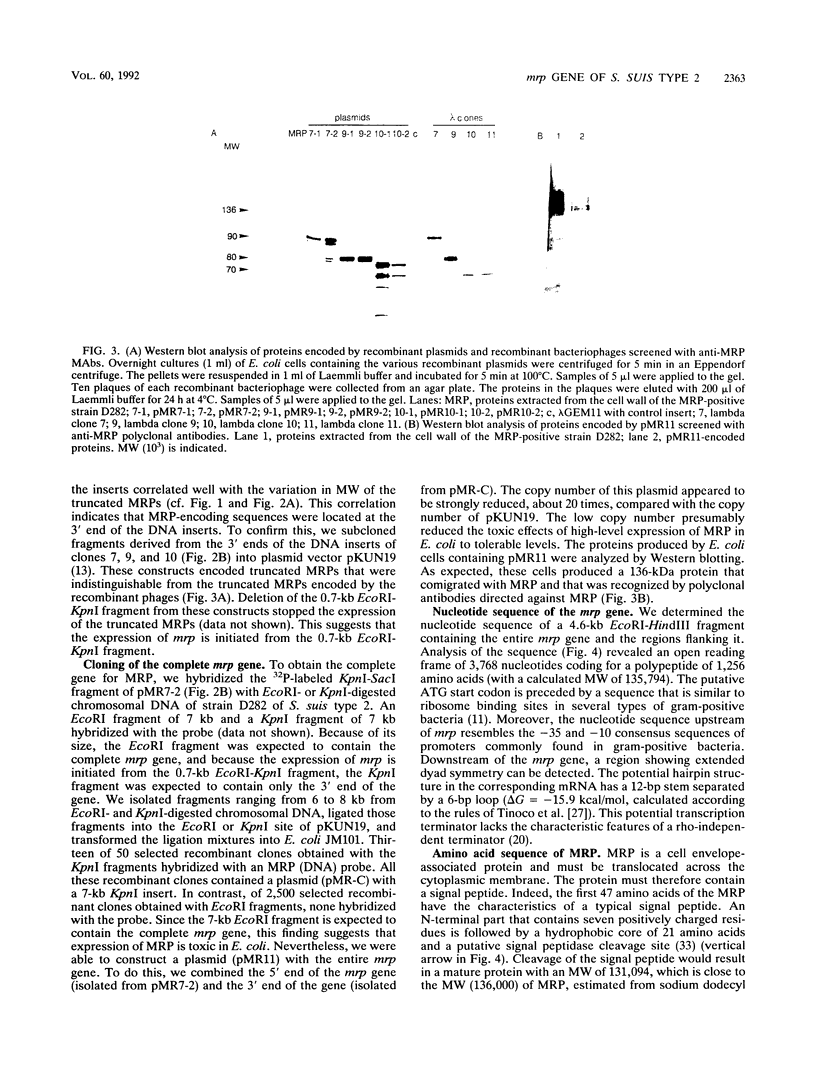
Abstract
We cloned and sequenced the gene encoding the muramidase-released protein (MRP) of a pathogenic Streptococcus suis type 2 strain to determine whether its amino acid sequence resembles that of proteins with known functions and to determine its function in virulence. The complete nucleotide sequence composing the gene and the regions flanking it was determined. The deduced amino acid sequence revealed the presence of a signal peptide at the N terminus and a cell envelope anchor at the C terminus, both of which resembled similar regions in several other surface proteins from gram-positive bacteria. The processed form of MRP has a length of 1,209 amino acids and a calculated molecular weight of 131,094. A highly repetitive region preceded the envelope anchor. The repeated units were preceded by a proline-rich stretch of amino acids (26 of 86). No overall homologies were observed between the amino acid sequence of MRP and protein sequences in the EMBL data bank. A particular region within the amino acid sequence, however, showed some similarity with the fibronectin-binding protein of Staphylococcus aureus. Binding of MRP to human fibronectin, however, could not be confirmed.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abou-Zeid C., Garbe T., Lathigra R., Wiker H. G., Harboe M., Rook G. A., Young D. B. Genetic and immunological analysis of Mycobacterium tuberculosis fibronectin-binding proteins. Infect Immun. 1991 Aug;59(8):2712–2718. doi: 10.1128/iai.59.8.2712-2718.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arends J. P., Zanen H. C. Meningitis caused by Streptococcus suis in humans. Rev Infect Dis. 1988 Jan-Feb;10(1):131–137. doi: 10.1093/clinids/10.1.131. [DOI] [PubMed] [Google Scholar]
- Clifton-Hadley F. A. Streptococcus suis type 2 infections. Br Vet J. 1983 Jan-Feb;139(1):1–5. doi: 10.1016/s0007-1935(17)30581-x. [DOI] [PubMed] [Google Scholar]
- Fahnestock S. R., Alexander P., Nagle J., Filpula D. Gene for an immunoglobulin-binding protein from a group G streptococcus. J Bacteriol. 1986 Sep;167(3):870–880. doi: 10.1128/jb.167.3.870-880.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferretti J. J., Russell R. R., Dao M. L. Sequence analysis of the wall-associated protein precursor of Streptococcus mutans antigen A. Mol Microbiol. 1989 Apr;3(4):469–478. doi: 10.1111/j.1365-2958.1989.tb00193.x. [DOI] [PubMed] [Google Scholar]
- Fischetti V. A., Pancholi V., Schneewind O. Conservation of a hexapeptide sequence in the anchor region of surface proteins from gram-positive cocci. Mol Microbiol. 1990 Sep;4(9):1603–1605. doi: 10.1111/j.1365-2958.1990.tb02072.x. [DOI] [PubMed] [Google Scholar]
- Fischetti V. A. Streptococcal M protein: molecular design and biological behavior. Clin Microbiol Rev. 1989 Jul;2(3):285–314. doi: 10.1128/cmr.2.3.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frithz E., Hedén L. O., Lindahl G. Extensive sequence homology between IgA receptor and M proteins in Streptococcus pyogenes. Mol Microbiol. 1989 Aug;3(8):1111–1119. doi: 10.1111/j.1365-2958.1989.tb00261.x. [DOI] [PubMed] [Google Scholar]
- Guss B., Eliasson M., Olsson A., Uhlén M., Frej A. K., Jörnvall H., Flock J. I., Lindberg M. Structure of the IgG-binding regions of streptococcal protein G. EMBO J. 1986 Jul;5(7):1567–1575. doi: 10.1002/j.1460-2075.1986.tb04398.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guss B., Uhlén M., Nilsson B., Lindberg M., Sjöquist J., Sjödahl J. Region X, the cell-wall-attachment part of staphylococcal protein A. Eur J Biochem. 1984 Jan 16;138(2):413–420. doi: 10.1111/j.1432-1033.1984.tb07931.x. [DOI] [PubMed] [Google Scholar]
- Hollingshead S. K., Fischetti V. A., Scott J. R. Complete nucleotide sequence of type 6 M protein of the group A Streptococcus. Repetitive structure and membrane anchor. J Biol Chem. 1986 Feb 5;261(4):1677–1686. [PubMed] [Google Scholar]
- Konings R. N., Verhoeven E. J., Peeters B. P. pKUN, vectors for the separate production of both DNA strands of recombinant plasmids. Methods Enzymol. 1987;153:12–34. doi: 10.1016/0076-6879(87)53045-2. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Murray N. E., Brammar W. J., Murray K. Lambdoid phages that simplify the recovery of in vitro recombinants. Mol Gen Genet. 1977 Jan 7;150(1):53–61. doi: 10.1007/BF02425325. [DOI] [PubMed] [Google Scholar]
- Pancholi V., Fischetti V. A. Isolation and characterization of the cell-associated region of group A streptococcal M6 protein. J Bacteriol. 1988 Jun;170(6):2618–2624. doi: 10.1128/jb.170.6.2618-2624.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platt T. Transcription termination and the regulation of gene expression. Annu Rev Biochem. 1986;55:339–372. doi: 10.1146/annurev.bi.55.070186.002011. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneewind O., Jones K. F., Fischetti V. A. Sequence and structural characteristics of the trypsin-resistant T6 surface protein of group A streptococci. J Bacteriol. 1990 Jun;172(6):3310–3317. doi: 10.1128/jb.172.6.3310-3317.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Signäs C., Raucci G., Jönsson K., Lindgren P. E., Anantharamaiah G. M., Hök M., Lindberg M. Nucleotide sequence of the gene for a fibronectin-binding protein from Staphylococcus aureus: use of this peptide sequence in the synthesis of biologically active peptides. Proc Natl Acad Sci U S A. 1989 Jan;86(2):699–703. doi: 10.1073/pnas.86.2.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjöbring U., Falkenberg C., Nielsen E., Akerström B., Björck L. Isolation and characterization of a 14-kDa albumin-binding fragment of streptococcal protein G. J Immunol. 1988 Mar 1;140(5):1595–1599. [PubMed] [Google Scholar]
- Talay S. R., Ehrenfeld E., Chhatwal G. S., Timmis K. N. Expression of the fibronectin-binding components of Streptococcus pyogenes in Escherichia coli demonstrates that they are proteins. Mol Microbiol. 1991 Jul;5(7):1727–1734. doi: 10.1111/j.1365-2958.1991.tb01921.x. [DOI] [PubMed] [Google Scholar]
- Tinoco I., Jr, Borer P. N., Dengler B., Levin M. D., Uhlenbeck O. C., Crothers D. M., Bralla J. Improved estimation of secondary structure in ribonucleic acids. Nat New Biol. 1973 Nov 14;246(150):40–41. doi: 10.1038/newbio246040a0. [DOI] [PubMed] [Google Scholar]
- Vecht U., Arends J. P., van der Molen E. J., van Leengoed L. A. Differences in virulence between two strains of Streptococcus suis type II after experimentally induced infection of newborn germ-free pigs. Am J Vet Res. 1989 Jul;50(7):1037–1043. [PubMed] [Google Scholar]
- Vecht U., Wisselink H. J., Jellema M. L., Smith H. E. Identification of two proteins associated with virulence of Streptococcus suis type 2. Infect Immun. 1991 Sep;59(9):3156–3162. doi: 10.1128/iai.59.9.3156-3162.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vecht U., Wisselink H. J., van Dijk J. E., Smith H. E. Virulence of Streptococcus suis type 2 strains in newborn germfree pigs depends on phenotype. Infect Immun. 1992 Feb;60(2):550–556. doi: 10.1128/iai.60.2.550-556.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vecht U., van Leengoed L. A., Verheijen E. R. Streptococcus suis infections in pigs in the Netherlands (Part I). Vet Q. 1985 Oct;7(4):315–321. doi: 10.1080/01652176.1985.9694005. [DOI] [PubMed] [Google Scholar]
- Vos P., Simons G., Siezen R. J., de Vos W. M. Primary structure and organization of the gene for a procaryotic, cell envelope-located serine proteinase. J Biol Chem. 1989 Aug 15;264(23):13579–13585. [PubMed] [Google Scholar]
- von Heijne G. A new method for predicting signal sequence cleavage sites. Nucleic Acids Res. 1986 Jun 11;14(11):4683–4690. doi: 10.1093/nar/14.11.4683. [DOI] [PMC free article] [PubMed] [Google Scholar]