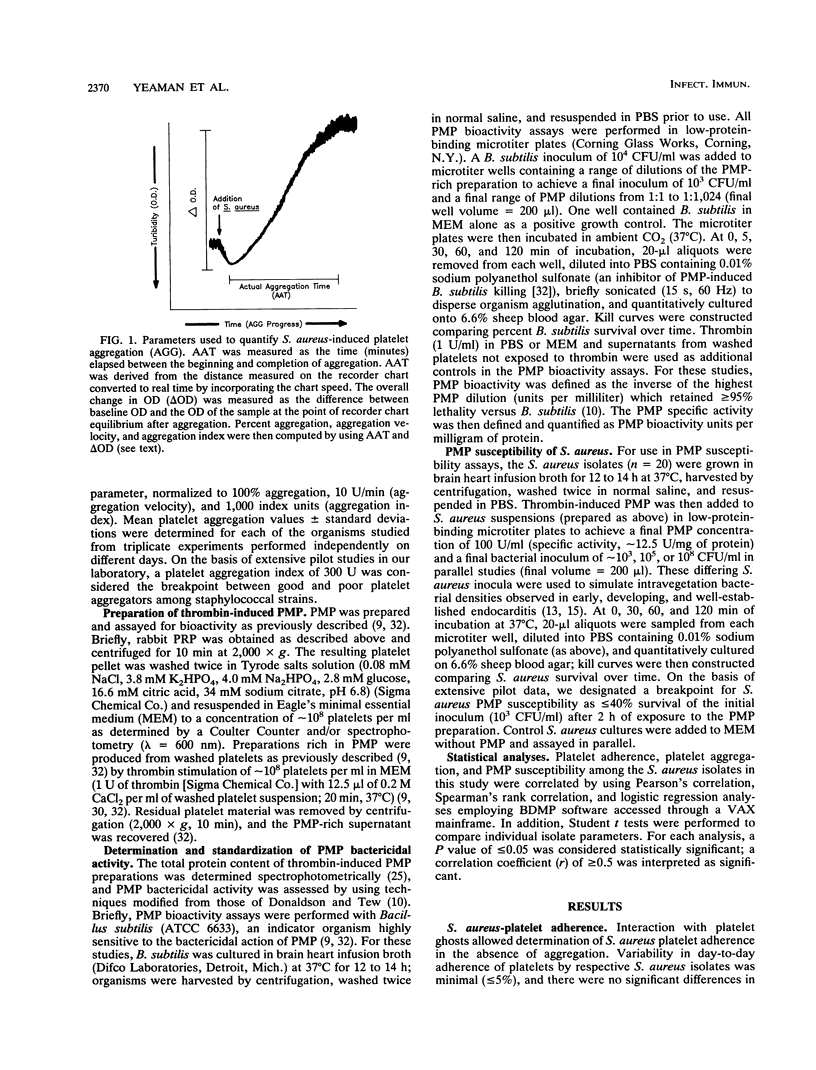
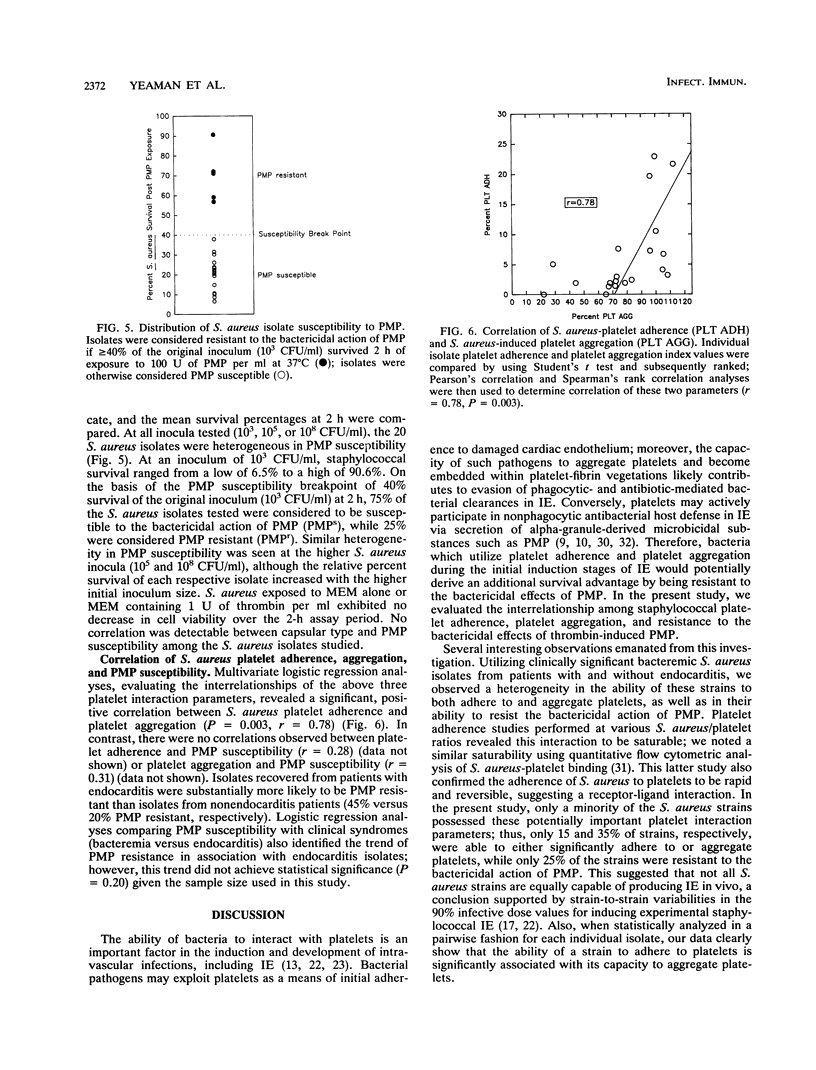
Abstract
Bacterium-platelet interactions at the cardiac valve surface represent an important initial step in the induction of infective endocarditis (IE). This cell-cell interaction may play either a protagonistic role in the induction of IE via bacterial adherence to and aggregation of platelets or an antagonistic role via secretion of platelet-derived microbicidal molecules. We examined the spectrum and interrelationship of three aspects of the interaction of 20 clinical Staphylococcus aureus isolates with rabbit platelets in vitro: (i) S. aureus adherence to platelets; (ii) S. aureus-induced platelet aggregation; and (iii) S. aureus resistance to the action of thrombin-induced platelet microbicidal protein (PMP; low-molecular-weight cationic peptides contained in alpha granules). Among the 20 S. aureus isolates (11 bacteremia, 9 endocarditis), there was a heterogeneous distribution profile for each of the bacterium-platelet interaction parameters studied. For S. aureus-platelet adherence and S. aureus-induced platelet aggregation, 3 of 20 and 7 of 20 isolates tested were considered highly active for each respective parameter; 5 of 20 staphylococcal strains were deemed resistant to the bactericidal action of PMP. In addition, more endocarditis isolates (45%) were PMP resistant than strains from patients without endocarditis (19%). When analyzed concomitantly, there was a significant, positive correlation between S. aureus-platelet adherence and S. aureus-induced platelet aggregation among isolates (P = 0.003; r = 0.78). In contrast, there were no statistically significant relationships between either platelet adherence or aggregation and PMP resistance among these 20 S. aureus isolates. These data suggest that platelet adherence and aggregation are related abilities of S. aureus, while resistance to thrombin-induced PMP is an independent phenotypic characteristic and potential virulence factor.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bayer A. S., Lam K., Ginzton L., Norman D. C., Chiu C. Y., Ward J. I. Staphylococcus aureus bacteremia. Clinical, serologic, and echocardiographic findings in patients with and without endocarditis. Arch Intern Med. 1987 Mar;147(3):457–462. doi: 10.1001/archinte.147.3.457. [DOI] [PubMed] [Google Scholar]
- Berney P., Francioli P. Successful prophylaxis of experimental streptococcal endocarditis with single-dose amoxicillin administered after bacterial challenge. J Infect Dis. 1990 Feb;161(2):281–285. doi: 10.1093/infdis/161.2.281. [DOI] [PubMed] [Google Scholar]
- Buiting A. G., Thompson J., van der Keur D., Schmal-Bauer W. C., Bertina R. M. Procoagulant activity of endocardial vegetations and blood monocytes in rabbits with Streptococcus sanguis endocarditis. Thromb Haemost. 1989 Nov 24;62(3):1029–1033. [PubMed] [Google Scholar]
- Cheung A. L., Krishnan M., Jaffe E. A., Fischetti V. A. Fibrinogen acts as a bridging molecule in the adherence of Staphylococcus aureus to cultured human endothelial cells. J Clin Invest. 1991 Jun;87(6):2236–2245. doi: 10.1172/JCI115259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clawson C. C. Platelet interaction with bacteria. 3. Ultrastructure. Am J Pathol. 1973 Mar;70(3):449–471. [PMC free article] [PubMed] [Google Scholar]
- Clawson C. C., White J. G. Platelet interaction with bacteria. I. Reaction phases and effects of inhibitors. Am J Pathol. 1971 Nov;65(2):367–380. [PMC free article] [PubMed] [Google Scholar]
- Clawson C. C., White J. G. Platelet interaction with bacteria. II. Fate of the bacteria. Am J Pathol. 1971 Nov;65(2):381–397. [PMC free article] [PubMed] [Google Scholar]
- Donaldson D. M., Tew J. G. beta-Lysin of platelet origin. Bacteriol Rev. 1977 Jun;41(2):501–513. doi: 10.1128/br.41.2.501-513.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drake T. A., Pang M. Effects of interleukin-1, lipopolysaccharide, and streptococci on procoagulant activity of cultured human cardiac valve endothelial and stromal cells. Infect Immun. 1989 Feb;57(2):507–512. doi: 10.1128/iai.57.2.507-512.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drake T. A., Pang M. Staphylococcus aureus induces tissue factor expression in cultured human cardiac valve endothelium. J Infect Dis. 1988 Apr;157(4):749–756. doi: 10.1093/infdis/157.4.749. [DOI] [PubMed] [Google Scholar]
- Durack D. T., Beeson P. B. Experimental bacterial endocarditis. I. Colonization of a sterile vegetation. Br J Exp Pathol. 1972 Feb;53(1):44–49. [PMC free article] [PubMed] [Google Scholar]
- Durack D. T., Beeson P. B., Petersdorf R. G. Experimental bacterial endocarditis. 3. Production and progress of the disease in rabbits. Br J Exp Pathol. 1973 Apr;54(2):142–151. [PMC free article] [PubMed] [Google Scholar]
- Durack D. T. Experimental bacterial endocarditis. IV. Structure and evolution of very early lesions. J Pathol. 1975 Feb;115(2):81–89. doi: 10.1002/path.1711150204. [DOI] [PubMed] [Google Scholar]
- Ganz T., Selsted M. E., Lehrer R. I. Defensins. Eur J Haematol. 1990 Jan;44(1):1–8. doi: 10.1111/j.1600-0609.1990.tb00339.x. [DOI] [PubMed] [Google Scholar]
- Greenberg D. P., Ward J. I., Bayer A. S. Influence of Staphylococcus aureus antibody on experimental endocarditis in rabbits. Infect Immun. 1987 Dec;55(12):3030–3034. doi: 10.1128/iai.55.12.3030-3034.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrmann M., Suchard S. J., Boxer L. A., Waldvogel F. A., Lew P. D. Thrombospondin binds to Staphylococcus aureus and promotes staphylococcal adherence to surfaces. Infect Immun. 1991 Jan;59(1):279–288. doi: 10.1128/iai.59.1.279-288.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrmann M., Vaudaux P. E., Pittet D., Auckenthaler R., Lew P. D., Schumacher-Perdreau F., Peters G., Waldvogel F. A. Fibronectin, fibrinogen, and laminin act as mediators of adherence of clinical staphylococcal isolates to foreign material. J Infect Dis. 1988 Oct;158(4):693–701. doi: 10.1093/infdis/158.4.693. [DOI] [PubMed] [Google Scholar]
- Herzberg M. C., Brintzenhofe K. L., Clawson C. C. Aggregation of human platelets and adhesion of Streptococcus sanguis. Infect Immun. 1983 Mar;39(3):1457–1469. doi: 10.1128/iai.39.3.1457-1469.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzberg M. C., Gong K., MacFarlane G. D., Erickson P. R., Soberay A. H., Krebsbach P. H., Manjula G., Schilling K., Bowen W. H. Phenotypic characterization of Streptococcus sanguis virulence factors associated with bacterial endocarditis. Infect Immun. 1990 Feb;58(2):515–522. doi: 10.1128/iai.58.2.515-522.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirano L., Bayer A. S. Beta-Lactam-beta-lactamase-inhibitor combinations are active in experimental endocarditis caused by beta-lactamase-producing oxacillin-resistant staphylococci. Antimicrob Agents Chemother. 1991 Apr;35(4):685–690. doi: 10.1128/aac.35.4.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holderbaum D., Spech R. A., Ehrhart L. A. Specific binding of collagen to Staphylococcus aureus. Coll Relat Res. 1985 Jun;5(3):261–271. doi: 10.1016/s0174-173x(85)80016-9. [DOI] [PubMed] [Google Scholar]
- Karakawa W. W., Fournier J. M., Vann W. F., Arbeit R., Schneerson R. S., Robbins J. B. Method for the serological typing of the capsular polysaccharides of Staphylococcus aureus. J Clin Microbiol. 1985 Sep;22(3):445–447. doi: 10.1128/jcm.22.3.445-447.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Ogawa S. K., Yurberg E. R., Hatcher V. B., Levitt M. A., Lowy F. D. Bacterial adherence to human endothelial cells in vitro. Infect Immun. 1985 Oct;50(1):218–224. doi: 10.1128/iai.50.1.218-224.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proctor R. A., Mosher D. F., Olbrantz P. J. Fibronectin binding to Staphylococcus aureus. J Biol Chem. 1982 Dec 25;257(24):14788–14794. [PubMed] [Google Scholar]
- Sullam P. M., Payan D. G., Dazin P. F., Valone F. H. Binding of viridans group streptococci to human platelets: a quantitative analysis. Infect Immun. 1990 Nov;58(11):3802–3806. doi: 10.1128/iai.58.11.3802-3806.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullam P. M., Valone F. H., Mills J. Mechanisms of platelet aggregation by viridans group streptococci. Infect Immun. 1987 Aug;55(8):1743–1750. doi: 10.1128/iai.55.8.1743-1750.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeaman M. R., Puentes S. M., Norman D. C., Bayer A. S. Partial characterization and staphylocidal activity of thrombin-induced platelet microbicidal protein. Infect Immun. 1992 Mar;60(3):1202–1209. doi: 10.1128/iai.60.3.1202-1209.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]



