Abstract
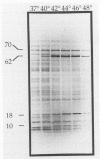
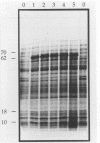
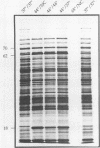
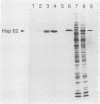
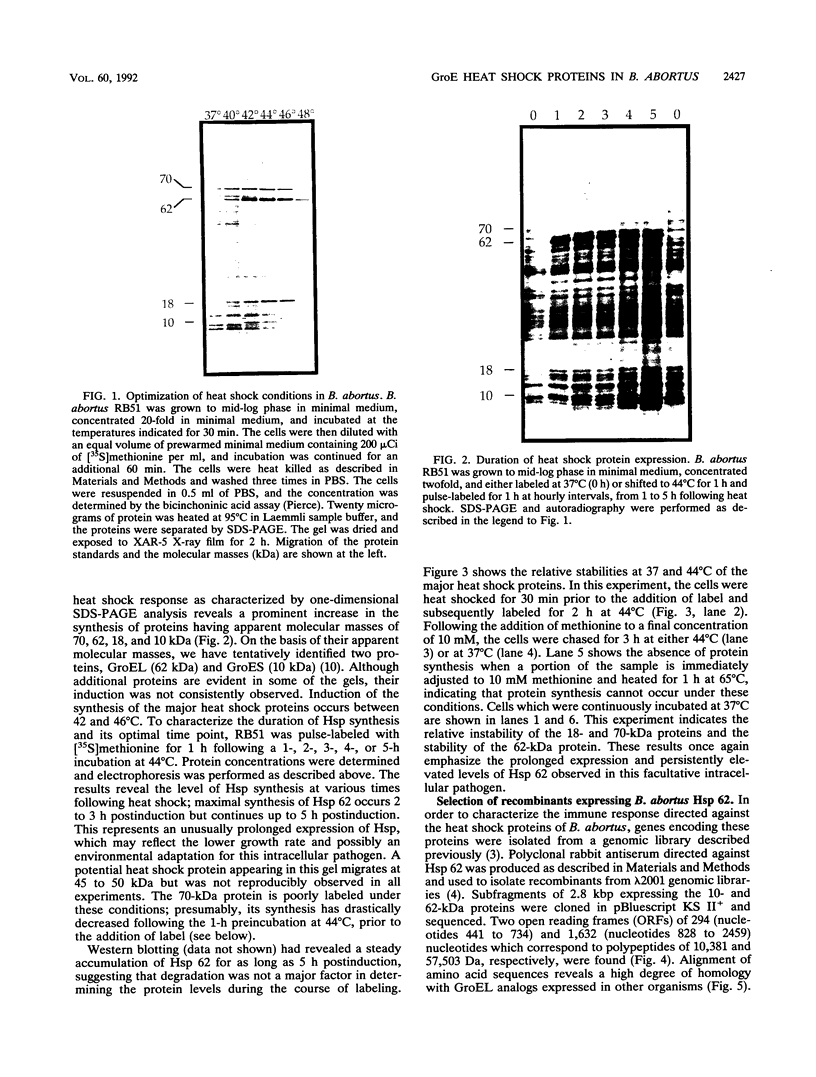
In an effort to define the heat shock response in the bovine intracellular pathogen Brucella abortus, a rough variant lacking extensive lipopolysaccharide was pulse-labeled with [35S]methionine following exposure to elevated temperatures. The major heat shock proteins observed following sodium dodecyl sulfate-polyacrylamide gel electrophoresis and autoradiography migrate at 70, 62, 18, and 10 kDa. The maximum response was observed between 42 and 46 degrees C and within 2 to 3 h of the shif in temperature and varied slightly for the different proteins. Accumulation of the 62-kDa heat shock protein (62-kDa Hsp) was observed to continue for up to 5 h following the shift in temperature. In an effort to better define the heat shock response and its potential relationship with protective immunity, genes encoding the major heat shock proteins were isolated from recombinant libraries constructed from B. abortus S19 and S2308 and sequenced. The 62-kDa Hsp shares more than 60% amino acid homology with members of the GroEL family and is immunoprecipitated with polyclonal antibodies to Escherichia coli GroEL and monoclonal antibodies to mycobacterial Hsp 65. Western blot (immunoblot) analysis with pooled sera from vaccinated and infected cattle revealed that the 62-kDa Hsp is a predominantly recognized antigen. The roles of these gene products during environmental stress and in protective immunity against brucellosis are under investigation.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson D. C., Barry M. E., Buchanan T. M. Exact definition of species-specific and cross-reactive epitopes of the 65-kilodalton protein of Mycobacterium leprae using synthetic peptides. J Immunol. 1988 Jul 15;141(2):607–613. [PubMed] [Google Scholar]
- Buchanan T. M., Nomaguchi H., Anderson D. C., Young R. A., Gillis T. P., Britton W. J., Ivanyi J., Kolk A. H., Closs O., Bloom B. R. Characterization of antibody-reactive epitopes on the 65-kilodalton protein of Mycobacterium leprae. Infect Immun. 1987 Apr;55(4):1000–1003. doi: 10.1128/iai.55.4.1000-1003.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ficht T. A., Bearden S. W., Sowa B. A., Adams L. G. A 36-kilodalton Brucella abortus cell envelope protein is encoded by repeated sequences closely linked in the genomic DNA. Infect Immun. 1988 Aug;56(8):2036–2046. doi: 10.1128/iai.56.8.2036-2046.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ficht T. A., Bearden S. W., Sowa B. A., Adams L. G. DNA sequence and expression of the 36-kilodalton outer membrane protein gene of Brucella abortus. Infect Immun. 1989 Nov;57(11):3281–3291. doi: 10.1128/iai.57.11.3281-3291.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GERHARDT P. The nutrition of brucellae. Bacteriol Rev. 1958 Jun;22(2):81–98. doi: 10.1128/br.22.2.81-98.1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guglielmi G., Mazodier P., Thompson C. J., Davies J. A survey of the heat shock response in four Streptomyces species reveals two groEL-like genes and three groEL-like proteins in Streptomyces albus. J Bacteriol. 1991 Nov;173(22):7374–7381. doi: 10.1128/jb.173.22.7374-7381.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hindersson P., Høiby N., Bangsborg J. Sequence analysis of the Legionella micdadei groELS operon. FEMS Microbiol Lett. 1991 Jan 1;61(1):31–38. doi: 10.1016/0378-1097(91)90009-y. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Morgan R. W., Christman M. F., Jacobson F. S., Storz G., Ames B. N. Hydrogen peroxide-inducible proteins in Salmonella typhimurium overlap with heat shock and other stress proteins. Proc Natl Acad Sci U S A. 1986 Nov;83(21):8059–8063. doi: 10.1073/pnas.83.21.8059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinnick T. M., Vodkin M. H., Williams J. C. The Mycobacterium tuberculosis 65-kilodalton antigen is a heat shock protein which corresponds to common antigen and to the Escherichia coli GroEL protein. Infect Immun. 1988 Feb;56(2):446–451. doi: 10.1128/iai.56.2.446-451.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoen C. O., Bruner J. A., Luchsinger D. W., Pietz D. E. Detection of brucella antibodies of different immunoglobulin classes in cow milk by enzyme-linked immunosorbent assay. Am J Vet Res. 1983 Feb;44(2):306–308. [PubMed] [Google Scholar]
- Thole J. E., Hindersson P., de Bruyn J., Cremers F., van der Zee J., de Cock H., Tommassen J., van Eden W., van Embden J. D. Antigenic relatedness of a strongly immunogenic 65 kDA mycobacterial protein antigen with a similarly sized ubiquitous bacterial common antigen. Microb Pathog. 1988 Jan;4(1):71–83. doi: 10.1016/0882-4010(88)90049-6. [DOI] [PubMed] [Google Scholar]
- Thole J. E., Keulen W. J., De Bruyn J., Kolk A. H., Groothuis D. G., Berwald L. G., Tiesjema R. H., van Embden J. D. Characterization, sequence determination, and immunogenicity of a 64-kilodalton protein of Mycobacterium bovis BCG expressed in escherichia coli K-12. Infect Immun. 1987 Jun;55(6):1466–1475. doi: 10.1128/iai.55.6.1466-1475.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter A. J., Duncan J. R., Santisteban C. G., Douglas J. T., Adams L. G. Capacity of passively administered antibody to prevent establishment of Brucella abortus infection in mice. Infect Immun. 1989 Nov;57(11):3438–3444. doi: 10.1128/iai.57.11.3438-3444.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods M. L., 2nd, Bonfiglioli R., McGee Z. A., Georgopoulos C. Synthesis of a select group of proteins by Neisseria gonorrhoeae in response to thermal stress. Infect Immun. 1990 Mar;58(3):719–725. doi: 10.1128/iai.58.3.719-725.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young R. A., Elliott T. J. Stress proteins, infection, and immune surveillance. Cell. 1989 Oct 6;59(1):5–8. doi: 10.1016/0092-8674(89)90861-1. [DOI] [PubMed] [Google Scholar]