Abstract
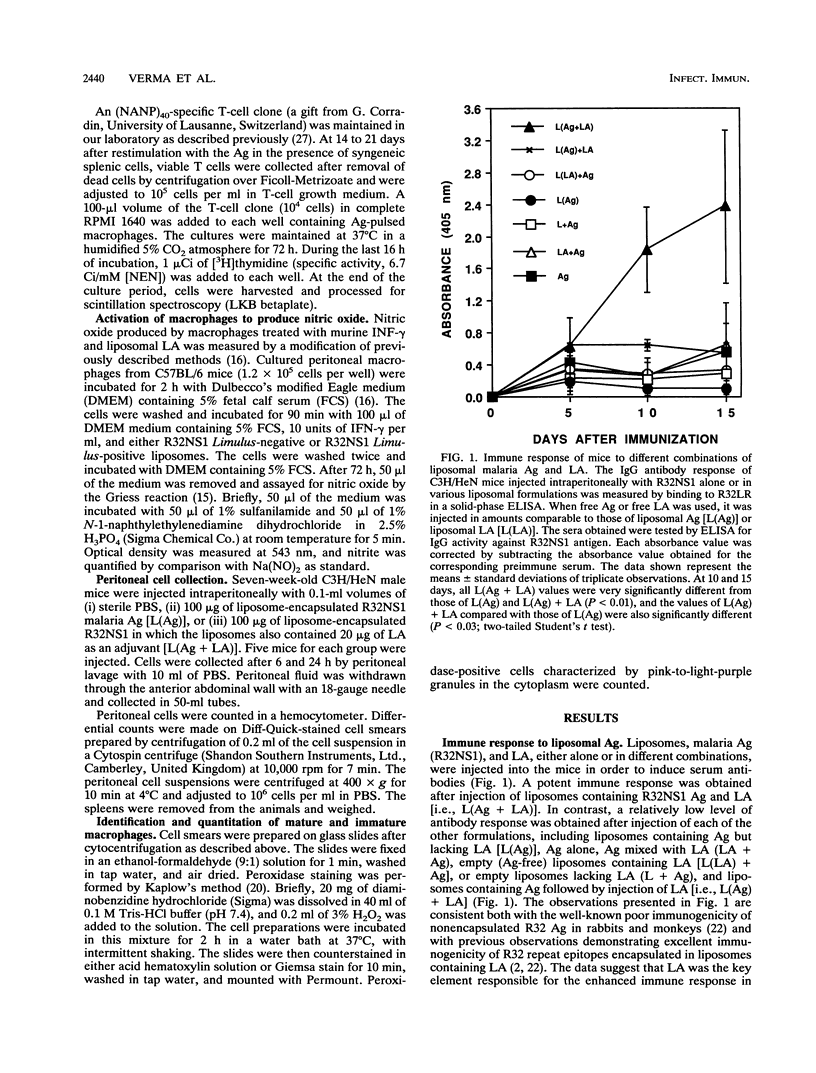
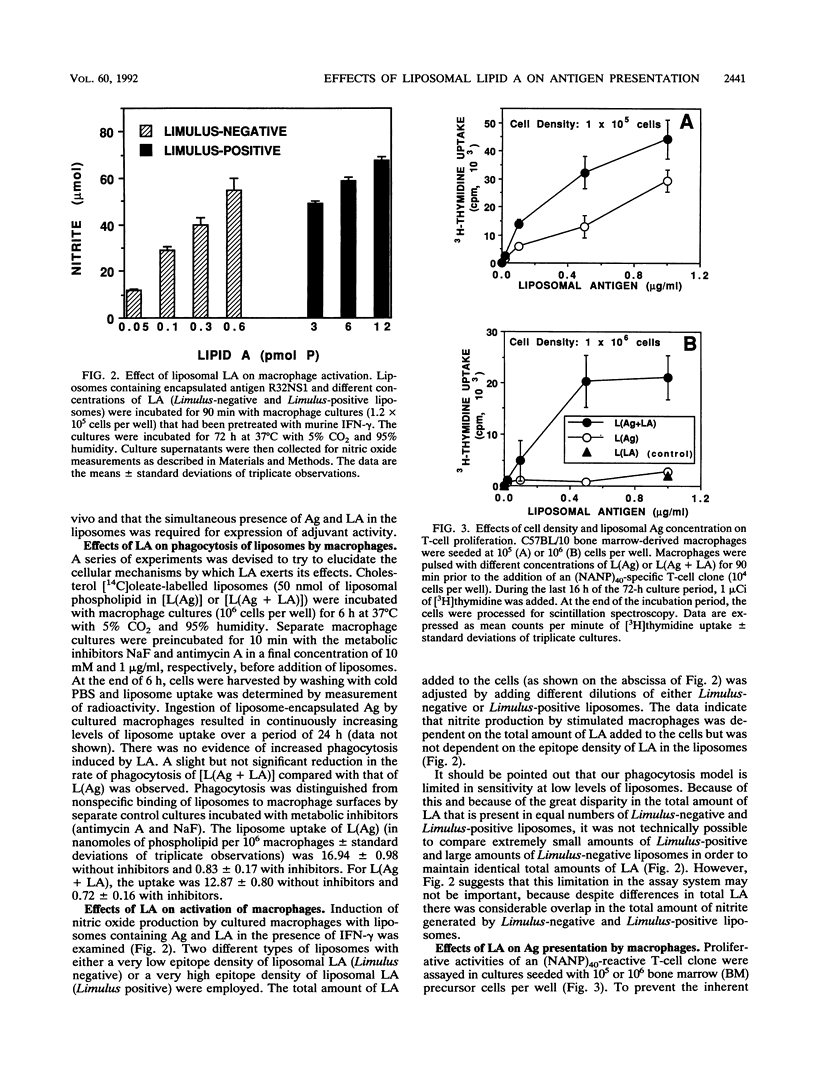
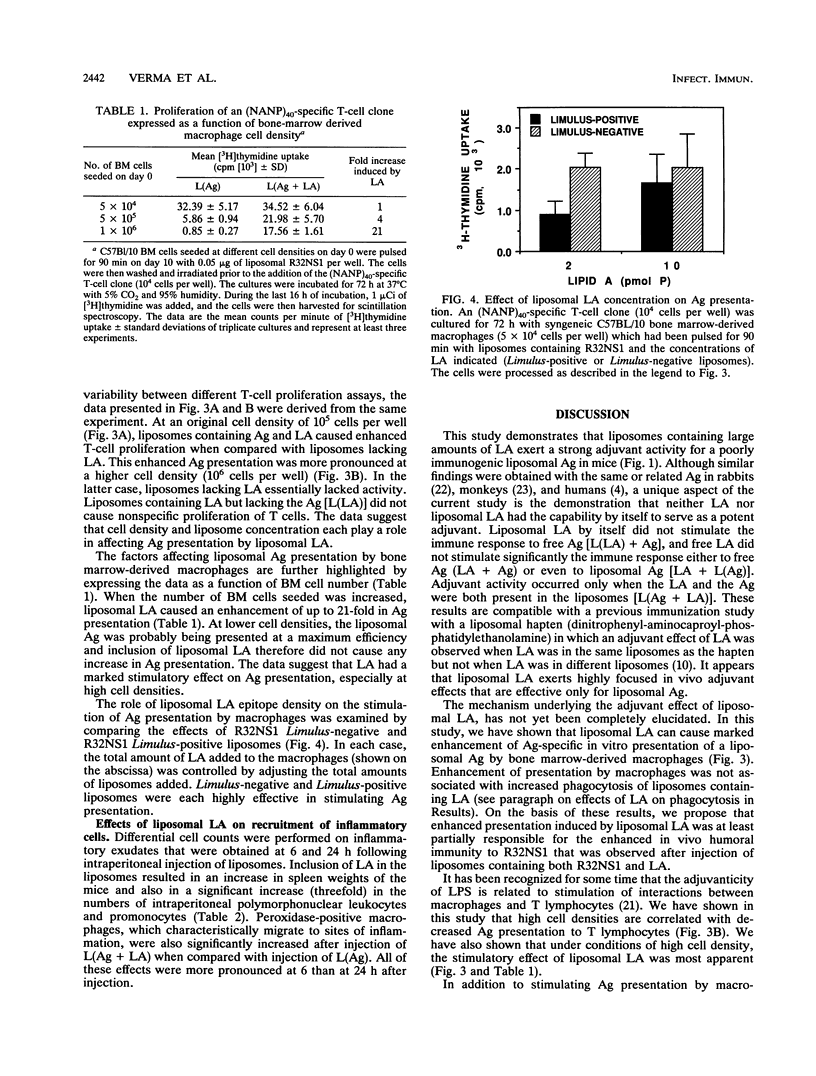
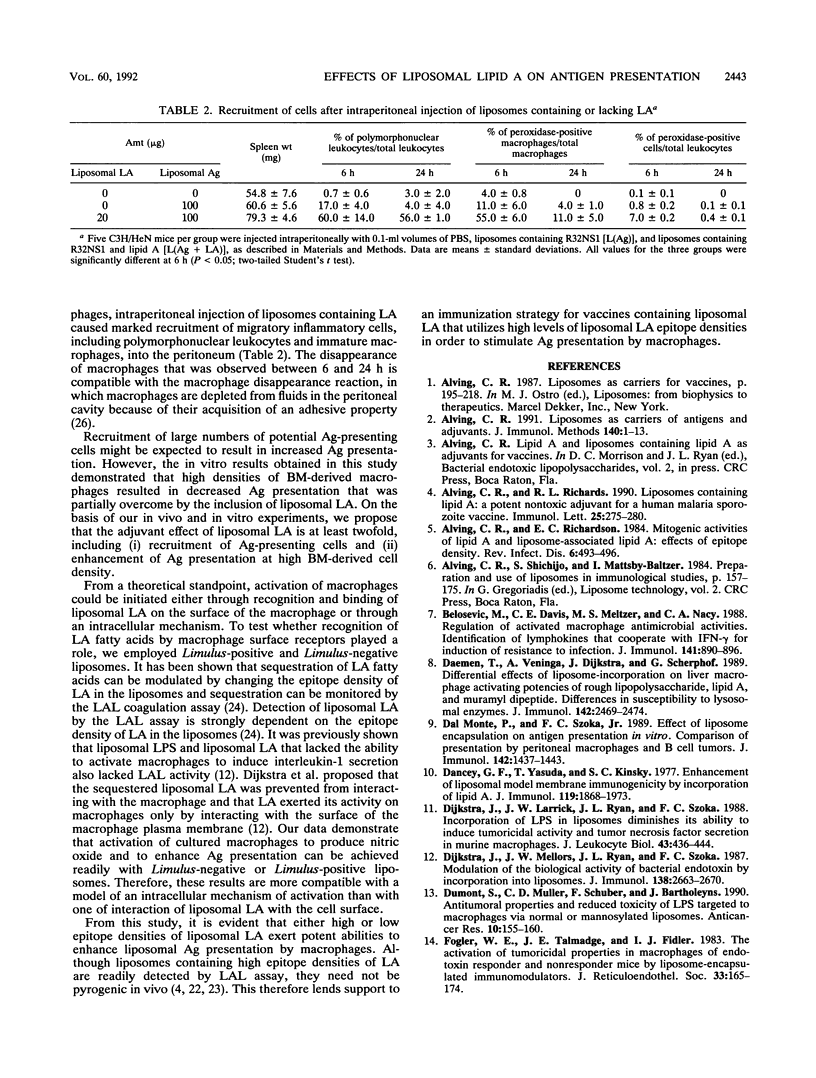
Liposomes containing lipid A induced potent humoral immune responses in mice against an encapsulated malaria antigen (R32NS1) containing NANP epitopes. The immune response was not enhanced by lipid A alone or by empty liposomes containing lipid A. Experiments to investigate the adjuvant mechanisms of liposomes and lipid A revealed that liposome-encapsulated R32NS1 was actively presented by bone marrow-derived macrophages to NANP-specific cloned T cells. The degree of presentation was related to the amount of liposomal antigen added per macrophage in the culture medium. At high cell densities, poor presentation occurred when liposomes lacked lipid A but excellent presentation occurred when the liposomes contained lipid A. Liposomes containing lipid A and encapsulated antigen also activated gamma interferon-treated macrophages to produce nitric oxide. Macrophage activation and antigen presentation occurred with liposomes that could not be detected by the Limulus amebocyte lysis assay. Intraperitoneal injection of liposomal lipid A caused a marked increase in the recruitment of immature (peroxidase-positive) macrophages to the peritoneum. On the basis of these experiments, we propose that the mechanism of the adjuvant action of liposomal lipid A is partly due to increased antigen presentation by macrophages and partly due to recruitment of an increased number of macrophages serving as antigen-presenting cells.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alving C. R. Liposomes as carriers of antigens and adjuvants. J Immunol Methods. 1991 Jun 24;140(1):1–13. doi: 10.1016/0022-1759(91)90120-5. [DOI] [PubMed] [Google Scholar]
- Alving C. R., Richards R. L. Liposomes containing lipid A: a potent nontoxic adjuvant for a human malaria sporozoite vaccine. Immunol Lett. 1990 Aug;25(1-3):275–279. doi: 10.1016/0165-2478(90)90127-c. [DOI] [PubMed] [Google Scholar]
- Alving C. R., Richardson E. C. Mitogenic activities of lipid A and liposome-associated lipid A: effects of epitope density. Rev Infect Dis. 1984 Jul-Aug;6(4):493–496. doi: 10.1093/clinids/6.4.493. [DOI] [PubMed] [Google Scholar]
- Belosevic M., Davis C. E., Meltzer M. S., Nacy C. A. Regulation of activated macrophage antimicrobial activities. Identification of lymphokines that cooperate with IFN-gamma for induction of resistance to infection. J Immunol. 1988 Aug 1;141(3):890–896. [PubMed] [Google Scholar]
- Daemen T., Veninga A., Dijkstra J., Scherphof G. Differential effects of liposome-incorporation on liver macrophage activating potencies of rough lipopolysaccharide, lipid A, and muramyl dipeptide. Differences in susceptibility to lysosomal enzymes. J Immunol. 1989 Apr 1;142(7):2469–2474. [PubMed] [Google Scholar]
- Dal Monte P., Szoka F. C., Jr Effect of liposome encapsulation on antigen presentation in vitro. Comparison of presentation by peritoneal macrophages and B cell tumors. J Immunol. 1989 Mar 1;142(5):1437–1443. [PubMed] [Google Scholar]
- Dancey G. F., Yasuda T., Kinsky S. C. Enhancement of liposomal model membrane immunogenicity by incorporation of lipid A. J Immunol. 1977 Dec;119(6):1868–1873. [PubMed] [Google Scholar]
- Dijkstra J., Larrick J. W., Ryan J. L., Szoka F. C. Incorporation of LPS in liposomes diminishes its ability to induce tumoricidal activity and tumor necrosis factor secretion in murine macrophages. J Leukoc Biol. 1988 May;43(5):436–444. doi: 10.1002/jlb.43.5.436. [DOI] [PubMed] [Google Scholar]
- Dijkstra J., Mellors J. W., Ryan J. L., Szoka F. C. Modulation of the biological activity of bacterial endotoxin by incorporation into liposomes. J Immunol. 1987 Apr 15;138(8):2663–2670. [PubMed] [Google Scholar]
- Dumont S., Muller C. D., Schuber F., Bartholeyns J. Antitumoral properties and reduced toxicity of LPS targeted to macrophages via normal or mannosylated liposomes. Anticancer Res. 1990 Jan-Feb;10(1):155–160. [PubMed] [Google Scholar]
- Fogler W. E., Talmadge J. E., Fidler I. J. The activation of tumoricidal properties in macrophages of endotoxin responder and nonresponder mice by liposome-encapsulated immunomodulators. J Reticuloendothel Soc. 1983 Mar;33(3):165–174. [PubMed] [Google Scholar]
- Green L. C., Wagner D. A., Glogowski J., Skipper P. L., Wishnok J. S., Tannenbaum S. R. Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids. Anal Biochem. 1982 Oct;126(1):131–138. doi: 10.1016/0003-2697(82)90118-x. [DOI] [PubMed] [Google Scholar]
- Green S. J., Crawford R. M., Hockmeyer J. T., Meltzer M. S., Nacy C. A. Leishmania major amastigotes initiate the L-arginine-dependent killing mechanism in IFN-gamma-stimulated macrophages by induction of tumor necrosis factor-alpha. J Immunol. 1990 Dec 15;145(12):4290–4297. [PubMed] [Google Scholar]
- Gregoriadis G. Immunological adjuvants: a role for liposomes. Immunol Today. 1990 Mar;11(3):89–97. doi: 10.1016/0167-5699(90)90034-7. [DOI] [PubMed] [Google Scholar]
- Harding C. V., Collins D. S., Slot J. W., Geuze H. J., Unanue E. R. Liposome-encapsulated antigens are processed in lysosomes, recycled, and presented to T cells. Cell. 1991 Jan 25;64(2):393–401. doi: 10.1016/0092-8674(91)90647-h. [DOI] [PubMed] [Google Scholar]
- Hoffman S. L., Cannon L. T., Sr, Berzofsky J. A., Majarian W. R., Young J. F., Maloy W. L., Hockmeyer W. T. Plasmodium falciparum: sporozoite boosting of immunity due to a T-cell epitope on a sporozoite vaccine. Exp Parasitol. 1987 Aug;64(1):64–70. doi: 10.1016/0014-4894(87)90009-9. [DOI] [PubMed] [Google Scholar]
- KAPLOW L. S. SIMPLIFIED MYELOPEROXIDASE STAIN USING BENZIDINE DIHYDROCHLORIDE. Blood. 1965 Aug;26:215–219. [PubMed] [Google Scholar]
- McGhee J. R., Farrar J. J., Michalek S. M., Mergenhagen S. E., Rosenstreich D. L. Cellular requirements for lipopolysaccharide adjuvanticity. A role for both T lymphocytes and macrophages for in vitro responses to particulate antigens. J Exp Med. 1979 Apr 1;149(4):793–807. doi: 10.1084/jem.149.4.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards R. L., Hayre M. D., Hockmeyer W. T., Alving C. R. Liposomes, lipid A, and aluminum hydroxide enhance the immune response to a synthetic malaria sporozoite antigen. Infect Immun. 1988 Mar;56(3):682–686. doi: 10.1128/iai.56.3.682-686.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards R. L., Swartz G. M., Jr, Schultz C., Hayre M. D., Ward G. S., Ballou W. R., Chulay J. D., Hockmeyer W. T., Berman S. L., Alving C. R. Immunogenicity of liposomal malaria sporozoite antigen in monkeys: adjuvant effects of aluminium hydroxide and non-pyrogenic liposomal lipid A. Vaccine. 1989 Dec;7(6):506–512. doi: 10.1016/0264-410x(89)90274-0. [DOI] [PubMed] [Google Scholar]
- Richardson E. C., Banerji B., Seid R. C., Jr, Levin J., Alving C. R. Interactions of lipid a and liposome-associated lipid A with Limulus polyphemus amoebocytes. Infect Immun. 1983 Mar;39(3):1385–1391. doi: 10.1128/iai.39.3.1385-1391.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rickman L. S., Gordon D. M., Wistar R., Jr, Krzych U., Gross M., Hollingdale M. R., Egan J. E., Chulay J. D., Hoffman S. L. Use of adjuvant containing mycobacterial cell-wall skeleton, monophosphoryl lipid A, and squalane in malaria circumsporozoite protein vaccine. Lancet. 1991 Apr 27;337(8748):998–1001. doi: 10.1016/0140-6736(91)92659-p. [DOI] [PubMed] [Google Scholar]
- Shannon B. T., Love S. H., Myrvik Q. N. Participation of hyaluronic acid in the macrophage disappearance reaction. Immunol Commun. 1980;9(4):357–370. doi: 10.3109/08820138009052982. [DOI] [PubMed] [Google Scholar]
- Verma J. N., Wassef N. M., Wirtz R. A., Atkinson C. T., Aikawa M., Loomis L. D., Alving C. R. Phagocytosis of liposomes by macrophages: intracellular fate of liposomal malaria antigen. Biochim Biophys Acta. 1991 Jul 22;1066(2):229–238. doi: 10.1016/0005-2736(91)90191-a. [DOI] [PubMed] [Google Scholar]
- Wassef N. M., Alving C. R. Complement-dependent phagocytosis of liposomes by macrophages. Methods Enzymol. 1987;149:124–134. doi: 10.1016/0076-6879(87)49050-2. [DOI] [PubMed] [Google Scholar]
- Wirtz R. A., Zavala F., Charoenvit Y., Campbell G. H., Burkot T. R., Schneider I., Esser K. M., Beaudoin R. L., Andre R. G. Comparative testing of monoclonal antibodies against Plasmodium falciparum sporozoites for ELISA development. Bull World Health Organ. 1987;65(1):39–45. [PMC free article] [PubMed] [Google Scholar]



