Abstract
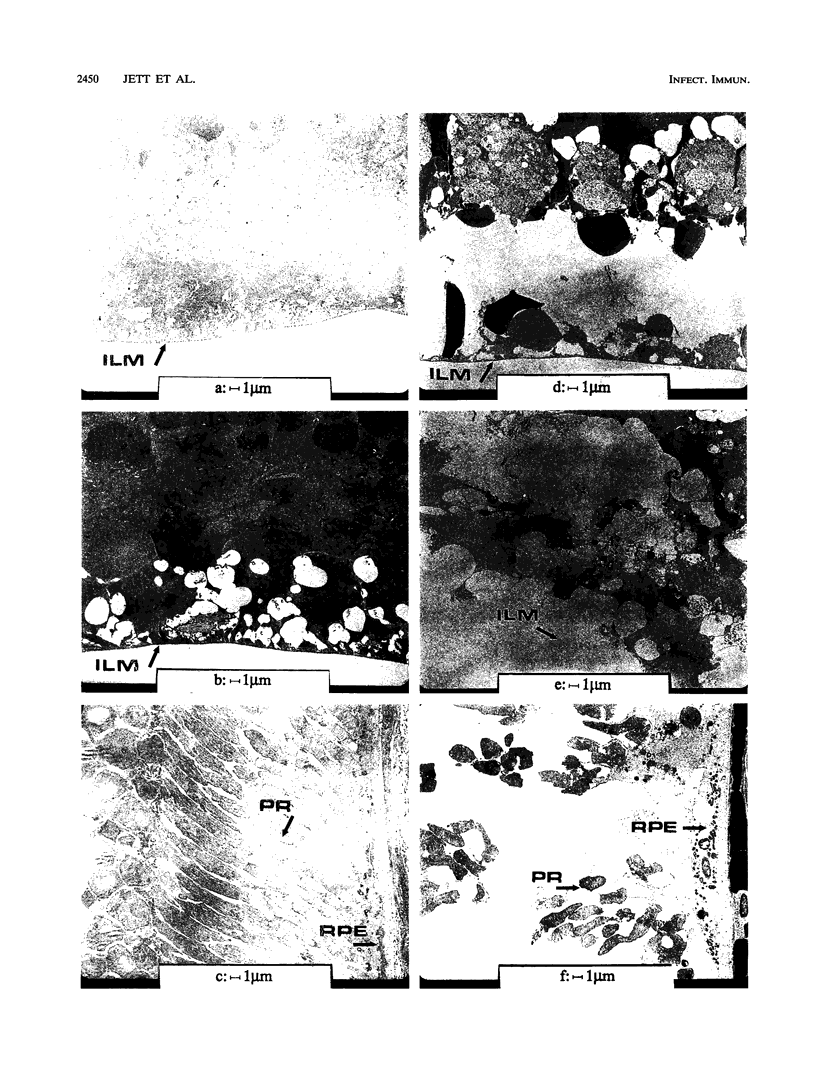
The contribution of the pAD1-encoded cytolysin to Enterococcus faecalis virulence in an experimental endophthalmitis model was studied by using isogenic strains differing only in the location of transposon Tn917. The course of experimental endophthalmitis in New Zealand White rabbits was evaluated by postoperative reduction in retinal neuroresponsiveness, thin-section histopathology, and transmission electron microscopy. Infections caused by cytolytic E. faecalis resulted in 99% loss of retinal function at postoperative day 3, while isogenic, noncytolytic strains produced reductions of only 74.2%. Light microscopy revealed near-total destruction of retinal architecture at 24 h postinfection with cytolytic E. faecalis, while noncytolytic strains produced few or no destructive changes. Transmission electron microscopy revealed tissue destruction in retinal layers as early as 6 h postinfection with cytolytic E. faecalis. In vivo and in vitro growth rates of cytolytic and noncytolytic E. faecalis showed similar kinetics. These data demonstrate the contribution of the pAD1-encoded cytolysin to both the course and the severity of experimental E. faecalis endophthalmitis.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abel R., Jr, Binder P. S., Bellows R. Postoperative bacterial endophthalmitis: section III. Ann Ophthalmol. 1976 Oct;8(10):1253–1265. [PubMed] [Google Scholar]
- Affeldt J. C., Flynn H. W., Jr, Forster R. K., Mandelbaum S., Clarkson J. G., Jarus G. D. Microbial endophthalmitis resulting from ocular trauma. Ophthalmology. 1987 Apr;94(4):407–413. doi: 10.1016/s0161-6420(87)33447-5. [DOI] [PubMed] [Google Scholar]
- Basinger S. F., Jackson R. W. Bacteriocin (hemolysin) of Streptococcus zymogenes. J Bacteriol. 1968 Dec;96(6):1895–1902. doi: 10.1128/jb.96.6.1895-1902.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clewell D. B. Plasmids, drug resistance, and gene transfer in the genus Streptococcus. Microbiol Rev. 1981 Sep;45(3):409–436. doi: 10.1128/mr.45.3.409-436.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clewell D. B., Tomich P. K., Gawron-Burke M. C., Franke A. E., Yagi Y., An F. Y. Mapping of Streptococcus faecalis plasmids pAD1 and pAD2 and studies relating to transposition of Tn917. J Bacteriol. 1982 Dec;152(3):1220–1230. doi: 10.1128/jb.152.3.1220-1230.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clewell D. B., Weaver K. E. Sex pheromones and plasmid transfer in Enterococcus faecalis. Plasmid. 1989 May;21(3):175–184. doi: 10.1016/0147-619x(89)90041-3. [DOI] [PubMed] [Google Scholar]
- Davey R. T., Jr, Tauber W. B. Posttraumatic endophthalmitis: the emerging role of Bacillus cereus infection. Rev Infect Dis. 1987 Jan-Feb;9(1):110–123. doi: 10.1093/clinids/9.1.110. [DOI] [PubMed] [Google Scholar]
- Driebe W. T., Jr, Mandelbaum S., Forster R. K., Schwartz L. K., Culbertson W. W. Pseudophakic endophthalmitis. Diagnosis and management. Ophthalmology. 1986 Apr;93(4):442–448. doi: 10.1016/s0161-6420(86)33722-9. [DOI] [PubMed] [Google Scholar]
- Engstrom R. E., Jr, Mondino B. J., Glasgow B. J., Pitchekian-Halabi H., Adamu S. A. Immune response to Staphylococcus aureus endophthalmitis in a rabbit model. Invest Ophthalmol Vis Sci. 1991 Apr;32(5):1523–1533. [PubMed] [Google Scholar]
- Forrester J. V., Worgul B. V., Merriam G. R., Jr Endotoxin-induced uveitis in the rat. Albrecht Von Graefes Arch Klin Exp Ophthalmol. 1980;213(4):221–233. doi: 10.1007/BF00417543. [DOI] [PubMed] [Google Scholar]
- Gaillard J. L., Berche P., Sansonetti P. Transposon mutagenesis as a tool to study the role of hemolysin in the virulence of Listeria monocytogenes. Infect Immun. 1986 Apr;52(1):50–55. doi: 10.1128/iai.52.1.50-55.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galli D., Lottspeich F., Wirth R. Sequence analysis of Enterococcus faecalis aggregation substance encoded by the sex pheromone plasmid pAD1. Mol Microbiol. 1990 Jun;4(6):895–904. doi: 10.1111/j.1365-2958.1990.tb00662.x. [DOI] [PubMed] [Google Scholar]
- Gilmore M. S., Segarra R. A., Booth M. C. An HlyB-type function is required for expression of the Enterococcus faecalis hemolysin/bacteriocin. Infect Immun. 1990 Dec;58(12):3914–3923. doi: 10.1128/iai.58.12.3914-3923.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huycke M. M., Spiegel C. A., Gilmore M. S. Bacteremia caused by hemolytic, high-level gentamicin-resistant Enterococcus faecalis. Antimicrob Agents Chemother. 1991 Aug;35(8):1626–1634. doi: 10.1128/aac.35.8.1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ike Y., Clewell D. B. Genetic analysis of the pAD1 pheromone response in Streptococcus faecalis, using transposon Tn917 as an insertional mutagen. J Bacteriol. 1984 Jun;158(3):777–783. doi: 10.1128/jb.158.3.777-783.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ike Y., Clewell D. B., Segarra R. A., Gilmore M. S. Genetic analysis of the pAD1 hemolysin/bacteriocin determinant in Enterococcus faecalis: Tn917 insertional mutagenesis and cloning. J Bacteriol. 1990 Jan;172(1):155–163. doi: 10.1128/jb.172.1.155-163.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ike Y., Hashimoto H., Clewell D. B. Hemolysin of Streptococcus faecalis subspecies zymogenes contributes to virulence in mice. Infect Immun. 1984 Aug;45(2):528–530. doi: 10.1128/iai.45.2.528-530.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ike Y., Hashimoto H., Clewell D. B. High incidence of hemolysin production by Enterococcus (Streptococcus) faecalis strains associated with human parenteral infections. J Clin Microbiol. 1987 Aug;25(8):1524–1528. doi: 10.1128/jcm.25.8.1524-1528.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs D. R., Cohen H. B. The inflammatory role of endotoxin in rabbit gram-negative bacterial endophthalmitis. Invest Ophthalmol Vis Sci. 1984 Sep;25(9):1074–1079. [PubMed] [Google Scholar]
- Jett B. D., Gilmore M. S. The growth-inhibitory effect of the Enterococcus faecalis bacteriocin encoded by pAD1 extends to the oral streptococci. J Dent Res. 1990 Oct;69(10):1640–1645. doi: 10.1177/00220345900690100301. [DOI] [PubMed] [Google Scholar]
- Jones S., Cohen E. J., Arentsen J. J., Laibson P. R. Ocular streptococcal infections. Cornea. 1988;7(4):295–299. [PubMed] [Google Scholar]
- Katz L. J., Cantor L. B., Spaeth G. L. Complications of surgery in glaucoma. Early and late bacterial endophthalmitis following glaucoma filtering surgery. Ophthalmology. 1985 Jul;92(7):959–963. doi: 10.1016/s0161-6420(85)33948-9. [DOI] [PubMed] [Google Scholar]
- Klimek J. J., Ajemian E., Andrews L., Hryb K., Hill D. A. Outbreak of bacterial endophthalmitis after cataract surgery and lens implantation: lack of direct evidence for exogenous contributing factors. Am J Infect Control. 1986 Aug;14(4):184–187. doi: 10.1016/0196-6553(86)90099-4. [DOI] [PubMed] [Google Scholar]
- Kohno T., Sorgente N., Ishibashi T., Goodnight R., Ryan S. J. Immunofluorescent studies of fibronectin and laminin in the human eye. Invest Ophthalmol Vis Sci. 1987 Mar;28(3):506–514. [PubMed] [Google Scholar]
- Leveille A. S., McMullan F. D., Cavanagh H. D. Endophthalmitis following penetrating keratoplasty. Ophthalmology. 1983 Jan;90(1):38–39. doi: 10.1016/s0161-6420(83)34601-7. [DOI] [PubMed] [Google Scholar]
- Mandelbaum S., Forster R. K., Gelender H., Culbertson W. Late onset endophthalmitis associated with filtering blebs. Ophthalmology. 1985 Jul;92(7):964–972. doi: 10.1016/s0161-6420(85)33947-7. [DOI] [PubMed] [Google Scholar]
- Meredith T. A., Aguilar H. E., Miller M. J., Gardner S. K., Trabelsi A., Wilson L. A. Comparative treatment of experimental Staphylococcus epidermidis endophthalmitis. Arch Ophthalmol. 1990 Jun;108(6):857–860. doi: 10.1001/archopht.1990.01070080101043. [DOI] [PubMed] [Google Scholar]
- Meredith T. A., Trabelsi A., Miller M. J., Aguilar E., Wilson L. A. Spontaneous sterilization in experimental Staphylococcus epidermidis endophthalmitis. Invest Ophthalmol Vis Sci. 1990 Jan;31(1):181–186. [PubMed] [Google Scholar]
- Meyers-Elliott R. H., Dethlefs B. A. Experimental Klebsiella-induced endophthalmitis in the rabbit. Arch Ophthalmol. 1982 Dec;100(12):1959–1963. doi: 10.1001/archopht.1982.01030040939015. [DOI] [PubMed] [Google Scholar]
- Mobley H. L., Chippendale G. R., Swihart K. G., Welch R. A. Cytotoxicity of the HpmA hemolysin and urease of Proteus mirabilis and Proteus vulgaris against cultured human renal proximal tubular epithelial cells. Infect Immun. 1991 Jun;59(6):2036–2042. doi: 10.1128/iai.59.6.2036-2042.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peyman G. A., Vastine D. W., Raichand M. Experimental aspects and their clinical application. Ophthalmology. 1978 Apr;85(4):374–385. doi: 10.1016/s0161-6420(78)35659-1. [DOI] [PubMed] [Google Scholar]
- Rowsey J. J., Jensen H., Sexton D. J. Clinical diagnosis of endophthalmitis. Int Ophthalmol Clin. 1987 Summer;27(2):82–88. doi: 10.1097/00004397-198702720-00004. [DOI] [PubMed] [Google Scholar]
- Rowsey J. J., Newsom D. L., Sexton D. J., Harms W. K. Endophthalmitis: current approaches. Ophthalmology. 1982 Sep;89(9):1055–1066. doi: 10.1016/s0161-6420(82)34691-6. [DOI] [PubMed] [Google Scholar]
- Segarra R. A., Booth M. C., Morales D. A., Huycke M. M., Gilmore M. S. Molecular characterization of the Enterococcus faecalis cytolysin activator. Infect Immun. 1991 Apr;59(4):1239–1246. doi: 10.1128/iai.59.4.1239-1246.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanford M. R., Robbins J., Kasp E., Dumonde D. C. Passive administration of antibody against retinal S-antigen induces electroretinographic supernormality. Invest Ophthalmol Vis Sci. 1992 Jan;33(1):30–35. [PubMed] [Google Scholar]
- Tomich P. K., An F. Y., Clewell D. B. Properties of erythromycin-inducible transposon Tn917 in Streptococcus faecalis. J Bacteriol. 1980 Mar;141(3):1366–1374. doi: 10.1128/jb.141.3.1366-1374.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber D. J., Hoffman K. L., Thoft R. A., Baker A. S. Endophthalmitis following intraocular lens implantation: report of 30 cases and review of the literature. Rev Infect Dis. 1986 Jan-Feb;8(1):12–20. doi: 10.1093/clinids/8.1.12. [DOI] [PubMed] [Google Scholar]
- Weiser J. N., Rubens C. E. Transposon mutagenesis of group B streptococcus beta-hemolysin biosynthesis. Infect Immun. 1987 Sep;55(9):2314–2316. doi: 10.1128/iai.55.9.2314-2316.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch R. A., Dellinger E. P., Minshew B., Falkow S. Haemolysin contributes to virulence of extra-intestinal E. coli infections. Nature. 1981 Dec 17;294(5842):665–667. doi: 10.1038/294665a0. [DOI] [PubMed] [Google Scholar]