Abstract
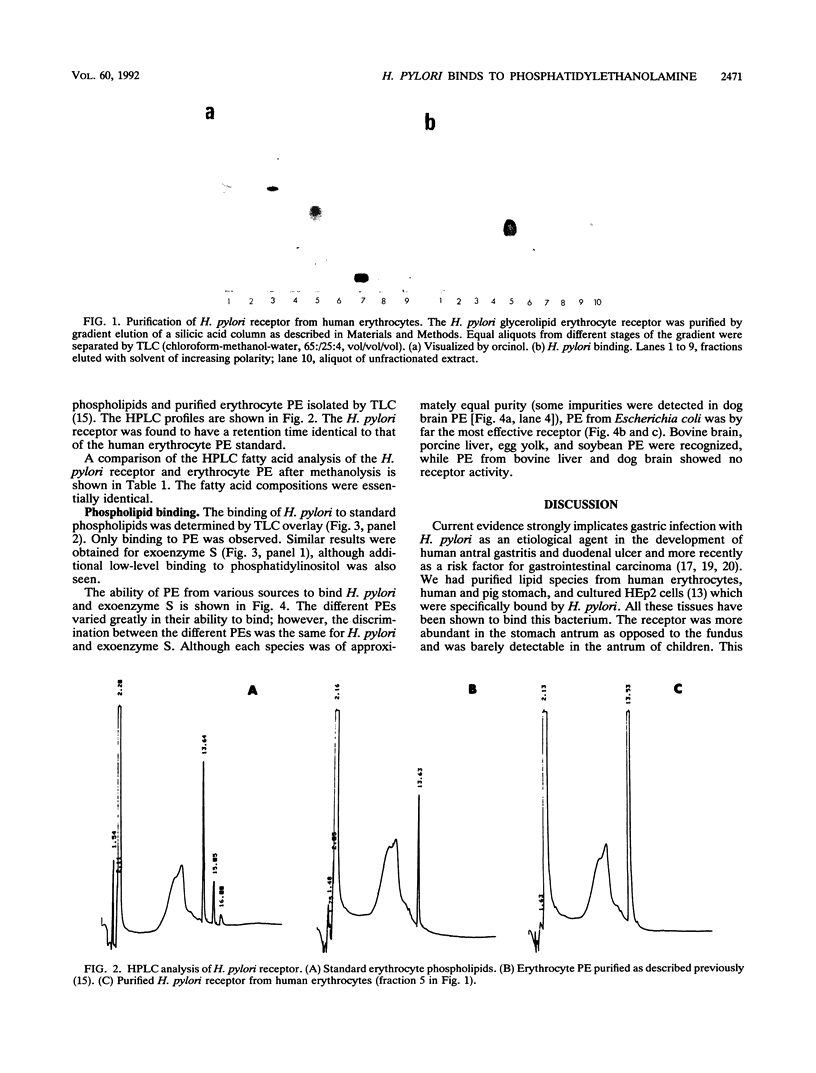
We have previously shown that Helicobacter pylori specifically binds to a glycerolipid species preferentially found in the antrum of the human stomach. We now show by high-pressure liquid chromatographic analysis that this species is a form of phosphatidylethanolamine and that H. pylori specifically binds to bona fide phosphatidylethanolamine as detected by a thin-layer chromatogram overlay procedure. Considerable variation in the binding of H. pylori to phosphatidylethanolamine from different sources was observed, however, suggesting the importance of the nature of the long-chain hydrophobic moiety. A similar binding specificity was shown by exoenzyme S from Pseudomonas aeruginosa, consistent with our hypothesis that that an exoenzyme S-like adhesin is responsible for the binding of H. pylori to its lipid receptors.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armstrong J. A., Cooper M., Goodwin C. S., Robinson J., Wee S. H., Burton M., Burke V. Influence of soluble haemagglutinins on adherence of Helicobacter pylori to HEp-2 cells. J Med Microbiol. 1991 Mar;34(3):181–187. doi: 10.1099/00222615-34-3-181. [DOI] [PubMed] [Google Scholar]
- Baker N. R., Minor V., Deal C., Shahrabadi M. S., Simpson D. A., Woods D. E. Pseudomonas aeruginosa exoenzyme S is an adhesion. Infect Immun. 1991 Sep;59(9):2859–2863. doi: 10.1128/iai.59.9.2859-2863.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dick J. D. Helicobacter (Campylobacter) pylori: a new twist to an old disease. Annu Rev Microbiol. 1990;44:249–269. doi: 10.1146/annurev.mi.44.100190.001341. [DOI] [PubMed] [Google Scholar]
- Evans D. G., Evans D. J., Jr, Moulds J. J., Graham D. Y. N-acetylneuraminyllactose-binding fibrillar hemagglutinin of Campylobacter pylori: a putative colonization factor antigen. Infect Immun. 1988 Nov;56(11):2896–2906. doi: 10.1128/iai.56.11.2896-2906.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fauchère J. L., Blaser M. J. Adherence of Helicobacter pylori cells and their surface components to HeLa cell membranes. Microb Pathog. 1990 Dec;9(6):427–439. doi: 10.1016/0882-4010(90)90061-t. [DOI] [PubMed] [Google Scholar]
- Graham D. Y. Campylobacter pylori and peptic ulcer disease. Gastroenterology. 1989 Feb;96(2 Pt 2 Suppl):615–625. doi: 10.1016/s0016-5085(89)80057-5. [DOI] [PubMed] [Google Scholar]
- Kiss Z., Anderson W. B. ATP stimulates the hydrolysis of phosphatidylethanolamine in NIH 3T3 cells. Potentiating effects of guanosine triphosphates and sphingosine. J Biol Chem. 1990 May 5;265(13):7345–7350. [PubMed] [Google Scholar]
- Kiss Z., Anderson W. B. Phorbol ester stimulates the hydrolysis of phosphatidylethanolamine in leukemic HL-60, NIH 3T3, and baby hamster kidney cells. J Biol Chem. 1989 Jan 25;264(3):1483–1487. [PubMed] [Google Scholar]
- Krivan H. C., Nilsson B., Lingwood C. A., Ryu H. Chlamydia trachomatis and Chlamydia pneumoniae bind specifically to phosphatidylethanolamine in HeLa cells and to GalNAc beta 1-4Gal beta 1-4GLC sequences-found in asialo-GM1 and asial-GM2. Biochem Biophys Res Commun. 1991 Mar 29;175(3):1082–1089. doi: 10.1016/0006-291x(91)91676-4. [DOI] [PubMed] [Google Scholar]
- Krivan H. C., Roberts D. D., Ginsburg V. Many pulmonary pathogenic bacteria bind specifically to the carbohydrate sequence GalNAc beta 1-4Gal found in some glycolipids. Proc Natl Acad Sci U S A. 1988 Aug;85(16):6157–6161. doi: 10.1073/pnas.85.16.6157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lingwood C. A., Cheng M., Krivan H. C., Woods D. Glycolipid receptor binding specificity of exoenzyme S from Pseudomonas aeruginosa. Biochem Biophys Res Commun. 1991 Mar 29;175(3):1076–1081. doi: 10.1016/0006-291x(91)91675-3. [DOI] [PubMed] [Google Scholar]
- Lingwood C. A., Law H., Pellizzari A., Sherman P., Drumm B. Gastric glycerolipid as a receptor for Campylobacter pylori. Lancet. 1989 Jul 29;2(8657):238–241. doi: 10.1016/s0140-6736(89)90428-5. [DOI] [PubMed] [Google Scholar]
- Lingwood C. A., Law H., Richardson S., Petric M., Brunton J. L., De Grandis S., Karmali M. Glycolipid binding of purified and recombinant Escherichia coli produced verotoxin in vitro. J Biol Chem. 1987 Jun 25;262(18):8834–8839. [PubMed] [Google Scholar]
- Myher J. J., Kuksis A., Pind S. Molecular species of glycerophospholipids and sphingomyelins of human erythrocytes: improved method of analysis. Lipids. 1989 May;24(5):396–407. doi: 10.1007/BF02535147. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. The role of protein kinase C in cell surface signal transduction and tumour promotion. Nature. 1984 Apr 19;308(5961):693–698. doi: 10.1038/308693a0. [DOI] [PubMed] [Google Scholar]
- Nomura A., Stemmermann G. N., Chyou P. H., Kato I., Perez-Perez G. I., Blaser M. J. Helicobacter pylori infection and gastric carcinoma among Japanese Americans in Hawaii. N Engl J Med. 1991 Oct 17;325(16):1132–1136. doi: 10.1056/NEJM199110173251604. [DOI] [PubMed] [Google Scholar]
- Op den Kamp J. A. Lipid asymmetry in membranes. Annu Rev Biochem. 1979;48:47–71. doi: 10.1146/annurev.bi.48.070179.000403. [DOI] [PubMed] [Google Scholar]
- Parsonnet J., Friedman G. D., Vandersteen D. P., Chang Y., Vogelman J. H., Orentreich N., Sibley R. K. Helicobacter pylori infection and the risk of gastric carcinoma. N Engl J Med. 1991 Oct 17;325(16):1127–1131. doi: 10.1056/NEJM199110173251603. [DOI] [PubMed] [Google Scholar]
- Parsonnet J., Vandersteen D., Goates J., Sibley R. K., Pritikin J., Chang Y. Helicobacter pylori infection in intestinal- and diffuse-type gastric adenocarcinomas. J Natl Cancer Inst. 1991 May 1;83(9):640–643. doi: 10.1093/jnci/83.9.640. [DOI] [PubMed] [Google Scholar]
- Pellizzari A., Pang H., Lingwood C. A. Binding of verocytotoxin 1 to its receptor is influenced by differences in receptor fatty acid content. Biochemistry. 1992 Feb 11;31(5):1363–1370. doi: 10.1021/bi00120a011. [DOI] [PubMed] [Google Scholar]
- Robinson J., Goodwin C. S., Cooper M., Burke V., Mee B. J. Soluble and cell-associated haemagglutinins of Helicobacter (Campylobacter) pylori. J Med Microbiol. 1990 Dec;33(4):277–284. doi: 10.1099/00222615-33-4-277. [DOI] [PubMed] [Google Scholar]
- Rosenberry T. L., Krall J. A., Dever T. E., Haas R., Louvard D., Merrick W. C. Biosynthetic incorporation of [3H]ethanolamine into protein synthesis elongation factor 1 alpha reveals a new post-translational protein modification. J Biol Chem. 1989 May 5;264(13):7096–7099. [PubMed] [Google Scholar]
- Saitoh T., Natomi H., Zhao W. L., Okuzumi K., Sugano K., Iwamori M., Nagai Y. Identification of glycolipid receptors for Helicobacter pylori by TLC-immunostaining. FEBS Lett. 1991 May 6;282(2):385–387. doi: 10.1016/0014-5793(91)80519-9. [DOI] [PubMed] [Google Scholar]
- Slomiany B. L., Piotrowski J., Samanta A., VanHorn K., Murty V. L., Slomiany A. Campylobacter pylori colonization factor shows specificity for lactosylceramide sulfate and GM3 ganglioside. Biochem Int. 1989 Oct;19(4):929–936. [PubMed] [Google Scholar]
- Whiteheart S. W., Shenbagamurthi P., Chen L., Cotter R. J., Hart G. W. Murine elongation factor 1 alpha (EF-1 alpha) is posttranslationally modified by novel amide-linked ethanolamine-phosphoglycerol moieties. Addition of ethanolamine-phosphoglycerol to specific glutamic acid residues on EF-1 alpha. J Biol Chem. 1989 Aug 25;264(24):14334–14341. [PubMed] [Google Scholar]
- Woods D. E., Que J. U. Purification of Pseudomonas aeruginosa exoenzyme S. Infect Immun. 1987 Mar;55(3):579–586. doi: 10.1128/iai.55.3.579-586.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods D. E., To M., Sokol P. A. Pseudomonas aeruginosa exoenzyme S as a pathogenic determinant in respiratory infections. Antibiot Chemother (1971) 1989;42:27–35. doi: 10.1159/000417600. [DOI] [PubMed] [Google Scholar]
- Wyatt J. I., Rathbone B. J., Dixon M. F., Heatley R. V. Campylobacter pyloridis and acid induced gastric metaplasia in the pathogenesis of duodenitis. J Clin Pathol. 1987 Aug;40(8):841–848. doi: 10.1136/jcp.40.8.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwaal R. F., Roelofsen B., Colley C. M. Localization of red cell membrane constituents. Biochim Biophys Acta. 1973 Sep 10;300(2):159–182. doi: 10.1016/0304-4157(73)90003-8. [DOI] [PubMed] [Google Scholar]