Abstract
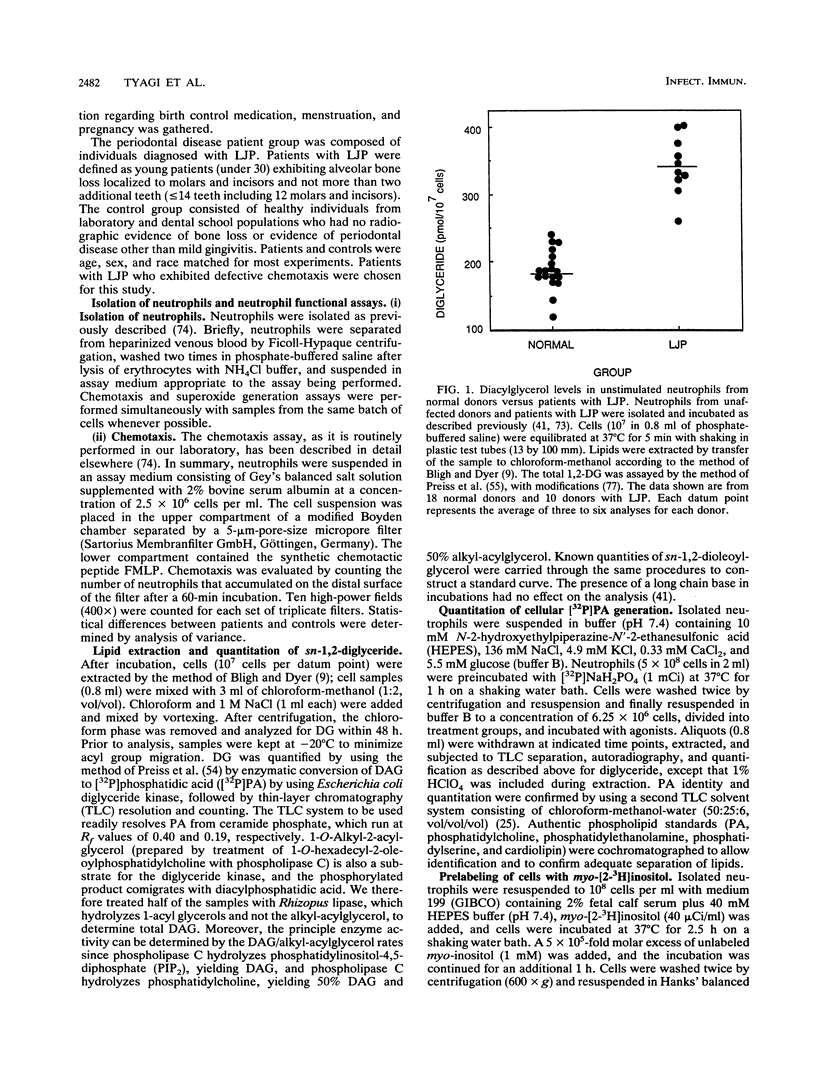
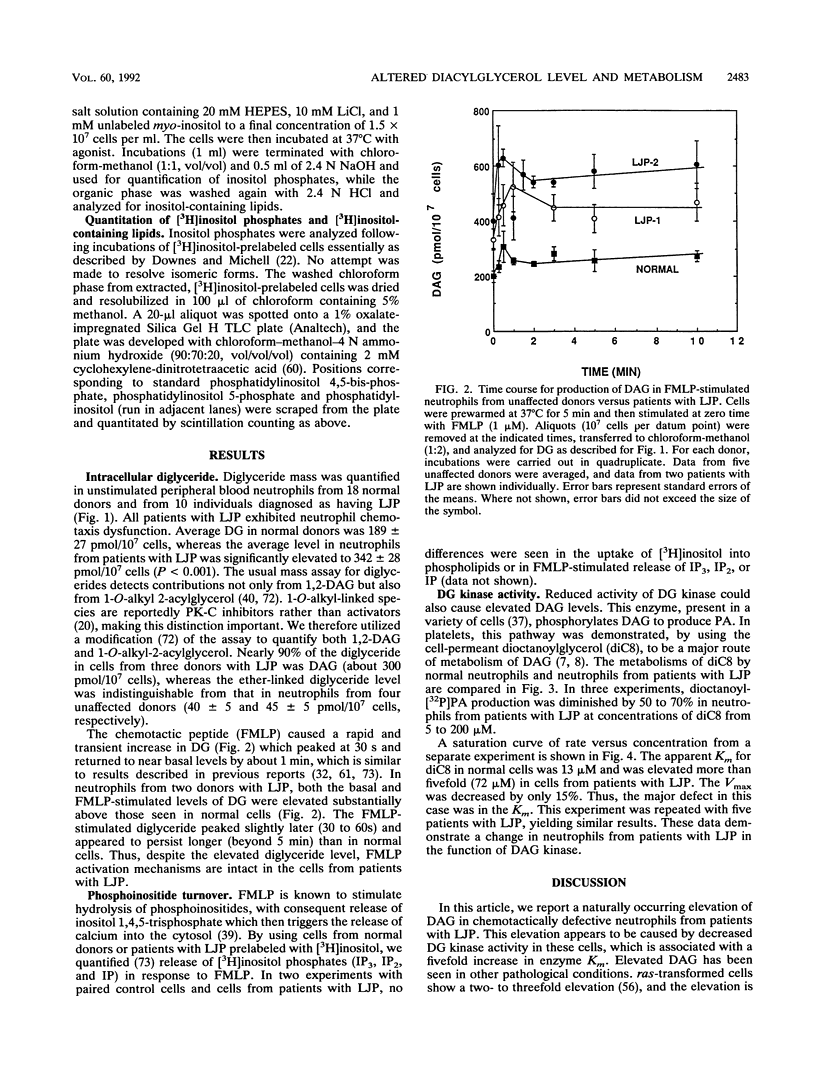
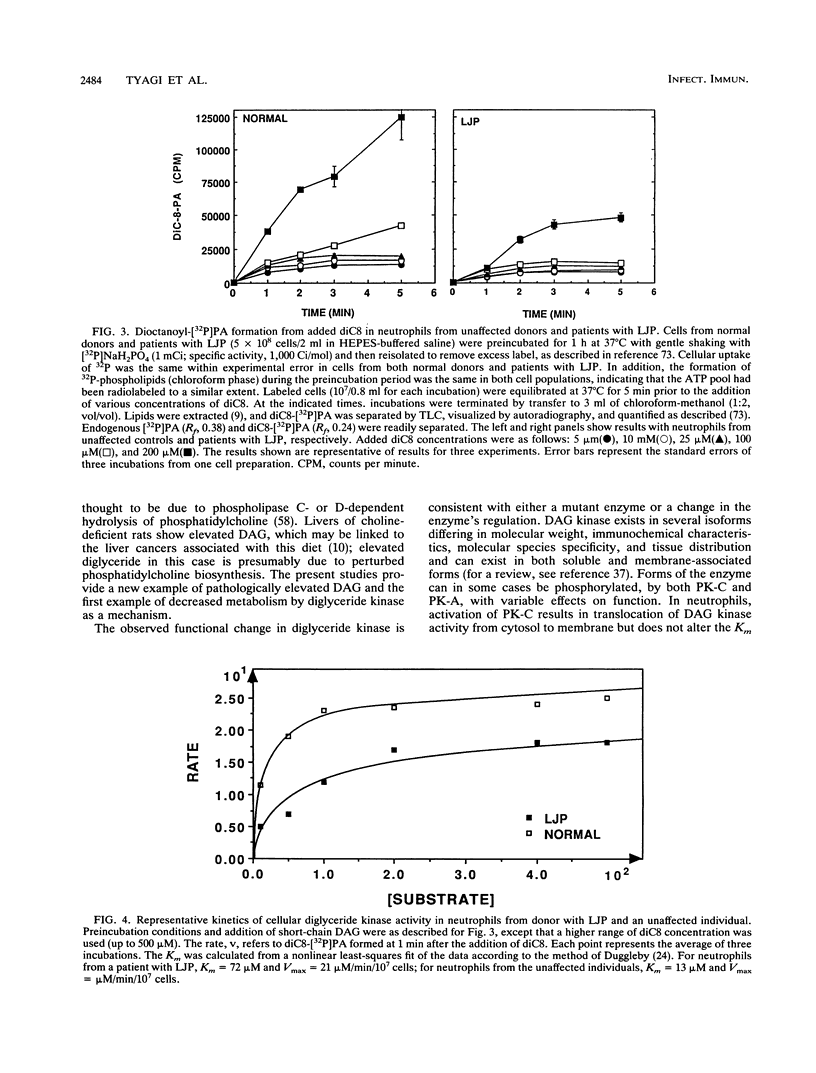
Diacylglycerol, a physiological activator of protein kinase C, was elevated nearly twofold in unstimulated peripheral blood neutrophils from patients with localized juvenile periodontitis compared with cells from normal individuals. These cells also showed an enhanced and prolonged elevation of diglyceride in response to N-formylmethionylleucylphenylalanine. The metabolism of a cell-permeant diacylglycerol by diglyceride kinase was significantly decreased, because of a fivefold or higher elevation in the apparent Km of cellular diglyceride kinase.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agarwal S., Reynolds M. A., Duckett L. D., Suzuki J. B. Altered free cytosolic calcium changes and neutrophil chemotaxis in patients with juvenile periodontitis. J Periodontal Res. 1989 Mar;24(2):149–154. doi: 10.1111/j.1600-0765.1989.tb00870.x. [DOI] [PubMed] [Google Scholar]
- Allan D., Michell R. H. The relationship between Ca2+-mediated polyphosphoinositide phosphodiesterase activity, 1, 2-diacylglycerol accumulation, and microvesiculation in erythrocytes. Prog Clin Biol Res. 1979;30:523–529. [PubMed] [Google Scholar]
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Berridge M. J., Dawson R. M., Downes C. P., Heslop J. P., Irvine R. F. Changes in the levels of inositol phosphates after agonist-dependent hydrolysis of membrane phosphoinositides. Biochem J. 1983 May 15;212(2):473–482. doi: 10.1042/bj2120473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M. J. Inositol trisphosphate and diacylglycerol as second messengers. Biochem J. 1984 Jun 1;220(2):345–360. doi: 10.1042/bj2200345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M. J., Irvine R. F. Inositol trisphosphate, a novel second messenger in cellular signal transduction. Nature. 1984 Nov 22;312(5992):315–321. doi: 10.1038/312315a0. [DOI] [PubMed] [Google Scholar]
- Berridge M. J. Rapid accumulation of inositol trisphosphate reveals that agonists hydrolyse polyphosphoinositides instead of phosphatidylinositol. Biochem J. 1983 Jun 15;212(3):849–858. doi: 10.1042/bj2120849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besterman J. M., Duronio V., Cuatrecasas P. Rapid formation of diacylglycerol from phosphatidylcholine: a pathway for generation of a second messenger. Proc Natl Acad Sci U S A. 1986 Sep;83(18):6785–6789. doi: 10.1073/pnas.83.18.6785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop W. R., Bell R. M. Attenuation of sn-1,2-diacylglycerol second messengers. Metabolism of exogenous diacylglycerols by human platelets. J Biol Chem. 1986 Sep 25;261(27):12513–12519. [PubMed] [Google Scholar]
- Bishop W. R., Ganong B. R., Bell R. M. Attenuation of sn-1,2-diacylglycerol second messengers by diacylglycerol kinase. Inhibition by diacylglycerol analogs in vitro and in human platelets. J Biol Chem. 1986 May 25;261(15):6993–7000. [PubMed] [Google Scholar]
- Blusztajn J. K., Zeisel S. H. 1,2-sn-diacylglycerol accumulates in choline-deficient liver. A possible mechanism of hepatic carcinogenesis via alteration in protein kinase C activity? FEBS Lett. 1989 Jan 30;243(2):267–270. doi: 10.1016/0014-5793(89)80142-5. [DOI] [PubMed] [Google Scholar]
- Bocckino S. B., Blackmore P. F., Exton J. H. Stimulation of 1,2-diacylglycerol accumulation in hepatocytes by vasopressin, epinephrine, and angiotensin II. J Biol Chem. 1985 Nov 15;260(26):14201–14207. [PubMed] [Google Scholar]
- Cainciola L. J., Genco R. J., Patters M. R., McKenna J., van Oss C. J. Defective polymorphonuclear leukocyte function in a human periodontal disease. Nature. 1977 Feb 3;265(5593):445–447. doi: 10.1038/265445a0. [DOI] [PubMed] [Google Scholar]
- Charest R., Prpić V., Exton J. H., Blackmore P. F. Stimulation of inositol trisphosphate formation in hepatocytes by vasopressin, adrenaline and angiotensin II and its relationship to changes in cytosolic free Ca2+. Biochem J. 1985 Apr 1;227(1):79–90. doi: 10.1042/bj2270079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cockcroft S., Baldwin J. M., Allan D. The Ca2+-activated polyphosphoinositide phosphodiesterase of human and rabbit neutrophil membranes. Biochem J. 1984 Jul 15;221(2):477–482. doi: 10.1042/bj2210477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cockcroft S., Gomperts B. D. Role of guanine nucleotide binding protein in the activation of polyphosphoinositide phosphodiesterase. Nature. 1985 Apr 11;314(6011):534–536. doi: 10.1038/314534a0. [DOI] [PubMed] [Google Scholar]
- Daniel L. W., Small G. W., Schmitt J. D., Marasco C. J., Ishaq K., Piantadosi C. Alkyl-linked diglycerides inhibit protein kinase C activation by diacylglycerols. Biochem Biophys Res Commun. 1988 Feb 29;151(1):291–297. doi: 10.1016/0006-291x(88)90592-x. [DOI] [PubMed] [Google Scholar]
- Dougherty R. W., Godfrey P. P., Hoyle P. C., Putney J. W., Jr, Freer R. J. Secretagogue-induced phosphoinositide metabolism in human leucocytes. Biochem J. 1984 Sep 1;222(2):307–314. doi: 10.1042/bj2220307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downes C. P., Michell R. H. The polyphosphoinositide phosphodiesterase of erythrocyte membranes. Biochem J. 1981 Jul 15;198(1):133–140. doi: 10.1042/bj1980133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downes C. P., Mussat M. C., Michell R. H. The inositol trisphosphate phosphomonoesterase of the human erythrocyte membrane. Biochem J. 1982 Apr 1;203(1):169–177. doi: 10.1042/bj2030169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duggleby R. G. A nonlinear regression program for small computers. Anal Biochem. 1981 Jan 1;110(1):9–18. doi: 10.1016/0003-2697(81)90104-4. [DOI] [PubMed] [Google Scholar]
- Farese R. V., Sabir A. M., Vandor S. L. Adrenocorticotropin acutely increases adrenal polyphosphoinositides. J Biol Chem. 1979 Aug 10;254(15):6842–6844. [PubMed] [Google Scholar]
- Genco R. J., Van Dyke T. E., Park B., Ciminelli M., Horoszewicz H. Neutrophil chemotaxis impairment in juvenile periodontitis: evaluation of specificity, adherence, deformability, and serum factors. J Reticuloendothel Soc. 1980 Dec;28(Suppl):81s–91s. [PubMed] [Google Scholar]
- Griendling K. K., Rittenhouse S. E., Brock T. A., Ekstein L. S., Gimbrone M. A., Jr, Alexander R. W. Sustained diacylglycerol formation from inositol phospholipids in angiotensin II-stimulated vascular smooth muscle cells. J Biol Chem. 1986 May 5;261(13):5901–5906. [PubMed] [Google Scholar]
- HOKIN M. R., HOKIN L. E. Enzyme secretion and the incorporation of P32 into phospholipides of pancreas slices. J Biol Chem. 1953 Aug;203(2):967–977. [PubMed] [Google Scholar]
- Hamilton R. E., Jr, Giansanti J. S. The Chediak-Higashi syndrome. Report of a case and review of the literature. Oral Surg Oral Med Oral Pathol. 1974 May;37(5):754–761. doi: 10.1016/0030-4220(74)90141-8. [DOI] [PubMed] [Google Scholar]
- Harvath L., McCall C. E., Bass D. A., McPhail L. C. Inhibition of human neutrophil chemotaxis by the protein kinase inhibitor, 1-(5-isoquinolinesulfonyl) piperazine. J Immunol. 1987 Nov 1;139(9):3055–3061. [PubMed] [Google Scholar]
- Hill H. R., Gerrard J. M., Hogan N. A., Quie P. G. Hyperactivity of neutrophil leukotactic responses during active bacterial infection. J Clin Invest. 1974 Apr;53(4):996–1002. doi: 10.1172/JCI107666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inhorn R. C., Bansal V. S., Majerus P. W. Pathway for inositol 1,3,4-trisphosphate and 1,4-bisphosphate metabolism. Proc Natl Acad Sci U S A. 1987 Apr;84(8):2170–2174. doi: 10.1073/pnas.84.8.2170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inhorn R. C., Majerus P. W. Inositol polyphosphate 1-phosphatase from calf brain. Purification and inhibition by Li+, Ca2+, and Mn2+. J Biol Chem. 1987 Nov 25;262(33):15946–15952. [PubMed] [Google Scholar]
- Irving H. R., Exton J. H. Phosphatidylcholine breakdown in rat liver plasma membranes. Roles of guanine nucleotides and P2-purinergic agonists. J Biol Chem. 1987 Mar 15;262(8):3440–3443. [PubMed] [Google Scholar]
- Ishitoya J., Yamakawa A., Takenawa T. Translocation of diacylglycerol kinase in response to chemotactic peptide and phorbol ester in neutrophils. Biochem Biophys Res Commun. 1987 Apr 29;144(2):1025–1030. doi: 10.1016/s0006-291x(87)80066-9. [DOI] [PubMed] [Google Scholar]
- Kanoh H., Yamada K., Sakane F. Diacylglycerol kinase: a key modulator of signal transduction? Trends Biochem Sci. 1990 Feb;15(2):47–50. doi: 10.1016/0968-0004(90)90172-8. [DOI] [PubMed] [Google Scholar]
- Kikkawa U., Nishizuka Y. The role of protein kinase C in transmembrane signalling. Annu Rev Cell Biol. 1986;2:149–178. doi: 10.1146/annurev.cb.02.110186.001053. [DOI] [PubMed] [Google Scholar]
- Krause K. H., Schlegel W., Wollheim C. B., Andersson T., Waldvogel F. A., Lew P. D. Chemotactic peptide activation of human neutrophils and HL-60 cells. Pertussis toxin reveals correlation between inositol trisphosphate generation, calcium ion transients, and cellular activation. J Clin Invest. 1985 Oct;76(4):1348–1354. doi: 10.1172/JCI112109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambeth J. D. Activation of the respiratory burst oxidase in neutrophils: on the role of membrane-derived second messengers, Ca++, and protein kinase C. J Bioenerg Biomembr. 1988 Dec;20(6):709–733. doi: 10.1007/BF00762549. [DOI] [PubMed] [Google Scholar]
- Lambeth J. D., Burnham D. N., Tyagi S. R. Sphinganine effects on chemoattractant-induced diacylglycerol generation, calcium fluxes, superoxide production, and on cell viability in the human neutrophil. Delivery of sphinganine with bovine serum albumin minimizes cytotoxicity without affecting inhibition of the respiratory burst. J Biol Chem. 1988 Mar 15;263(8):3818–3822. [PubMed] [Google Scholar]
- Lavine W. S., Maderazo E. G., Stolman J., Ward P. A., Cogen R. B., Greenblatt I., Robertson P. B. Impaired neutrophil chemotaxis in patients with juvenile and rapidly progressing periodontitis. J Periodontal Res. 1979 Jan;14(1):10–19. doi: 10.1111/j.1600-0765.1979.tb00213.x. [DOI] [PubMed] [Google Scholar]
- Lew P. D., Monod A., Krause K. H., Waldvogel F. A., Biden T. J., Schlegel W. The role of cytosolic free calcium in the generation of inositol 1,4,5-trisphosphate and inositol 1,3,4-trisphosphate in HL-60 cells. Differential effects of chemotactic peptide receptor stimulation at distinct Ca2+ levels. J Biol Chem. 1986 Oct 5;261(28):13121–13127. [PubMed] [Google Scholar]
- Lindhe J., Helldén L. Neutrophil chemotactic activity elaborated by dental plaque. J Periodontal Res. 1972;7(4):297–303. doi: 10.1111/j.1600-0765.1972.tb01718.x. [DOI] [PubMed] [Google Scholar]
- Liscovitch M., Blusztajn J. K., Freese A., Wurtman R. J. Stimulation of choline release from NG108-15 cells by 12-O-tetradecanoylphorbol 13-acetate. Biochem J. 1987 Jan 1;241(1):81–86. doi: 10.1042/bj2410081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas D. O., Bajjalieh S. M., Kowalchyk J. A., Martin T. F. Direct stimulation by thyrotropin-releasing hormone (TRH) of polyphosphoinositide hydrolysis in GH3 cell membranes by a guanine nucleotide-modulated mechanism. Biochem Biophys Res Commun. 1985 Oct 30;132(2):721–728. doi: 10.1016/0006-291x(85)91192-1. [DOI] [PubMed] [Google Scholar]
- Mergenhagen S. E., Tempel T. R., Snyderman R. Immunologic reactions and periodontal inflammation. J Dent Res. 1970 Mar-Apr;49(2):256–261. doi: 10.1177/00220345700490020901. [DOI] [PubMed] [Google Scholar]
- Michell R. H. Inositol phospholipids and cell surface receptor function. Biochim Biophys Acta. 1975 Mar 25;415(1):81–47. doi: 10.1016/0304-4157(75)90017-9. [DOI] [PubMed] [Google Scholar]
- Miller M. E., Oski F. A., Harris M. B. Lazy-leucocyte syndrome. A new disorder of neutrophil function. Lancet. 1971 Apr 3;1(7701):665–669. doi: 10.1016/s0140-6736(71)92679-1. [DOI] [PubMed] [Google Scholar]
- Mowat A. G., Baum J. Polymorphonuclear leucocyte chemotaxis in patients with bacterial infections. Br Med J. 1971 Sep 11;3(5775):617–619. doi: 10.1136/bmj.3.5775.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishizuka Y. Studies and perspectives of protein kinase C. Science. 1986 Jul 18;233(4761):305–312. doi: 10.1126/science.3014651. [DOI] [PubMed] [Google Scholar]
- Page R. C., Schroeder H. E. Pathogenesis of inflammatory periodontal disease. A summary of current work. Lab Invest. 1976 Mar;34(3):235–249. [PubMed] [Google Scholar]
- Pandol S. J., Schoeffield M. S. 1,2-Diacylglycerol, protein kinase C, and pancreatic enzyme secretion. J Biol Chem. 1986 Apr 5;261(10):4438–4444. [PubMed] [Google Scholar]
- Preiss J. E., Bell R. M., Niedel J. E. Diacylglycerol mass measurements in stimulated HL-60 phagocytes. J Immunol. 1987 Mar 1;138(5):1542–1545. [PubMed] [Google Scholar]
- Preiss J., Loomis C. R., Bishop W. R., Stein R., Niedel J. E., Bell R. M. Quantitative measurement of sn-1,2-diacylglycerols present in platelets, hepatocytes, and ras- and sis-transformed normal rat kidney cells. J Biol Chem. 1986 Jul 5;261(19):8597–8600. [PubMed] [Google Scholar]
- Pribluda V. S., Metzger H. Calcium-independent phosphoinositide breakdown in rat basophilic leukemia cells. Evidence for an early rise in inositol 1,4,5-trisphosphate which precedes the rise in other inositol phosphates and in cytoplasmic calcium. J Biol Chem. 1987 Aug 25;262(24):11449–11454. [PubMed] [Google Scholar]
- Price B. D., Morris J. D., Marshall C. J., Hall A. Stimulation of phosphatidylcholine hydrolysis, diacylglycerol release, and arachidonic acid production by oncogenic ras is a consequence of protein kinase C activation. J Biol Chem. 1989 Oct 5;264(28):16638–16643. [PubMed] [Google Scholar]
- Ragab-Thomas J. M., Hullin F., Chap H., Douste-Blazy L. Pathways of arachidonic acid liberation in thrombin and calcium ionophore A23187-stimulated human endothelial cells: respective roles of phospholipids and triacylglycerol and evidence for diacylglycerol generation from phosphatidylcholine. Biochim Biophys Acta. 1987 Feb 23;917(3):388–397. doi: 10.1016/0005-2760(87)90117-2. [DOI] [PubMed] [Google Scholar]
- Rebecchi M. J., Gershengorn M. C. Thyroliberin stimulates rapid hydrolysis of phosphatidylinositol 4,5-bisphosphate by a phosphodiesterase in rat mammotropic pituitary cells. Evidence for an early Ca2+-independent action. Biochem J. 1983 Nov 15;216(2):287–294. doi: 10.1042/bj2160287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rider L. G., Dougherty R. W., Niedel J. E. Phorbol diesters and dioctanoylglycerol stimulate accumulation of both diacylglycerols and alkylacylglycerols in human neutrophils. J Immunol. 1988 Jan 1;140(1):200–207. [PubMed] [Google Scholar]
- Rittenhouse-Simmons S. Production of diglyceride from phosphatidylinositol in activated human platelets. J Clin Invest. 1979 Apr;63(4):580–587. doi: 10.1172/JCI109339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schacht J., Agranoff B. W. Effects of acetylcholine on labeling of phosphatidate and phosphoinositides by ( 32 P) orthophosphate in nerve ending fractions of guinea pig cortex. J Biol Chem. 1972 Feb 10;247(3):771–777. [PubMed] [Google Scholar]
- Shears S. B., Storey D. J., Morris A. J., Cubitt A. B., Parry J. B., Michell R. H., Kirk C. J. Dephosphorylation of myo-inositol 1,4,5-trisphosphate and myo-inositol 1,3,4-triphosphate. Biochem J. 1987 Mar 1;242(2):393–402. doi: 10.1042/bj2420393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith C. D., Lane B. C., Kusaka I., Verghese M. W., Snyderman R. Chemoattractant receptor-induced hydrolysis of phosphatidylinositol 4,5-bisphosphate in human polymorphonuclear leukocyte membranes. Requirement for a guanine nucleotide regulatory protein. J Biol Chem. 1985 May 25;260(10):5875–5878. [PubMed] [Google Scholar]
- Storey D. J., Shears S. B., Kirk C. J., Michell R. H. Stepwise enzymatic dephosphorylation of inositol 1,4,5-trisphosphate to inositol in liver. Nature. 1984 Nov 22;312(5992):374–376. doi: 10.1038/312374a0. [DOI] [PubMed] [Google Scholar]
- Streb H., Irvine R. F., Berridge M. J., Schulz I. Release of Ca2+ from a nonmitochondrial intracellular store in pancreatic acinar cells by inositol-1,4,5-trisphosphate. Nature. 1983 Nov 3;306(5938):67–69. doi: 10.1038/306067a0. [DOI] [PubMed] [Google Scholar]
- Tempel T. R., Kimball H. R., Kakenashi S., Amen C. R. Host factors in periodontal disease: periodontal manifestations of Chediak-Higashi Syndrome. J Periodontal Res. 1972;(10):26–27. [PubMed] [Google Scholar]
- Tilly B. C., van Paridon P. A., Verlaan I., Wirtz K. W., de Laat S. W., Moolenaar W. H. Inositol phosphate metabolism in bradykinin-stimulated human A431 carcinoma cells. Relationship to calcium signalling. Biochem J. 1987 May 15;244(1):129–135. doi: 10.1042/bj2440129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trimble E. R., Bruzzone R., Meehan C. J., Biden T. J. Rapid increases in inositol 1,4,5-trisphosphate, inositol 1,3,4,5-tetrakisphosphate and cytosolic free Ca2+ in agonist-stimulated pancreatic acini of the rat. Effect of carbachol, caerulein and secretin. Biochem J. 1987 Feb 15;242(1):289–292. doi: 10.1042/bj2420289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyagi S. R., Burnham D. N., Lambeth J. D. On the biological occurrence and regulation of 1-acyl and 1-O-alkyl-diradylglycerols in human neutrophils. Selective destruction of diacyl species using Rhizopus lipase. J Biol Chem. 1989 Aug 5;264(22):12977–12982. [PubMed] [Google Scholar]
- Tyagi S. R., Tamura M., Burnham D. N., Lambeth J. D. Phorbol myristate acetate (PMA) augments chemoattractant-induced diglyceride generation in human neutrophils but inhibits phosphoinositide hydrolysis. Implications for the mechanism of PMA priming of the respiratory burst. J Biol Chem. 1988 Sep 15;263(26):13191–13198. [PubMed] [Google Scholar]
- Van Dyke T. E., Horoszewicz H. U., Cianciola L. J., Genco R. J. Neutrophil chemotaxis dysfunction in human periodontitis. Infect Immun. 1980 Jan;27(1):124–132. doi: 10.1128/iai.27.1.124-132.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dyke T. E., Levine M. J., Tabak L. A., Genco R. J. Juvenile periodontitis as a model for neutrophil function: reduced binding of the complement chemotactic fragment, C5a. J Dent Res. 1983 Aug;62(8):870–872. doi: 10.1177/00220345830620080301. [DOI] [PubMed] [Google Scholar]
- Van Dyke T. E., Levine M. J., Tabak L. A., Genco R. J. Reduced chemotactic peptide binding in juvenile periodontitis: a model for neutrophil function. Biochem Biophys Res Commun. 1981 Jun 16;100(3):1278–1284. doi: 10.1016/0006-291x(81)91962-8. [DOI] [PubMed] [Google Scholar]
- Van Dyke T. E., Schweinebraten M., Cianciola L. J., Offenbacher S., Genco R. J. Neutrophil chemotaxis in families with localized juvenile periodontitis. J Periodontal Res. 1985 Sep;20(5):503–514. doi: 10.1111/j.1600-0765.1985.tb00834.x. [DOI] [PubMed] [Google Scholar]
- Volpe P., Salviati G., Di Virgilio F., Pozzan T. Inositol 1,4,5-trisphosphate induces calcium release from sarcoplasmic reticulum of skeletal muscle. Nature. 1985 Jul 25;316(6026):347–349. doi: 10.1038/316347a0. [DOI] [PubMed] [Google Scholar]


