Abstract
F41, K88, and CS31A are fimbrial adhesins associated with enterotoxigenic Escherichia coli. These adhesins are distinct from one another in the composition of their structural subunits and the adherence characteristics associated with their expression. Despite these differences, extensive homology exists between the genetic determinants mediating the expression of these adhesins, extending throughout the region of each determinant encoding the accessory proteins involved in adhesin biogenesis. This suggests that the regulatory and assembly systems mediating expression of these adhesins may be functionally interchangeable. In the present study we demonstrated that the accessory systems of the F41, K88, and CS31A determinants are able to mediate the functional expression of heterologous fimbrial subunit proteins. Plasmid constructs containing the isolated fimbrial subunit gene of the F41 or CS31A determinant were prepared and introduced into E. coli harboring the F41, K88, and CS31A accessory genes contained on compatible plasmid vectors. The ability of each of the three accessory systems to mediate stable expression of the F41 or CS31A fimbrial subunit peptide was demonstrated by Western blot (immunoblot) analysis. Functional expression of the F41 or CS31A subunit on the bacterial cell surface was demonstrated by the ability of these proteins to confer mannose-resistant hemagglutination of human erythrocytes or in vitro adherence to epithelial cells, respectively. The accessory system of an unrelated adhesin determinant, F1845, did not mediate functional expression of F41 adherence. Taken together, these data indicate that the genetic determinants mediating expression of the F41, K88, and CS31A adhesins are members of a closely related family and suggest that a mechanism exists in the family for the more rapid divergence of genes encoding antigenic and adhesive determinants.
Full text
PDF


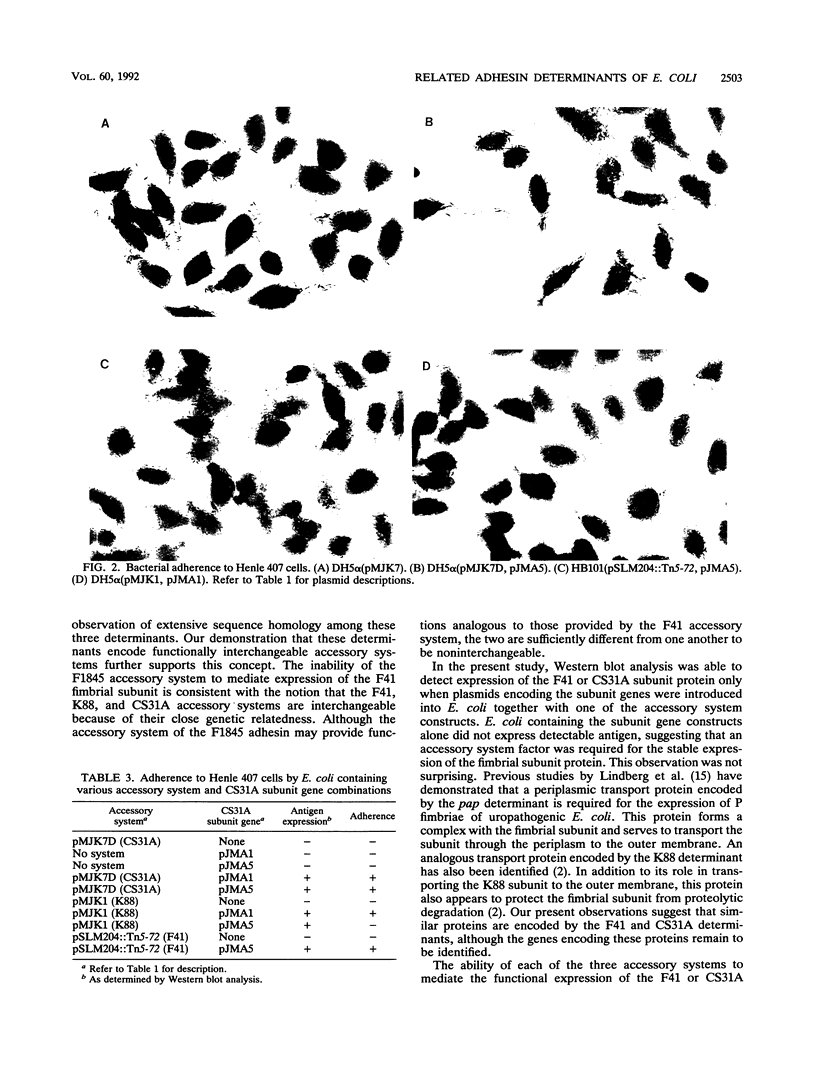


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson D. G., Moseley S. L. Escherichia coli F41 adhesin: genetic organization, nucleotide sequence, and homology with the K88 determinant. J Bacteriol. 1988 Oct;170(10):4890–4896. doi: 10.1128/jb.170.10.4890-4896.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakker D., Vader C. E., Roosendaal B., Mooi F. R., Oudega B., de Graaf F. K. Structure and function of periplasmic chaperone-like proteins involved in the biosynthesis of K88 and K99 fimbriae in enterotoxigenic Escherichia coli. Mol Microbiol. 1991 Apr;5(4):875–886. doi: 10.1111/j.1365-2958.1991.tb00761.x. [DOI] [PubMed] [Google Scholar]
- Bilge S. S., Clausen C. R., Lau W., Moseley S. L. Molecular characterization of a fimbrial adhesin, F1845, mediating diffuse adherence of diarrhea-associated Escherichia coli to HEp-2 cells. J Bacteriol. 1989 Aug;171(8):4281–4289. doi: 10.1128/jb.171.8.4281-4289.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer H. W., Roulland-Dussoix D. A complementation analysis of the restriction and modification of DNA in Escherichia coli. J Mol Biol. 1969 May 14;41(3):459–472. doi: 10.1016/0022-2836(69)90288-5. [DOI] [PubMed] [Google Scholar]
- Casey T. A., Moseley S. L., Moon H. W. Characterization of bovine septicemic, bovine diarrheal, and human enteroinvasive Escherichia coli that hybridize with K88 and F41 accessory gene probes but do not express these adhesins. Microb Pathog. 1990 Jun;8(6):383–392. doi: 10.1016/0882-4010(90)90025-l. [DOI] [PubMed] [Google Scholar]
- Chang A. C., Cohen S. N. Construction and characterization of amplifiable multicopy DNA cloning vehicles derived from the P15A cryptic miniplasmid. J Bacteriol. 1978 Jun;134(3):1141–1156. doi: 10.1128/jb.134.3.1141-1156.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerlach G. F., Clegg S., Allen B. L. Identification and characterization of the genes encoding the type 3 and type 1 fimbrial adhesins of Klebsiella pneumoniae. J Bacteriol. 1989 Mar;171(3):1262–1270. doi: 10.1128/jb.171.3.1262-1270.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girardeau J. P., Bertin Y., Martin C., Der Vartanian M., Boeuf C. Sequence analysis of the clpG gene, which codes for surface antigen CS31A subunit: evidence of an evolutionary relationship between CS31A, K88, and F41 subunit genes. J Bacteriol. 1991 Dec;173(23):7673–7683. doi: 10.1128/jb.173.23.7673-7683.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girardeau J. P., Der Vartanian M., Ollier J. L., Contrepois M. CS31A, a new K88-related fimbrial antigen on bovine enterotoxigenic and septicemic Escherichia coli strains. Infect Immun. 1988 Aug;56(8):2180–2188. doi: 10.1128/iai.56.8.2180-2188.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs A. A., Venema J., Leeven R., van Pelt-Heerschap H., de Graaf F. K. Inhibition of adhesive activity of K88 fibrillae by peptides derived from the K88 adhesin. J Bacteriol. 1987 Feb;169(2):735–741. doi: 10.1128/jb.169.2.735-741.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kehoe M., Sellwood R., Shipley P., Dougan G. Genetic analysis of K88-mediated adhesion of enterotoxigenic Escherichia coli. Nature. 1981 May 14;291(5811):122–126. doi: 10.1038/291122a0. [DOI] [PubMed] [Google Scholar]
- Korth M. J., Schneider R. A., Moseley S. L. An F41-K88-related genetic determinant of bovine septicemic Escherichia coli mediates expression of CS31A fimbriae and adherence to epithelial cells. Infect Immun. 1991 Jul;59(7):2333–2340. doi: 10.1128/iai.59.7.2333-2340.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lindberg F., Tennent J. M., Hultgren S. J., Lund B., Normark S. PapD, a periplasmic transport protein in P-pilus biogenesis. J Bacteriol. 1989 Nov;171(11):6052–6058. doi: 10.1128/jb.171.11.6052-6058.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund B., Marklund B. I., Strömberg N., Lindberg F., Karlsson K. A., Normark S. Uropathogenic Escherichia coli can express serologically identical pili of different receptor binding specificities. Mol Microbiol. 1988 Mar;2(2):255–263. doi: 10.1111/j.1365-2958.1988.tb00027.x. [DOI] [PubMed] [Google Scholar]
- Martin C., Boeuf C., Bousquet F. Escherichia coli CS31A fimbriae: molecular cloning, expression and homology with the K88 determinant. Microb Pathog. 1991 Jun;10(6):429–442. doi: 10.1016/0882-4010(91)90108-m. [DOI] [PubMed] [Google Scholar]
- Mooi F. R., Wouters C., Wijfjes A., de Graaf F. K. Construction and characterization of mutants impaired in the biosynthesis of the K88ab antigen. J Bacteriol. 1982 May;150(2):512–521. doi: 10.1128/jb.150.2.512-521.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon H. W. Colonization factor antigens of enterotoxigenic Escherichia coli in animals. Curr Top Microbiol Immunol. 1990;151:147–165. doi: 10.1007/978-3-642-74703-8_8. [DOI] [PubMed] [Google Scholar]
- Moon H. W., Isaacson R. E., Pohlenz J. Mechanisms of association of enteropathogenic Escherichia coli with intestinal epithelium. Am J Clin Nutr. 1979 Jan;32(1):119–127. doi: 10.1093/ajcn/32.1.119. [DOI] [PubMed] [Google Scholar]
- Moseley S. L., Dougan G., Schneider R. A., Moon H. W. Cloning of chromosomal DNA encoding the F41 adhesin of enterotoxigenic Escherichia coli and genetic homology between adhesins F41 and K88. J Bacteriol. 1986 Sep;167(3):799–804. doi: 10.1128/jb.167.3.799-804.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ott M., Hoschützky H., Jann K., Van Die I., Hacker J. Gene clusters for S fimbrial adhesin (sfa) and F1C fimbriae (foc) of Escherichia coli: comparative aspects of structure and function. J Bacteriol. 1988 Sep;170(9):3983–3990. doi: 10.1128/jb.170.9.3983-3990.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oudega B., De Graaf F. K. Genetic organization and biogenesis of adhesive fimbriae of Escherichia coli. Antonie Van Leeuwenhoek. 1988;54(4):285–299. doi: 10.1007/BF00393521. [DOI] [PubMed] [Google Scholar]
- Swanson T. N., Bilge S. S., Nowicki B., Moseley S. L. Molecular structure of the Dr adhesin: nucleotide sequence and mapping of receptor-binding domain by use of fusion constructs. Infect Immun. 1991 Jan;59(1):261–268. doi: 10.1128/iai.59.1.261-268.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira J., Messing J. The pUC plasmids, an M13mp7-derived system for insertion mutagenesis and sequencing with synthetic universal primers. Gene. 1982 Oct;19(3):259–268. doi: 10.1016/0378-1119(82)90015-4. [DOI] [PubMed] [Google Scholar]




