Abstract
Fibroblast growth factor-2 (FGF-2) immobilized on non-tissue culture plastic promotes adhesion and spreading of bovine and human endothelial cells that are inhibited by anti-FGF-2 antibody. Heat-inactivated FGF-2 retains its cell-adhesive activity despite its incapacity to bind to tyrosine-kinase FGF receptors or to cell-surface heparan sulfate proteoglycans. Recombinant glutathione-S-transferase-FGF-2 chimeras and synthetic FGF-2 fragments identify two cell-adhesive domains in FGF-2 corresponding to amino acid sequences 38–61 and 82–101. Both regions are distinct from the FGF-receptor-binding domain of FGF-2 and contain a DGR sequence that is the inverse of the RGD cell-recognition sequence. Calcium deprivation, RGD-containing eptapeptides, soluble vitronectin (VN), but not fibronectin (FN), inhibit cell adhesion to FGF-2. Conversely, soluble FGF-2 prevents cell adhesion to VN but not FN, thus implicating VN receptor in the cell-adhesive activity of FGF-2. Accordingly, monoclonal and polyclonal anti-αvβ3 antibodies prevent cell adhesion to FGF-2. Also, purified human αvβ3 binds to immobilized FGF-2 in a cation-dependent manner, and this interaction is competed by soluble VN but not by soluble FN. Finally, anti-αvβ3 monoclonal and polyclonal antibodies specifically inhibit mitogenesis and urokinase-type plasminogen activator (uPA) up-regulation induced by free FGF-2 in endothelial cells adherent to tissue culture plastic. These data demonstrate that FGF-2 interacts with αvβ3 integrin and that this interaction mediates the capacity of the angiogenic growth factor to induce cell adhesion, mitogenesis, and uPA up-regulation in endothelial cells.
INTRODUCTION
Angiogenesis, the growth of new blood vessels, plays a key role in different physiological and pathological conditions, including embryonic development, wound repair, inflammation, tumor growth, and angiogenesis-dependent diseases (Folkman, 1995). Neovascularization is a multi-step process. It begins with the degradation of the basement membrane by proteases secreted by activated endothelial cells that will migrate and proliferate, leading to the formation of solid endothelial cell sprouts into the stromal space. Then, vascular loops are formed and capillary tubes develop with formation of tight junctions and deposition of new basement membrane (Ausprunk and Folkman, 1977). A close interaction exists among cell-adhesive proteins of the extracellular matrix (ECM), their integrin receptors, and soluble angiogenesic growth factors during each step of the angiogenesis process (Ingber and Folkman, 1989a, 1989b; Davis et al., 1993; Brooks et al., 1994; Plopper et al., 1995).
One of the best characterized modulators of angiogenesis is the heparin-binding basic fibroblast growth factor (FGF-2). FGF-2 has been demonstrated to induce neovascularization in vivo in different experimental models (Basilico and Moscatelli, 1992) and to be implicated in the growth of new blood vessels during wound healing and chick embryo development (Broadley et al., 1989; Ribatti et al., 1995). In vitro, FGF-2 induces cell proliferation, migration, and production of proteases in endothelial cells (Moscatelli et al., 1986) by interacting with specific tyrosine kinase receptors (FGFRs) and with heparan sulfate proteoglycans (HSPGs) of the cell surface (Johnson and Williams, 1993). In addition, FGF-2 modulates integrin expression in endothelium (Enenstein et al., 1992; Klein et al., 1993).
Integrins are a family of transmembrane, heterodimeric adhesion receptors comprised of α and β subunits. The combination of different subunits produces distinct integrin molecules that mediate cell adhesion to a variety of adhesive proteins of the ECM such as fibronectin (FN), vitronectin (VN), thrombospondin (TSP), laminin, and collagens (Albelda and Buck, 1990; Hynes, 1992; Ginsberg et al., 1992). In addition to mediating cell adhesion, the interaction of integrins with cell-adhesive proteins plays a crucial role in regulating the response of endothelial cells to soluble growth factors, including FGF-2 (Ingber et al., 1986, 1987, 1990; Ingber and Folkman, 1988, 1989a, 1989b). Also, it has been demonstrated that αvβ3 integrin is highly expressed by endothelial cells during angiogenesis, and it is specifically required to sustain neovascularization induced in vivo by FGF-2 (Brooks et al., 1994; Friedlander et al., 1995). Despite these observations, the molecular mechanism(s) underlying the relationship between FGF-2 and the cell adhesion machinery are not fully elucidated.
A first point of convergence between FGF-2 and the cell-adhesion machinery may occur intracellularly and is represented by the signal transduction mechanism(s) activated by two biological effectors. For instance, binding of cell-adhesive proteins to integrins results in the activation of focal adhesion kinase pp125FAK, that can be tyrosine phosphorylated also by growth factors, including FGF-2 (Hatai et al., 1994). Integrins and FGF-2 also share the activation of phospholipase C (Banga et al., 1986; Peters et al., 1992), mitogen-activated protein kinases (Chen et al., 1994; Schlaepfer et al., 1994; Besser et al., 1995), inositol lipids turnover (Banga et al., 1986; Peters et al., 1992), calcium channel (Pelletier et al., 1992; Peters et al., 1992; Schwartz, 1993), and protein kinase C (Vuori and Ruoslahti, 1993; Presta et al., 1989a) as common downstream targets for their intracellular signaling systems. Interestingly, FGFRs, integrins, and intracellular transducers, including pp125FAK and protein kinase C, colocalize in focal adhesion contacts (Plopper et al., 1995). This may facilitate the cross-talk between signaling pathways that has long been viewed as separate systems.
Alternatively, the interplay between FGF-2 and the cell adhesion machinery may occur extracellularly by distinct mechanisms. 1) Adhesive proteins may signal through FGFRs, as suggested by the presence in FGFR of the trypeptide His-Ala-Val, implicated in homophilic cadherin interaction (Byers et al., 1992). Also, FGFR contains regions characterized by a high homology with the neuronal adhesion molecule L1 and with the variant alternatively spliced exon NCAM isoform (Mason, 1994), and it is involved in neurite outgrowth stimulated by NCAM, N-cadherin, and L1 (Williams et al., 1994). 2) FGF-2 binds directly to TSP (Taraboletti et al., 1997) and may interact also with other adhesive proteins including FN, laminin, and collagen (Feige et al., 1989). 3) FGF-2 may interact with cell-adhesive receptors, as indicated by its capacity to bind the E-selectin-ligand ESL-1B in a myeloid cell line (Steegmaler et al., 1995).
Large amounts of FGF-2 are present in ECM both in vivo and in vitro (Vlodavsky et al., 1987; Folkman et al., 1988). Collagen-bound FGF-2 is mitogenically active in situ for BALB/c-3T3 fibroblasts (Smith et al., 1982) and FGF-2 immobilized onto heparin-coated surfaces promotes endothelial cell adhesion (Baird et al., 1988) and PC12 cell adhesion and differentiation (Schubert et al., 1987). Finally, FGF-2 immobilized to a plastic substrate retains the capacity to induce cell proliferation and uPA production in adherent endothelial cells (Presta et al., 1992). It is therefore tempting to hypothesize that ECM-bound FGF-2 may induce endothelial cell adhesion and act at the same time as a localized, persistent stimulus for angiogenesis by interacting with different cell-surface molecules.
In the present paper, we investigated the mechanisms responsible for the endothelial cell-adhesive capacity of immobilized FGF-2. The results indicate that surface-bound FGF-2 induces cell adhesion of cultured endothelial cells of different origin. This depends on the interaction of immobilized FGF-2 with the VN receptor αvβ3. VN receptor plays a pivotal role also in mediating the mitogenic activity and the uPA-inducing capacity of soluble FGF-2, underlying the complexity of the interaction among ECM components, various endothelial cell-surface receptors (i.e., FGFRs, HSPGs, and integrins), and soluble and/or immobilized FGF-2 during angiogenesis.
MATERIALS AND METHODS
Materials
Human recombinant FGF-2 was expressed and purified to homogenity from transformed Escherichia coli cells by heparin-Sepharose affinity chromatography (Isacchi et al., 1991). The production and characterization of the synthetic peptides representing fragments of human FGF-2 were described previously (Presta et al., 1991). Peptides GRGDSPK and GRADSPK were from Neosystem Laboratoire (Strasbourg, France). Bovine FN and VN were from Sigma (St. Louis, MO). Anti-αvβ3 integrin antiserum was from Telios (San Diego, CA). Immunopurified anti-FGF-2 antibody was a gift from D.B. Rifkin (New York University, New York, NY). Anti-αvβ3 monoclonal LM 609 antibody was from Chemicon International (Temecula, CA). Anti-α5β1 integrin antiserum, anti-bovine FN antiserum, human VN, and anti-human VN monoclonal antibody were gifts from E. Dejana (Istituto Mario Negri, Milan, Italy). Highly specific antisera directed to αv subunit, to β3 subunit purified from human platelets, and to a synthetic peptide representing the COOH terminus of the β5 subunit were gifts from G. Tarone (University “La Sapienza,” Rome, Italy). Bovine TSP and anti-bovine TSP antiserum were gifts from G. Taraboletti (Istituto Mario Negri, Bergamo, Italy).
Production and Purification of Recombinant Glutathione-S-transferase (GST)-FGF-2 Fusion Proteins
Human FGF-2 cDNA coding for amino acid residues FGF-2(20–156) and two Fok-I fragments coding for amino acid residues FGF-2(20–103) and FGF-2(104–156) were cloned in frame in pGEX-2T vector (Pharmacia, Uppsala, Sweden) at the 3′ end of cDNA. The recombinant plasmids were introduced in E. coli. After induction of GST fusion proteins with 0.1 mM isopropyl β-d-thiogalactopyranoside, the bacterial transformants were screened by Western blot analysis using an anti-FGF-2 antiserum. Positive clones were grown on a large scale, and FGF-2-GST chimeric proteins were purified on a glutathione-agarose affinity chromatography column according to manufacturer’s instructions.
Cell Cultures
Fetal bovine aortic endothelial GM 7373 cells were obtained from the NIGMS Human Genetic Mutant Cell Repository (Institute for Medical Research, Camden, NJ). They correspond to the BFA-1c multilayered transformed clone described by Grinspan et al. (1983). GM 7373 cells were grown in Eagle’s MEM containing 10% fetal calf serum (FCS), vitamins, and essential and nonessential amino acids. Human endothelium-derived EAhy 926 cells (Edgell, et al., 1983) were provided by A. Albini (IST, Genova, Italy) and were grown in DMEM containing 10% heat inactivated FCS, vitamins, and essential and nonessential amino acids. Chinese hamster ovary (CHO) cells were a gift from D. Di Lorenzo (Spedali Civili, Brescia, Italy). CHOflg7G clone expressing FGFR-1/flg was obtained by transfection of parental CHO cells with the plasmid 91023b-flg as described (Rusnati et al., 1996). Both parental and CHOflg7G cells were grown in Ham’s F-12 medium supplemented with 10% FCS.
Cell Adhesion Assay
Aliquots (100 μl) of 100 mM NaHCO3, pH 9.6 (carbonate buffer), containing the adhesive molecule being tested were added to polystyrene non-tissue culture microtiter plates. After 16 h of incubation at 4°C the solution was removed, and wells were washed three times with cold phosphate- buffered saline (PBS). For the cell-adhesion assay, confluent cultures of GM 7373 cells or EAhy 926 cells were trypsinized, washed, and resuspended with the appropriate medium. Preliminary observations had indicated that low concentrations of serum were required in some experiments for optimal cell adhesion to FGF-2–coated plastic. For this reason, 1% FCS was utilized routinely in cell-adhesion experiments. Fifty thousand GM 7373 cells or 6,000 EAhy 926 cells were resuspended in 200 μl of medium and were immediately seeded onto wells coated with the molecule being tested or were mixed for 2 h at 4°C with RGD-containing peptides, anti-integrin antibodies, or soluble adhesive proteins before seeding. Routinely, cell adhesion was allowed to occur for 2 h at 37°C. Then, wells were washed once with 2 mM EDTA in PBS and once in MEM (GM 7373 cells) or DMEM (EAhy 926 cells) without serum. The washing procedure was repeated three times. Adherent cells were trypsinized and counted in a Burker chamber.
Scanning Electron Microscopy
Glass coverslips (10 mm in diameter) were immersed in 65% HNO3 for 1 h, washed with distilled water, immersed in 7% NaOH for 1 more hour, washed with distilled water again, and dryed. Coverslips were then placed within 24-well tissue culture plates and coated overnight at 4°C with carbonate buffer containing 20 μg/ml of FGF-2, FN, or VN. Then, free molecules were removed by washing the plates three times with cold PBS. EAhy 926 cells were seeded at 20,000/cm2 and allowed to adhere onto glass coverslips. Adherent cells were then fixed with 2.5% glutaraldehyde in 0.1 M cacodylate buffer (pH 7.4) for 3 h. Coverslips were then washed, osmicated, dehydrated, critical point dried with a Balzer apparatus (BAL-TEC Liechtenstein, Principality of Liechtenstein) and sputter coated with an Edward apparatus (Edwards High Vacuum International, Wilmington, MA). Cells were then viewed under a Philips scanning electron microscope model XL 20 (Philips, Eindhoven, The Netherlands) at 30 kV and photographed at ×1,200 magnification.
Evaluation of the Mitogenic and uPA-inducing Activity of FGF-2
GM 7373 cells were seeded at 70,000 cells/cm2 onto 96-well tissue culture plastic and incubated for 16 h at 37°C with MEM containing 10% FCS. Then cells were washed with serum-free medium and incubated for 24 h in fresh MEM containing 0.4% FCS, the molecule under test, and increasing concentrations of anti-αvβ3 monoclonal antibody, irrelevant IgGs, nonimmune serum or antisera directed to human αvβ3, or to human α5β1. At the end of incubation, parallel cultures were trypsinized and counted in a Burker chamber. uPA activity was measured in the cell extracts as described (Presta et al., 1989a) by using the plasmin chromogenic substrate d-norleucyl-hexahydrotyrosyllysine p-nitroanilide acetate (American Diagnostica, Greenwich, CT). Human urokinase (Calbiochem, San Diego, CA) was used as a standard.
Isolation of αvβ3 Integrin
Human αvβ3 integrin was purified from term placenta according to the method of Pytela et al. (1987) with modifications. The affinity matrix was prepared by coupling the eptapeptide Gly-Arg-Gly-Asp-Ser-Pro-Lys (GRGDSPK) (Neosystem Laboratories, Strasbourg, France) to cyanogen bromide-activated Sepharose. Human placenta (∼250 g) was extensively rinsed with cold PBS and homogenized at 4°C in a food processor in PBS containing 100 mM octylglucoside, 1 mM CaCl2, 1 mM MgCl2, and protease inhibitors (1 mM phenylmethylsulfonylfluoride and 1 μg/ml leupeptin). The homogenized tissue was centrifuged at 10,000 × g for 30 min, dialyzed against PBS containing 0.1% NP 40, 1 mM CaCl2, 1 MgCl2, and protease inhibitors, and loaded onto a wheat germ lectin-Sepharose column (1.5 × 6 cm, Pharmacia) equilibrated in the same buffer. After extensive washing, the column was eluted with PBS containing 200 mM N-acetyl-d-glucosamine, 1 mM CaCl2, 1 mM MgCl2, and protease inhibitors. Eluted fractions were pooled, dialyzed against PBS containing 1 mM CaCl2, 1 mM MgCl2, and protease inhibitors, and then loaded onto the GRGDSPK-Sepharose column (1 × 5 cm) equilibrated in the same buffer. After extensive washing, the column was eluted with PBS containing 10 mM EDTA, 1 mM CaCl2, 1 mM MgCl2, and protease inhibitors. Eluted fractions were analyzed by SDS-PAGE followed by silver staining of the gel and by Western blot with specific antisera directed against α5β1 integrin or against αv, β3, and β5 integrin subunit. Purity of human αvβ3 integrin was routinely ≥ 95% as assessed by soft laser scanning of the silver-stained gel.
Cell-free αvβ3 Integrin/FGF-2 Interaction
Aliquots (1 ml) of carbonate buffer containing FGF-2, FN, or BSA (each at 20 μg/ml) were added to polystyrene non-tissue culture dishes (35 mm in diameter). After 16 h of incubation at 4°C, the solutions were removed, and dishes were washed three times with cold PBS and incubated for 30 min at 37°C with 1 mg/ml BSA. Aliquots of purified human αvβ3 integrin (6 μg/sample) were added to each dish and incubated for 4 h at 37°C on an orbital shaker. At the end of incubation the solution was removed and the dishes were washed three times with PBS containing 2 mM EDTA, added with 150 μl of nonreducing SDS-PAGE sample buffer and incubated for 1 h at 50°C. At the end of incubation dishes were scraped with a rubber policeman, and the sample buffer was recovered and analyzed on SDS-7% polyacrylamide gel under nonreducing conditions followed by Western blot using anti-β3 subunit and anti-αv subunit antisera. In some experiments, αvβ3 integrin interaction with immobilized FGF-2 was assessed in the presence of 20 mM EDTA or of 75 μg/ml of soluble FN or VN.
RESULTS
Substrate-bound FGF-2 Promotes Endothelial Cell Adhesion to Non-Tissue Culture Plastic
When non-tissue culture plates were incubated for 16 h at 4°C with 20 μg/ml of FGF-2 dissolved in carbonate buffer in the presence of tracer amounts of 125I-FGF-2, 5–8% of the growth factor remained adsorbed to the substrate. This amount corresponds approximately to 8.4 × 1011 molecules/cm2. FGF-2 bound to plastic resists extraction with 6 M urea, with methanol or ethanol both at 95%, but it is removed by drastic treatment with detergents, including incubation for 1 h at 37°C with 0.5% Triton X-100 or by boiling with 1% SDS.
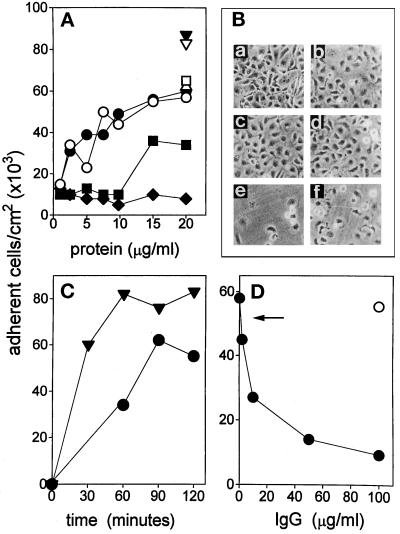
To evaluate the endothelial cell-adhesive capacity of FGF-2, fetal bovine aortic endothelial GM 7373 cells were seeded onto non-tissue culture plates coated with increasing concentrations of FGF-2. As shown in Figure 1A, FGF-2 promotes a dose-dependent adhesion of GM 7373 cells with a maximal effect observed at 20 μg/ml. Two hours after seeding, 53,000–60,000 cells/cm2 adhere to the immobilized growth factor. Under the same experimental conditions, FN, VN, and TSP promote adhesion of 87,000, 65,000, and 62,000 cells/cm2, respectively. No significant cell adhesion and spreading were observed on BSA-coated plastic for concentrations of the molecule up to 50 μg/ml. The cell-adhesive capacity of FGF-2 was fully retained when FGF-2-coated plates were incubated for 30 min at 37°C with 3% BSA before the cell-adhesion assay. Microscopic observation of GM 7373 cells adherent to FGF-2-coated plastic showed that most of the cells spread onto the substrate, as observed for FN- and VN-adherent cells (Figure 1B).
Figure 1.
GM 7373 cell adhesion to FGF-2–coated plastic. (A) Non-tissue culture plastic plates were incubated with carbonate buffer containing the indicated concentrations of native FGF-2 (•), heat-denaturated FGF-2 (○), BSA (♦), histone III-S (▪), native FN (▾), heat-denaturated FN (▿), VN (□), or TSP(Δ). GM 7373 cells were seeded onto coated plates and allowed to adhere for 2 h at 37°C. Then, the number of adherent cells was evaluated. Each point is the mean of six to seven determinations in duplicate. SEM does not exceed 11% of the values. (B) GM 7373 cells adherent to plastic coated with 20 μg/ml of FN (a), native (b), or heat-denaturated (c) FGF-2, VN (d), BSA (e), and histone III-S (f) were photographed under a phase-contrast microscope. Original magnification 256×. (C) GM 7373 cells were seeded on FN- (▾) or on FGF-2- (•) coated plates and allowed to adhere at 37°C for the indicated times. Then, the number of adherent cells was evaluated. Each point is the mean of two determinations in duplicate. SEM does not exceed 16% of the values. (D) GM 7373 cells were seeded on FGF-2-coated plates in the presence of the indicated concentrations of anti-FGF-2 antibody (•) or of nonimmune IgG (O). After 2 h of incubation at 37°C, the number of adherent cells was evaluated. Each point is the mean of four to five determinations in duplicate. SEM does not exceed 9% of the values. Arrow points to the number of cells adherent to FGF-2-coated plastic in the presence of anti-FN antiserum (1:100), anti-TSP antiserum (1:100), or anti-VN monoclonal antibody (1:5). Under the same experimental conditions, these antibodies fully inhibited GM 7373 cell adhesion to plastic coated with their corresponding antigens.
GM 7373 cell adhesion to FGF-2 is time-dependent, with half-maximal and maximal number of cells adherent to the substrate 60 min and 90 min after seeding, respectively (Figure 1C). Cell spreading was apparent 60 min after seeding. Also, neutralizing affinity-purified anti-FGF-2 antibodies inhibited cell adhesion to FGF-2 coated plastic in a dose-dependent manner while antibodies directed to FN, VN, or TSP and irrelevant IgGs were ineffective (Figure 1D). Conversely, antiFGF-2 antibody did not affect GM 7373 cell adhesion to plastic coated with FN, VN, or TSP.
To investigate the role of protein synthesis and secretion in the process of endothelial cell adhesion to the substrate, cells were treated with 20 μM cycloheximide or 1 μM monensin for 1 h at 37°C before the adhesion assay (Dejana et al., 1988). Inhibitors were also added to the medium during the assay. In our experimental conditions these molecules caused a limited decrease (10–30%) in the number of cells adherent to FGF-2 or to FN, indicating that de novo protein synthesis and secretion do not play a major role in cell adhesion.
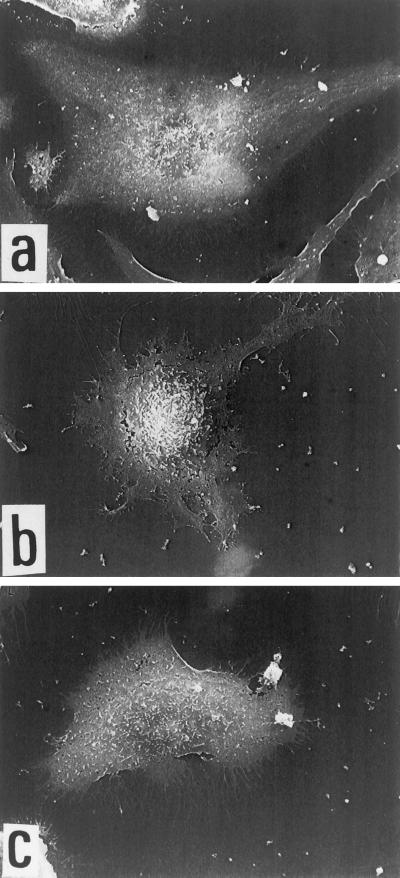
The capacity to adhere onto bFGF-coated plastic was not limited to endothelial GM 7373 cells being shared by adult bovine aortic endothelial cells (E. Tanghetti, unpublished observations) and by human endothelial EAhy 926 cells (Figure 2). When observed by scanning electron microscopy, EAhy 926 cells adherent to FGF-Z show a flattened morphology representative of well spread cells with pseudopodia and short filapodial extensions distributed all around the cell. Irregular margin with filapodial extensions are present also in VN-adherent cells, while FN-adherent cells appear cobblestone-shaped with more regular cell margins.
Figure 2.
Scanning electronic microscopy of EAhy 926 endothelial cells adherent to different substrata. Cells were allowed to adhere onto glass coverslips coated with 20 μg/ml of FN (a), FGF-2 (b), or VN (c). Then, cells were fixed and photographed as described in MATERIALS AND METHODS.
Mapping of the Cell-adhesive Region(s) of FGF-2
To assess the possibility that the net positive charge of cationic FGF-2 was responsible for its cell-adhesive activity, we compared the cell-adhesive capacity of FGF-2 with that of histone III-S, a molecule that shares similar charge and molecular weight with the growth factor. Also, to evaluate whether an appropriate three-dimensional structure was required for FGF-2 to exert its cell-adhesive activity, heat-denaturated FGF-2 was included in the cell adhesion assay. As shown in Figure 1, histone III-S promotes only a limited adhesion and spreading of GM 7373 cells when compared with FGF-2. In contrast, as observed for heat-denaturated FN, heat-denaturated FGF-2 exerts a cell-adhesive capacity similar to that shown by the native molecule. Thus, in analogy with different cell-adhesive proteins, our data suggest that specific primary amino acid sequence(s), rather than 3-D structure and/or net positive charge, mediate the cell-adhesive capacity of FGF-2.
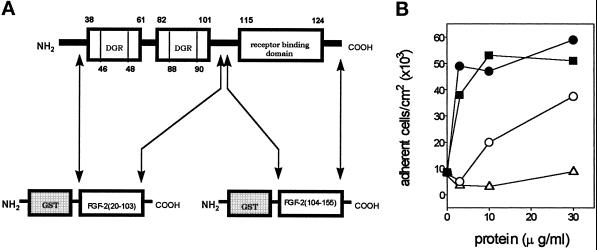
To assess this hypothesis, recombinant GST-fusion proteins were produced in which the C terminus was represented either by the fragment FGF-2(20–103) or by the fragment FGF-2(104–155) that contains the putative FGFR-binding domain (Baird et al., 1988) (Figure 3A). As shown in Figure 3B, GST-FGF-2(20–103) protein is up to 30 times more potent than GST-FGF-2(104–155) in promoting endothelial cell adhesion. A very limited cell-adhesive capacity was shown by GST alone. These data suggest that amino acid sequence(s) within FGF-2(20–103) mediate the cell-adhesive activity of the growth factor.
Figure 3.
Cell-adhesive capacity of GST-FGF-2 fusion proteins. (A) Schematic representation of the recombinant GST-FGF-2 fusion proteins. GST-FGF-2(20–103) contains the two cell-adhesive fragments FGF-2(38–61) and FGF-2(82–101), both bearing the trypeptide DGR (see text). GST-FGF-2(104–155) contains the putative FGFR-binding domain (Baird et al., 1988). (B) GM 7373 cells were seeded and allowed to adhere onto non-tissue culture plastic coated with the indicated concentrations of FGF-2 (▪), recombinant GST (Δ), GST-FGF-2(20–103) fusion protein (•), or GST-FGF-2(104–155) fusion protein (O). The number of adherent cells was evaluated after 2 h of incubation at 37°C. Each point is the mean of four determinations in duplicate. SEM does not exceed 18% of the values.
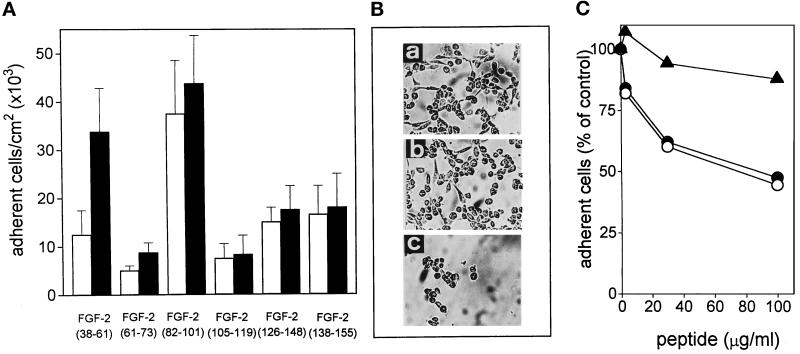
To identify these amino acid sequence(s), GM 7373 cells were allowed to adhere onto non-tissue culture plastic coated with different synthetic peptides corresponding to various regions of the FGF-2 molecule (Figure 4A, B). Among the peptides tested, only those corresponding to amino acid sequences FGF-2(38–61) and FGF-2(82–101) promote cell adhesion and a limited spreading. At 300 μg/ml the two peptides allow the adhesion of 33,000 and 44,000 cells/cm2, respectively. Both cell-adhesive peptides are included within the FGF-2 region comprised in the cell-adhesive chimera GST-FGF-2(20–103) and are distinct from the putative receptor-binding domain of FGF-2 (Figure 3A). To rule out the possibility that the above data may be the mere consequence of differences in the capacity of FGF-2 fragments to adhere to the substratum rather than reflect differences in cell-adhesive capacity of the peptides, FGF-2 fragments were tested in solution for the ability to prevent cells adhesion to FGF-2. As shown in Figure 4C, preincubation of GM 7373 cells in suspension with increasing concentrations of FGF-2(38–61) or FGF-2(82–101) caused a significant decrease in the number of cells that were able to adhere to FGF-2-coated plastic. Peptide FGF-2(138–154) was instead ineffective. In conclusion, the data identify the primary amino acid sequences FGF-2(38–61) and FGF-2(82–101) as those involved in the cell-adhesive capacity of the growth factor.
Figure 4.
Mapping of FGF-2 cell-adhesive domains. (A) GM 7373 cells were allowed to adhere onto non-tissue culture plastic coated with 100 μg/ml (white bars) or 300 μg/ml (black bars) of the indicated synthetic fragments of human FGF-2. The number of adherent cells was evaluated after 2 h of incubation at 37°C. Each point is the mean ± SEM of three to five determinations in duplicate. (B) Phase-contrast microphotographs of cells adherent to plastic coated with 300 μg/ml of peptides FGF-2(38–61) (a), FGF-2(82–101) (b), and FGF-2(138–155) (c). Original magnification 128×. (C) GM 7373 cells were incubated in suspension for 90 min at 37°C with increasing concentrations of peptides FGF-2(38–61) (•), FGF-2(82–101) (O), or FGF-2(138–155) (▴). Then, cells were centrifuged to remove unbound peptides and seeded onto non-tissue culture plates coated with 20 μg/ml of FGF-2. The number of adherent cells was evaluated after 2 h of incubation at 37°C, corrected for the nonspecific cell adhesion measured onto BSA-coated wells, and expressed as percentage of peptide-untreated cells specifically bound to FGF-2-coated plates. Each point is the mean of five to seven determinations in duplicate. SEM does not exceed 14% of the values.
αvβ3 Integrin Mediates the Cell-adhesive Activity of FGF-2
FGF-2 is known to bind to FGFRs and HSPGs of the cell surface. However, the above data indicate that the receptor-binding domain of FGF-2 does not mediate cell adhesion to the growth factor. Furthermore, heparin causes only a limited inhibition of endothelial cell adhesion to FGF-2 even when administered at 1 mg/ml (Figure 5), a dose that is 1000 times higher than that required to prevent the binding of 125I-FGF-2 to its low-affinity sites (Rusnati et al., 1996). Accordingly, undersulfation of cell-associated HSPGs by cell treatment with 4-methyl-umbelliferyl-β-d-xyloside (Schor and Schor, 1988; Saksela and Rifkin, 1990) induces a 60% reduction in the amount of 125I-FGF-2 that binds to low-affinity sites without affecting cell adhesion to FGF-2-coated plastic (E. Tanghetti, unpublished observations). Thus, the data indicate that FGFRs and cell-associated HSPGs do not play a major role in mediating endothelial cell-adhesion to FGF-2. These findings are in keeping with the capacity of heat-denaturated FGF-2 to promote endothelial cell adhesion (see Figure 1), despite its incapacity to bind to FGFRs and to cell-surface HSPGs.
Figure 5.
Effect of calcium ion, RGD-containing peptides, and heparin on GM 7373 cell adhesion to FGF-2–coated plastic. Cells were seeded onto non-tissue culture plates coated with 20 μg/ml of FGF-2 or FN in the presence of 10 mM EGTA (black bars), 10 mM EGTA added with 20 mM CaCl2 (white bars), 30 μg/ml of the peptide GRGDSPK (hatched bars), 30 μg/ml of the peptide GRADSPK (shaded bars), or 1 mg/ml of heparin (cross-hatched bars). The number of adherent cells was evaluated after 2 h of incubation at 37°C, corrected for the nonspecific cell adhesion measured onto BSA-coated wells, and expressed as percentage of cells adherent to FGF-2 or to FN in the absence of any addition. Each point is the mean ± SEM of three to six determinations in duplicate.
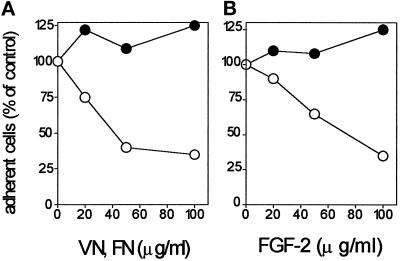
Integrins are cell surface receptors that mediate cell adhesion to different molecules. These receptors recognize Arg-Gly-Asp (RGD) sequences in their ligands in a calcium-dependent manner (Ruoslahti and Pierschbacher, 1987). Examination of the primary sequences of the cell-adhesive peptides FGF-2(38–61) and FGF-2(82–101) shows the presence of the amino acid sequence DGR at position 46–48 and 88–90, respectively (Figure 3A). Interestingly, both RGD- and DGR-containing peptides have been demonstrated to compete with adhesive proteins for integrin interaction (Humphries et al., 1986; Yamada and Kennedy, 1987; Koivunen et al., 1993). On this basis, the possibility that integrins are involved in the cell-adhesive activity of FGF-2 was investigated. To this purpose, we evaluated the effect of calcium and of RGD-containing eptapeptides on endothelial cell adhesion to FGF-2. As shown in Figure 5, calcium deprivation inhibits endothelial cells’ adhesion to FGF-2 and to FN, which was completely restored by addition of an excess of CaCl2 to the medium during the assay. Also, the synthetic peptide GRGDSPK, but not GRADSPK, inhibits endothelial cell adhesion to FGF-2 and to FN. It must be pointed out that calcium deprivation and RGD-containing peptides do not affect the binding of 125I-FGF-2 to HSPGs and FGFRs in GM 7373 cells (Presta et al., 1991). Finally, we evaluated the capacity of soluble FN and VN to inhibit GM 7373 cell adhesion to immobilized FGF-2. As shown in Figure 6A, a 90 min-incubation of GM 7373 cells in suspension with soluble VN before the assay prevented cell adhesion and spreading onto FGF-2, while preincubation with soluble FN was ineffective. Conversely, soluble FGF-2 inhibits GM 7373 cell adhesion to VN but not to FN (Figure 6B). Taken together, the data support the hypothesis that integrins, possibly VN receptors, are involved in cell adhesion to FGF-2.
Figure 6.
Effect of soluble VN and FGF-2 on GM 7373 cell adhesion. (A) GM 7373 cells were incubated in suspension for 90 min at 37°C with increasing concentrations of VN (O) or FN (•). Then, cells were centrifuged to remove unbound molecules and seeded onto non-tissue culture plates coated with 10 μg/ml of FGF-2 and saturated with 10 mg/ml BSA. (B) In a parallel experiment cells were incubated for 90 min at 37°C with increasing concentrations of FGF-2, centrifuged, and seeded onto non-tissue culture plates coated with 10 μg/ml of VN (O) or FN (•) and saturated with 10 mg/ml BSA. In both experiments, the number of adherent cells was evaluated after 2 h of incubation at 37°C, corrected for the nonspecific cell adhesion measured onto BSA-coated wells, and expressed as percentage of cells adherent to the different substrata when preincubated the in absence of soluble molecules. Each point is the mean of three determinations in duplicate. SEM does not exceed 13% of the values.
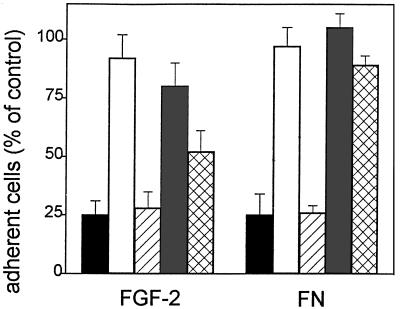
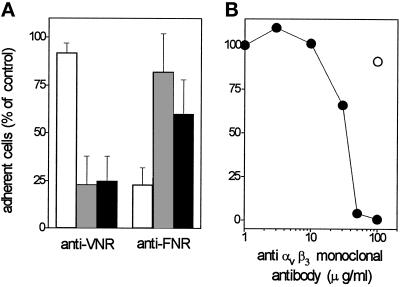
On this basis, we evaluated the effect of neutralizing antisera directed to the human VN receptor αvβ3 or to the human FN receptor α5β1 on endothelial cell adhesion to FGF-2. Preliminary experiments demonstrated that anti-αvβ3 antiserum does not cross-react with the 100-kDa β1 subunit or with the 85-kDa β5 subunit in endothelial cells, while anti-α5β1 antiserum shows a limited cross-reactivity for the β3 subunit (E. Tanghetti, unpublished observations). As shown in Figure 7A, antiserum to αvβ3 inhibits endothelial cell adhesion to FGF-2 and to VN, without affecting the adhesion to FN. Conversely, antiserum to α5β1 inhibits the adhesion of endothelial cells to FN-coated plastic, exerting only a limited effect on VN- or FGF-2-dependent adhesion.
Figure 7.
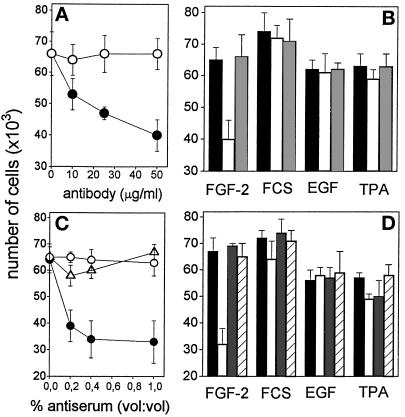
Effect of anti-αvβ3 antibodies on endothelial cell adhesion to FGF-2-coated plastic. (A) EAhy 926 cells were incubated for 30 min at 37°C with neutralizing antisera directed to human α5β1 integrin (FNR) or αvβ3 integrin (VNR) at 1:400 or 1:200 dilution (vol/vol), respectively. Then, cells were seeded onto non-tissue culture plates coated with 20 μg/ml of FN (white bar), VN (shaded bars), or FGF-2 (black bars). The number of adherent cells was evaluated after 2 h of incubation at 37°C, subtracted from the nonspecific cell adhesion, measured onto BSA-coated wells, and expressed as percentage of cells adherent to the different substrata in the presence of nonimmune serum. Each point is the mean ± SEM of four determinations in duplicate. (B) GM 7373 cells were incubated for 30 min at 37°C with the indicated concentrations of irrelevant IgG (O) or of monoclonal anti-αvβ3 antibody (•). Then, cells were seeded onto non-tissue culture plates coated with 20 μg/ml of FGF-2. The number of adherent cells was evaluated after 2 h of incubation at 37°C, corrected for the nonspecific cell adhesion measured onto BSA-coated wells and expressed as percentage of cells adherent to the different substrata in the absence of any addition. Each point is the mean of three determinations in duplicate. SEM does not exceed 13% of the values.
In agreement with these observations, the highly specific monoclonal LM 609 antibody directed to αvβ3 (Cheresh, 1987) completely prevented endothelial cell adhesion to FGF-2-coated plastic while irrelevant IgGs were ineffective (Figure 7B). In conclusion, the data demonstrate that αvβ3 mediates the cell-adhesive capacity of immobilized FGF-2.
In Vitro Interaction of FGF-2 with αvβ3
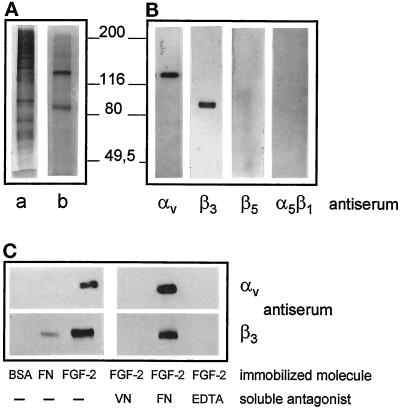
The above observations prompted us to assess whether FGF-2 can interact with αvβ3 integrin in vitro. To this purpose, αvβ3 integrin was purified from human term placenta (see MATERIALS AND METHODS for details). As shown in Figure 8A, SDS-PAGE analysis of the purified material followed by silver staining of the gel shows the presence of two bands with apparent molecular masses of 138 and 85 kDa. They were identified as the αv and β3 subunits of the VN receptor because of their molecular mass and immunoreactivity with specific anti-αv and anti-β3 antibodies, respectively. These bands do not cross-react instead with anti-β5 and anti-α5β1 antibodies (Figure 8B). Prolonged time of exposure of the film revealed only trace amounts of this latter integrin in the αvβ3 preparation.
Figure 8.
Purification of αvβ3 integrin from human placenta and its interaction with immobilized FGF-2. Human term placenta extract was applied onto a wheat germ lectin-Sepharose column that was eluted with N-acetyl-d-glucosamine. Eluted proteins (a) were loaded onto a GRGDSPK-Sepharose column that was then eluted with EDTA (b). Proteins (15 and 0.5 μg/sample for a and b, respectively) were analyzed by SDS-PAGE on 8% polyacrylamide gel under reducing conditions and visualized by silver staining (A). In panel B, aliquots (0.1 μg) of purified αvβ3 integrin (corresponding to the material visualized in panel A, lane b) were analyzed by Western blotting using the indicated polyclonal antibodies. (C) Aliquots (6 μg) of purified human αvβ3 were incubated onto plastic dishes coated with FN, BSA, or FGF-2 in the absence or in the presence of soluble FN or VN (both at 75 μg/ml), or in the presence of 20 mM EDTA. At the end of incubation proteins bound to plastic were extracted and analyzed by Western blotting with anti-β3 and anti-αv antibodies. Molecular weights are in thousands.
The purified human αvβ3 integrin was then assessed for its capacity to interact with FGF-2 in a cell-free system. To this purpose the growth factor was immobilized onto non-tissue culture plastic and assessed for its capacity to bind the purified VN receptor. As shown in Figure 8C, αvβ3 binds to immobilized FGF-2 but not to FN or BSA. Moreover, interaction of αvβ3 with immobilized FGF-2 is prevented by soluble VN but not by soluble FN. Finally, EDTA prevents the formation of the FGF-2/αvβ3 complex.
Anti-αvβ3 Antibodies Inhibit the Biological Activity of Soluble FGF-2
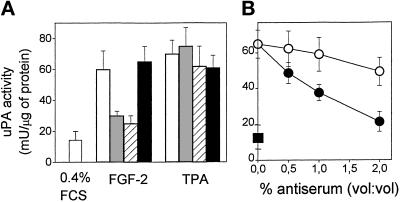
The above data demonstrate the interaction of immobilized FGF-2 with αvβ3 integrin located at the basal site of the endothelial cell. However, in vitro and in vivo studies have shown that αvβ3 integrin is present also at the luminal aspect of endothelium (Conforti et al., 1992), raising the possibility that also free FGF-2 may interact with the VN receptor. On this basis, to assess the role of VN receptor in mediating the biological activity of FGF-2, we evaluated the effect of monoclonal and polyclonal neutralizing anti-αvβ3 antibodies on the mitogenic and uPA-inducing activity exerted by soluble FGF-2 on GM 7373 cells adherent to tissue culture plastic. When added to the cell culture medium, monoclonal anti-αvβ3 antibody inhibits the mitogenic activity of FGF-2 in a dose-dependent manner (Figure 9A). The effect was specific, as demonstrated by the incapacity of this antibody to inhibit the mitogenic activity exerted by other mitogens, including FCS, epidermal growth factor (EGF), and the phorbol ester 12-O-tetradecanoyl phorbol 13-acetate (TPA) (Figure 9B). Accordingly, polyclonal anti-VN receptor antiserum, but not anti-FN receptor antiserum, specifically inhibits the mitogenic activity exerted by soluble FGF-2. Also, monoclonal anti-αvβ3 antibody, anti-VN receptor antiserum, but not anti-FN receptor antiserum fully prevent uPA up-regulation induced by soluble FGF-2 in GM 7373 cells without affecting the uPA-inducing activity of TPA (Figure 10A, B). As shown in Figure 10C, anti-VN receptor antiserum inhibits also uPA production induced by soluble FGF-2 in CHO cells transfected with FGFR-1/flg (Rusnati et al., 1996), thus suggesting that the involvement of αvβ3 integrin in mediating the biological activity of FGF-2 is not restricted to endothelial cells.
Figure 9.
Effect of anti-αvβ3 antibodies on the mitogenic activity of soluble FGF-2. (A) GM 7373 cells grown on tissue culture plates were incubated with FGF-2 (10 ng/ml) in the presence of the indicated concentrations of monoclonal antiαvβ3 antibody (black symbols) or irrelevant antibody (open symbols). (B) Cells were treated with 10% FCS, EGF (30 ng/ml), or TPA (100 ng/ml) in the absence (black bars) or in the presence of 50 μg/ml of monoclonal anti-αvβ3 antibody (white bars) or of irrelevant antibody (gray bars). (C) Cells grown on tissue culture plates were incubated with FGF-2 (10 ng/ml) in the presence of the indicated concentration of anti-αvβ3 antiserum (•), antiα5β1 antiserum (O), or irrelevant antiserum (Δ). (D) Cells were treated with 10% FCS, EGF, or TPA (doses as in panel B) in the absence (black bars) or in the presence of 1% (vol/vol) of anti-αvβ3 antiserum (white bars), anti-α5β1 antiserum (gray bars), or irrelevant antiserum (striped bars). After 24 h, all cell cultures were trypsinized and cells were counted in a Burker chamber. Control GM 7373 cells incubated with 0.4% FCS or 10% FCS in the absence of any addition were 37,000 ± 7,000 and 72,500 ± 5,600 cells/well, respectively. Each point is the mean ± SEM of two to three determinations in duplicate.
Figure 10.
Effect of anti-αvβ3 antibodies on the uPA-inducing activity of soluble FGF-2. (A) GM 7373 cells grown onto tissue culture plates were incubated in fresh medium containing 0.4% FCS in the absence (white bar) or in the presence of 10 ng/ml FGF-2 or 100 ng/ml TPA (crossed bars). One-half of FGF-2 or TPA-treated cell cultures was added also with 100 μg/ml of monoclonal anti-αvβ3 antibody (gray bars) or with a 4% dilution (vol/vol) of anti-αvβ3 antiserum (striped bars) or of anti-α5β1 antiserum (black bars). After 24 h, cell-associated uPA activity was evaluated and expressed as milliunits of uPA activity/mg of protein. (B) FGFR-1/flg transfected CHO cells grown onto tissue culture plates were incubated with fresh medium containing 0.4% FCS alone (▪) or added with 10 ng/ml FGF-2 in the absence or in the presence of the indicated dilutions of anti-αvβ3 (•) or of anti-α5β1 (O) antisera. Each point is the mean ± SEM of two to three determinations in duplicate.
DISCUSSION
In the present paper we demonstrate for the first time that immobilized FGF-2 interacts with a member of the integrin family, namely αvβ3, thus promoting endothelial cell adhesion and spreading. Also, anti-αvβ3 monoclonal and polyclonal antibodies specifically inhibit cell proliferation and uPA up-regulation induced by soluble FGF-2 in GM 7373 cells grown on tissue culture plastic. These data implicate αvβ3/FGF-2 interaction in mediating the biological activity of the growth factor and may explain and extend previous observations on the capacity of αvβ3 antibodies to selectively inhibit angiogenesis stimulated by FGF-2 (Friedlander et al., 1995).
Our findings are in keeping with the observation that the capacity to interact with αvβ3 and promote endothelial cell adhesion is not limited to typical ECM cell-adhesive proteins but is shared by a variety of molecules with different biological activities, including thrombin (Bar-Shavit et al., 1991), perlecan (Hayashi et al., 1992), matrix metalloproteinase MMP-2 (Brooks et al., 1996), and human immunodeficiency virus type 1 (HIV-1) Tat (Barillari et al., 1993; Voegel et al., 1993; Weeks et al., 1993). Interestingly, HIV-1 Tat, like FGF-2, is endowed with angiogenic capacity (Albini et al., 1996).
Immobilized FGF-2 induces cell adhesion and spreading of fetal bovine aortic endothelial GM 7373 cells and of human endothelial EAhy 926 cells. The effect is time- and dose-dependent and is fully prevented by neutralizing anti-FGF-2 antibodies. The cell-adhesive activity of immobilized FGF-2 is similar to that exerted by classic cell adhesion molecules like FN, VN, and TSP, even though FGF-2 may require the presence of low concentrations of serum (<1%) to exert an optimal cell-adhesive capacity. Experiments in progress in our laboratory indicate that lysophosphatidic acid, a phospholipid naturally occurring in serum and able to induce focal adhesion assembly and organization of actin stress fibers (Moolenaar, 1995; Ridley and Hall, 1992), is responsible for this effect (Tanghetti et al., manuscript in preparation).
Several experimental results indicate that the cell-adhesive capacity of FGF-2 is mediated by the VN receptor αvβ3. 1) Cell adhesion to FGF-2 is calcium-dependent and it is inhibited by RGD-containing peptides. 2) Soluble VN, but not soluble FN, inhibits endothelial cell adhesion to FGF-2. Conversely, soluble FGF-2 prevents cell adhesion to VN but not to FN. 3) Monoclonal and polyclonal anti-αvβ3 antibodies, but not anti-α5β1 antibody, inhibit endothelial cell adhesion to FGF-2. 4) Immobilized FGF-2 binds to purified human αvβ3 integrin in a cell-free system, and this interaction is competed by soluble VN but not by soluble FN. We cannot rule out the hypothesis that FGF-2 may interact also with other members of the integrin family, as it occurs for HIV-1 Tat protein that promiscuously interacts with both αvβ3 and αvβ5 integrins (Voegel et al., 1993; Weeks et al., 1993). Experiments are in progress to assess this possibility.
FGF-2 does not require its native three-dimensional conformation to exert a cell-adhesive activity, indicating that linear amino acid sequence(s) of the growth factor are involved in FGF-2/integrin interaction. Binding to several specific linear amino acid sequences, including the well known RGD sequence, is a typical feature of integrin-mediated cell adhesion (McCarthy et al., 1986; Humphires et al., 1986, 1987; Elices et al., 1990; Guan and Hynes, 1990; Isberg and Leong, 1990; Yamada, 1991; Koivunen et al., 1993, 1994). We have identified two cell-adhesion domains in FGF-2 corresponding to amino acid sequences 38–61 and 82–101. Both domains contain one DGR sequence that is exposed onto the surface of the native FGF-2 molecule (Eriksson et al., 1991). DGR is the inverse of the integrin recognition sequence RGD present on adhesive proteins. Since DGR-containing peptides inhibit integrin-mediated cell adhesion to FN (Humphries et al., 1986; Yamada and Kennedy, 1987; Koivunen et al., 1993), it is tempting to hypothesize that the two DGR sequences of FGF-2 are responsible for the integrin-mediated cell-adhesive activity of the growth factor. On the other hand, the two cell-adhesive regions of FGF-2 have a highly positive net charge that may be partially responsible for cell interaction. Indeed, positively charged amino acid sequences play an important role in integrin interaction. For instance, peptides containing RGD plus a basic segment bind more avidly to IIb/IIIa integrin than peptides containing RGD alone (Savage et al., 1990); a basic domain in VN plays a role in the interaction with αvβ4 (Voegel et al., 1993); α3β1 binds a basic peptide present within laminin (Gehlsen et al., 1992); α5β1 and α3β1 bind to poly-R or poly-K affinity columns (Voegel et al., 1993). All these observations point to a cooperation between integrin recognition sequences and basic amino acids in mediating the binding of adhesive proteins to integrin receptors. This kind of cooperation has been well demonstrated for the HIV-1 Tat protein in which one RGD sequence and the basic domain mediate integrin-dependent cell adhesion (Voegel et al., 1993; Weeks et al., 1993).
RGD- and DGR-containing tetra- and eptapeptides inhibit the mitogenic activity exerted by soluble FGF-2 in endothelial cells in a competitive manner without affecting the binding of the growth factor to FGFRs or to HSPGs (Presta et al., 1991). Moreover, the cell-adhesive fragments FGF-2(38–61) and FGF-2(82–101) antagonize the mitogenic activity of soluble FGF-2 without interacting with FGFRs (Presta et al., 1991). These data suggest that the binding of FGF-2 to FGFR is not sufficient to induce cell proliferation in endothelial cells and that an interaction of FGF-2 with a cell-surface integrin receptor is also required. This hypothesis is sustained by the observation that monoclonal and polyclonal anti-αvβ3 antibodies specifically inhibit the mitogenic and uPA-inducing activity exerted by soluble FGF-2 in endothelial cell cultures. These data are in keeping with the observation that anti-αvβ3 antibody inhibits the angiogenic activity exerted in vivo by FGF-2 without affecting neovascularization induced by vascular endothelial cell growth factor, transforming growth factor-α, or phorbol ester (Friedlander et al., 1995). Thus, the mechanism by which endothelial αvβ3 integrin mediates FGF-2-induced angiogenesis may consist in an interaction with the growth factor that promotes endothelial cell adhesion and that cooperates with FGFR in transducing the intracellular signals required for the induction of the angiogenic phenotype. FGFR and αvβ3 integrin may be favored in their cross-talk by their structural vicinity that can occur both at the basal aspect of the endothelium, where they colocalize in the focal adhesion contacts (Plopper et al., 1995), and at the luminal aspect of the endothelium, where αvβ3 is also expressed (Conforti et al., 1992).
αvβ3 integrin is highly expressed in endothelium during angiogenesis and is involved in neovascularization induced by FGF-2 (Brooks et al., 1994; Friedlander et al., 1995). We report here that FGF-2 interacts with αvβ3 integrin, affecting different aspects of the angiogenic phenotype of the endothelial cell, including cell adhesion, cell proliferation, and protease production. This novel interaction is part of the intimate cross-talking existing between cytokines and vascular cell adhesion events during angiogenesis.
ACKNOWLEDGMENTS
We thank Mr. F. Bonardi and Dr. N. Quirici for their help in performing scanning electron microscopy, Dr. D. Soligo (Fondazione Matarelli, Milan, Italy) for making the scanning electron microscope available, and Dr. G. Tarone for helpful discussion. This work was supported by C.N.R. (grant 95.02983.CT14 to M.R., grant 95.02880.CT14 to P.D.E, Progetto Finalizzato Biotecnologie e Biostrumentazioni “Sottoprogetto Biofarmaci” and grants 94.00316.CT14 and 95.02925.CT14 to M.P.); by M.U.R.S.T. (quota 60% to M.R and to M.P.); by the Associazione Italiana per la Ricerca sul Cancro (Special Project Angiogenesis), and Istituto Superiore di Sanità (AIDS Project) to M.P.
Footnotes
Abbreviations: ECM, extracellular matrix; EGF, epidermal growth factor; FCS, fetal calf serum; FGF-2, basic fibroblast growth factor; carbonate buffer, 100 mM NaHCO3 pH 9.6; FGFR, tyrosine kinase FGF receptor; FN, fibronectin; GST, glutathione-S-transferase; HSPG, heparan sulfate proteoglycan; TPA, phorbol ester 12-O-tetradecanoyl phorbol 13-acetate; TSP, thrombospondin; uPA, urokinase-type plasminogen activator; VN, vitronectin.
REFERENCES
- Albelda SM, Buck CA. Integrins and other cell adhesion molecules. FASEB J. 1990;4:2868–2880. [PubMed] [Google Scholar]
- Albini A, Benelli R, Presta M, Rusnati M, Ziche M, Rubartelli A, Paglialunga G, Bussolino F, Noonan D. HIV-tat protein is a heparin-binding angiogenic growth factor. Oncogene. 1996;12:289–297. [PubMed] [Google Scholar]
- Ausprunk DH, Folkman J. Migration and proliferation of endothelial cells in preformed and newly formed blood vessel during tumor angiogenesis. Microvasc Res. 1977;14:53–65. doi: 10.1016/0026-2862(77)90141-8. [DOI] [PubMed] [Google Scholar]
- Baird A, Schubert D, Ling N, Guillemin R. Receptor- and heparin-binding domains of basic fibroblast growth factor. Proc Natl Acad Sci USA. 1988;85:2324–2328. doi: 10.1073/pnas.85.7.2324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banga HS, Simons ER, Brass LF, Rittenhouse SE. Activation of phospholipases A and C in human platelets exposed to epinephrine: role of glycoproteins IIb/IIIa and dual role of epinephrine. Proc Acad Natl Sci USA. 1986;83:9197–9291. doi: 10.1073/pnas.83.23.9197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barillari G, Gendelman R, Gallo RC, Ensoli B. The tat protein of human immunodeficency virus type 1, a growth factor for AIDS Kaposi sarcoma and cytokine-activated vascular cells, induces adhesion of the same cell type by using integrin receptors recognizing the RGD amino acid sequence. Proc Natl Acad Sci USA. 1993;90:7941–7945. doi: 10.1073/pnas.90.17.7941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar-Shavit R, Sabbah V, Lampugnani MG, Marchisio PC, Fenton II JW, Vlodavsky I, Dejana E. An Arg-Gly-Asp sequence within thrombin promotes endothelial cell adhesion. J Cell Biol. 1991;112:335–344. doi: 10.1083/jcb.112.2.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basilico C, Moscatelli D. The FGF family of growth factor and oncogenes. Adv Cancer Res. 1992;59:115–165. doi: 10.1016/s0065-230x(08)60305-x. [DOI] [PubMed] [Google Scholar]
- Besser D, Presta M, Nagamine Y. Elucidation of a signaling pathway induced by FGF-2 leading to uPA gene expression in NIH 3T3 fibroblast. Cell Growth & Differ. 1995;6:1009–1017. [PubMed] [Google Scholar]
- Broadley KN, Aquino AM, Woodward SC, Buckley-Sturrock A, Sato Y, Rifkin DB, Davidson JM. Monospecific antibodies implicate basic fibroblast growth factor in normal wound repair. Lab Invest. 1989;61:571–575. [PubMed] [Google Scholar]
- Brooks PC, Richard AF, Cheresh DA. Requirement of vascular integrin αvβ3 for angiogenesis. Science. 1994;264:569–571. doi: 10.1126/science.7512751. [DOI] [PubMed] [Google Scholar]
- Brooks PC, Stromblad S, Sanders LC, von Schalscha TL, Aimes RT, Stetler-Stevenson WG, Quigley JP, Cheresh DA. Localization of matrix metalloproteinase MMP-2 to the surface of invasive cells by interaction with integrin αvβ3. Cell. 1996;85:683–693. doi: 10.1016/s0092-8674(00)81235-0. [DOI] [PubMed] [Google Scholar]
- Byers S, Amaya E, Munro S, Blaschuk O. Fibroblast growth factor receptor contain a conserved HAV region common to chaderins and influenza strain A hemagglutinins: a role in protein-protein interaction? Dev Biol. 1992;152:411–414. doi: 10.1016/0012-1606(92)90149-b. [DOI] [PubMed] [Google Scholar]
- Chen Q, Kinch MS, Lin TH, Burridge K, Juliano RL. Integrin-mediated cell adhesion activates mitogen-activated protein kinases. J Biol Chem. 1994;269:26602–26605. [PubMed] [Google Scholar]
- Cheresh DA. Human endothelial cells synthesize and express an Arg-Gly-Asp-directed adhesion receptor involved in attachment to fibrinogen and von Willebrand factor. Proc Natl Acad Sci USA. 1987;84:6471–6475. doi: 10.1073/pnas.84.18.6471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conforti G, Dominguez-Jimenez C, Zanetti A, Gimbrone MA, Jr, Cremona O, Marchisio PC, Dejana E. Human endothelial cells express integrin receptors on the luminal aspect of their membrane. Blood. 1992;80:437–446. [PubMed] [Google Scholar]
- Davis CM, Danehower SC, Laurenza A, Molony JL. Identification of a role of the vitronectin receptor and protein kinase C in the induction of endothelial cell vascular formation. J Cell Biochem. 1993;51:206–218. doi: 10.1002/jcb.240510213. [DOI] [PubMed] [Google Scholar]
- Dejana E, Colella S, Conforti G, Abbadini M, Gaboli M, Marchisio PC. Fibronectin and vitronectin regulate the organization of their respective Arg-Gly-Asp adhesion receptors in cultured human endothelial cells. J Cell Biol. 1988;107:1215–1223. doi: 10.1083/jcb.107.3.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgell CJ, McDonald CC, Graham JB. Permanent cell line expressing human factor VIII-related antigen estabilished by hybridization. Proc Natl Acad Sci USA. 1983;80:3734–3737. doi: 10.1073/pnas.80.12.3734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elices MJ, Osborn L, Takada Y, Crouse C, Luhowsky S, Hemier ME, Lobb RR. VCAM-1 on activated endothelium interacts with the leukocyte integrin VLA-4 at a site distinct from the VLA-4/fibronectin binding site. Cell. 1990;60:577–584. doi: 10.1016/0092-8674(90)90661-w. [DOI] [PubMed] [Google Scholar]
- Enenstein J, Waleh NS, Kramer RH. Basic FGF and TGF-β differentially modulate integrin expression of human microvascular endothelial cells. Exp Cell Res. 1992;203:499–503. doi: 10.1016/0014-4827(92)90028-7. [DOI] [PubMed] [Google Scholar]
- Eriksson AE, Cousens LS, Weaver LH, Matthews BW. Three-dimensional structure of human basic fibroblast growth factor. Proc Natl Acad Sci USA. 1991;88:3441–3445. doi: 10.1073/pnas.88.8.3441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feige JJ, Bradley JD, Fryburg K, Farris J, Cousens LC, Barr PJ, Baird A. Different effects of heparin, fibronectin and laminin on the phosphorylation of basic fibroblast growth factor by protein kinase C and the catalytic subunit of protein kinase A. J Cell Biol. 1989;109:3105–3114. doi: 10.1083/jcb.109.6.3105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folkman J. Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat Med. 1995;1:27–31. doi: 10.1038/nm0195-27. [DOI] [PubMed] [Google Scholar]
- Folkman J, Klagsbrun M, Sasse J, Wadzinsky M, Ingber D, Vlodavsky I. A heparin-binding angiogenic protein-basic fibroblast growth factor is stored within basement membrane. Am J Pathol. 1988;130:393–400. [PMC free article] [PubMed] [Google Scholar]
- Friedlander M, Brooks PC, Shaffer RW, Kincaid CM, Varner JA, Cheresh DA. Definition of two angiogenic pathways by distinct αv integrins. Science. 1995;270:1500–1502. doi: 10.1126/science.270.5241.1500. [DOI] [PubMed] [Google Scholar]
- Gehlsen KR, Striramaro P, Fuecht LT, Skubitz APN. A synthetic peptide derived from the carboxy terminus of the laminin A chain represent a binding site for the α3β1 integrin. J Cell Biol. 1992;117:449–459. doi: 10.1083/jcb.117.2.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginsberg MH, Du X, Plaw EF. Inside-out integrin signaling. Curr Opin Cell Biol. 1992;4:766–771. doi: 10.1016/0955-0674(92)90099-x. [DOI] [PubMed] [Google Scholar]
- Grinspan JB, Stephen NM, Levine EM. Bovine endothelial cells transformed in vitro with benzo(a)pyrene. J Cell Physiol. 1983;114:328–338. doi: 10.1002/jcp.1041140312. [DOI] [PubMed] [Google Scholar]
- Guan J, Hynes RO. Lymphoid cells recognize an alternatively spliced segment of fibronectin via the integrin receptor [α4β1] Cell. 1990;60:53–61. doi: 10.1016/0092-8674(90)90715-q. [DOI] [PubMed] [Google Scholar]
- Hatai M, Hashi H, Mogi A, Som H, Yokota I, Yaoi Y. Stimulation of tyrosine- and serine-phosphorylation of focal adhesion kinase in mouse 3T3 cells by fibronectin and fibroblast growth factor. FEBS Lett. 1994;350:113–116. doi: 10.1016/0014-5793(94)00745-4. [DOI] [PubMed] [Google Scholar]
- Hayashi K, Madri JA, Yurchenco PD. Endothelial cells interact with the core protein of basement membrane perlecan through β1 and β3 integrins: an adhesion modulated by glycosaminoglycan. J Cell Biol. 1992;119:945–959. doi: 10.1083/jcb.119.4.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphries MJ, Akiyama SK, Komoriya A, Olden K, Yamada KM. Identification of an alternatively spliced site in human plasma fibronectin that mediates cell type specific adhesion. J Cell Biol. 1986;103:2637–2647. doi: 10.1083/jcb.103.6.2637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphries MJ, Komoriya A, Akiyama SK, Olden K, Yamada KM. Identification of two distinct regions of the type III connecting segment of human plasma fibronectin that promote cell type-specific adhesion. J Biol Chem. 1987;262:6886–6892. [PubMed] [Google Scholar]
- Hynes RO. Integrins:versatility, modulation, and signaling in cell adhesion. Cell. 1992;69:11–25. doi: 10.1016/0092-8674(92)90115-s. [DOI] [PubMed] [Google Scholar]
- Ingber DE, Folkman J. Inhibition of angiogenesis through modulation of collagen metabolism. Lab Invest. 1988;59:44–51. [PubMed] [Google Scholar]
- Ingber DE, Folkman J. How does extracellular matrix control capillary morphogenesis? Cell. 1989a;58:803–805. doi: 10.1016/0092-8674(89)90928-8. [DOI] [PubMed] [Google Scholar]
- Ingber DE, Folkman J. Mechanochemical switching between growth and differentiation during fibroblast growth factor-stimulated angiogenesis in vitro: role of extracellular matrix. J Cell Biol. 1989b;109:317–330. doi: 10.1083/jcb.109.1.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingber DE, Madri JA, Folkman J. A possible mechanism for inhibition of angiogenesis by angiostatic steroids: induction of capillary basement membrane dissolution. Endocrinology. 1986;119:1768–1775. doi: 10.1210/endo-119-4-1768. [DOI] [PubMed] [Google Scholar]
- Ingber DE, Madri JA, Folkman J. Endothelial growth factor and extracellular matrix regulate DNA synthesis through modulation of cell and nuclear expansion. In Vitro Cell & Dev Biol. 1987;23:387–394. doi: 10.1007/BF02620997. [DOI] [PubMed] [Google Scholar]
- Ingber DE, Prusty D, Frangione J, Cragoe EJ, Jr, Lechene C, Schawartz M. Control of intracellular pH and growth by fibronectin in capillary endothelial cells. J Cell Biol. 1990;110:1803–1812. doi: 10.1083/jcb.110.5.1803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isacchi A, Statuto M, Chiesa R, Bergonzoni L, Rusnati M, Sarmientos P, Ragnotti G, Presta M. A six amino acid deletion in basic fibroblast growth factor dissociates its mitogenic activity from its plasminogen activator-inducing capacity. Proc Natl Acad Sci USA. 1991;88:2628–2632. doi: 10.1073/pnas.88.7.2628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isberg RR, Leong JM. Multiple β1 chain integrins are receptors for invasin, a protein that promotes bacterial penetration into mammalian cells. Cell. 1990;60:861–871. doi: 10.1016/0092-8674(90)90099-z. [DOI] [PubMed] [Google Scholar]
- Johnson DE, Williams LT. Structural and functional diversity in the FGF receptor multigene family. Adv Cancer Res. 1993;60:1–41. doi: 10.1016/s0065-230x(08)60821-0. [DOI] [PubMed] [Google Scholar]
- Klein S, Giancotti FG, Presta M, Albelda SM, Clayton AB, Rifkin DB. Basic fibroblast growth factor modulates integrin expression in microvascular endothelial cells. Mol Biol Cell. 1993;4:973–982. doi: 10.1091/mbc.4.10.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koivunen E, Gay DA, Ruoslahti E. Selection of peptides to the α5β1 integrin from phage display library. J Biol Chem. 1993;268:20205–20210. [PubMed] [Google Scholar]
- Koivunen E, Wang B, Ruoslahti E. Isolation of a highly specific ligand for the α5β1 integrin from a phage display library. J Cell Biol. 1994;124:373–380. doi: 10.1083/jcb.124.3.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason I. Do adhesion molecules signal via FGF receptors ? Curr Biol. 1994;4:1158–1161. doi: 10.1016/s0960-9822(00)00263-3. [DOI] [PubMed] [Google Scholar]
- McCarthy JB, Hagen ST, Furcht LT. Human fibronectin contains distinct adhesion- and motility-promoting domains for metastatic melanoma cells. J Cell Biol. 1986;102:179–188. doi: 10.1083/jcb.102.1.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moolenaar WH. Lysophosphatidic acid, a multifunctional phospholipid messenger. J Biol Chem. 1995;270:12949–12952. doi: 10.1074/jbc.270.22.12949. [DOI] [PubMed] [Google Scholar]
- Moscatelli D, Presta M, Rifkin DB. Purification of a factor from human placenta that stimulates capillary endothelial cell protease production, DNA synthesis, and migration. Proc Natl Acad Sci USA. 1986;83:2091–2095. doi: 10.1073/pnas.83.7.2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelletier AJ, Bodary SC, Levinson AD. Signal transduction by the platelet integrin alpha IIb/beta 3: induction of calcium oscillation required for protein-tyrosine phosphorylation and ligand-induced spreading of stably transformed cells. Mol Biol Cell. 1992;3:989–998. doi: 10.1091/mbc.3.9.989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters KG, Marie J, Wilson E, Ives HE, Escobedo J, Del Rosario M, Mirda D, Williams LT. Point mutation of an FGF receptor abolishes phosphatidylinositol turnover and Ca2+ flux but not mitogenesis. Nature. 1992;358:678–681. doi: 10.1038/358678a0. [DOI] [PubMed] [Google Scholar]
- Plopper GE, McNamee HP, Dike LE, Bojanowski K, Ingber DE. Convergence of integrin and growth factor receptor signaling pathways within the focal adhesion complex. Mol Biol Cell. 1995;6:1349–1365. doi: 10.1091/mbc.6.10.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Presta M, Mayer JAM, Ragnotti G. The mitogenic signaling pathway but not the plasminogen activator-inducing pathway of basic fibroblast growth factor is mediated though protein kinase C in fetal bovine aortic endothelial cells. J Cell Biol. 1989a;109:1877–1884. doi: 10.1083/jcb.109.4.1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Presta M, Rusnati M, Urbinati C, Sommer A, Ragnotti G. Biologically active synthetic fragments of human basic fibroblast growth factor (bFGF): identification of two Asp-Gly-Arg-containing domains involved in the mitogenic activity of bFGF in endothelial cells. J Cell Physiol. 1991;149:512–524. doi: 10.1002/jcp.1041490322. [DOI] [PubMed] [Google Scholar]
- Presta M, Rusnati M, Urbinati C, Tanghetti E, Statuto M, Pozzi A, Gualandris A, Ragnotti G. In: Basic fibroblast growth factor bound to cell-substrate promotes cell adhesion, proliferation, and protease production in cultured endothelial cells. Experientia Supplement on Angiogenesis: Key Principles-Science-Technology-Medicine. Stainer R, Weisz PB, Langer R, editors. Vol. 62. Basel, Switzerland: Birkauser Verlag; 1992. pp. 205–209. [DOI] [PubMed] [Google Scholar]
- Pytela R, Pierschbacher MD, Argraves S, Suzuki S, Ruoslahti E. Arginine-glycine-aspartic acid adhesion receptors. Methods Enzymol. 1987;144:475–489. doi: 10.1016/0076-6879(87)44196-7. [DOI] [PubMed] [Google Scholar]
- Ribatti D, Urbinati C, Nico B, Rusnati M, Roncali L, Presta M. Endogenous basic fibroblast growth factor is implicated in the vascularization of the chick embryo chorionallantoic membranes. Dev Biol. 1995;170:39–49. doi: 10.1006/dbio.1995.1193. [DOI] [PubMed] [Google Scholar]
- Ridley AJ, Hall A. The small GTP-binding protein rho regulates the assembly of focal adhesion and actin stress fibers in response to growth factors. Cell. 1992;70:389–399. doi: 10.1016/0092-8674(92)90163-7. [DOI] [PubMed] [Google Scholar]
- Ruoslahti E, Pierschbacher MD. New perspectives in cell adhesion: RGD and integrins. Science. 1987;238:439–497. doi: 10.1126/science.2821619. [DOI] [PubMed] [Google Scholar]
- Rusnati M, Dell’Era P, Urbinati C, Tanghetti E, Massardi ML, Nagamine Y, Monti E, Presta M. A distinct basic fibroblast growth factor (FGF-2)/FGF receptor interaction distinguishes urokinase-type plasminogen activator induction from mitogenicity in endothelial cells. Mol Biol Cell, 1996;7:369–381. doi: 10.1091/mbc.7.3.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saksela O, Rifkin DB. Release of basic fibroblast growth factor-heparan sulfate complexes from endothelial cells by plasminogen activator-mediated proteolytic activity. J Cell Biol. 1990;110:767–775. doi: 10.1083/jcb.110.3.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savage B, Marzec UM, Chao BH, Harker LA, Maraganore JM, Ruggeri ZM. Binding of the snake venom-derived proteins applaggin and echistatin to the arginin-glycine-aspartic acid recognition site(s) on platelet glycoprotein IIb/IIa complex inhibits receptor function. J Biol Chem. 1990;265:11766–11772. [PubMed] [Google Scholar]
- Schlaepfer DD, Hanks SK, Hunter T, van der Geer P. Integrin-mediated signal transduction linked to Ras pathway by GRB2 binding to focal adhesion kinase. Nature. 1994;372:786–789. doi: 10.1038/372786a0. [DOI] [PubMed] [Google Scholar]
- Schor AM, Schor SL. Inhibition of endothelial cell morphogenetic interactions in vitro by alpha- and beta-xylosides. In Vitro. 1988;24:659–668. doi: 10.1007/BF02623603. [DOI] [PubMed] [Google Scholar]
- Schubert D, Ling N, Baird A. Multiple influences of a heparin-binding growth factor in neuronal development. J Cell Biol. 1987;104:635–643. doi: 10.1083/jcb.104.3.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz MA. Spreading of human endothelial cells on fibronectin or vitronectin triggers elevation of intracellular free calcium. J Cell Biol. 1993;120:1003–1010. doi: 10.1083/jcb.120.4.1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JC, Singh JP, Lillquist JS, Goon DS, Stiles CD. Growth factors adherent to cell substrate are mitogenically active in situ. Nature. 1982;269:154–156. doi: 10.1038/296154a0. [DOI] [PubMed] [Google Scholar]
- Steegmaler M, Levinovitz A, Isenmann S, Borges E, Lenter M, Kocher HP, Kleuser B, Vestweber D. The E-selectin-ligand ESL-1 is a variant of a receptor for fibroblast growth factor. Nature. 1995;373:615–620. doi: 10.1038/373615a0. [DOI] [PubMed] [Google Scholar]
- Taraboletti G, Bellotti D, Borsotti P, Rusnati M, Presta M, Giavazzi R. The 140 kD, anti-angiogenic fragment of thrombospondin binds and sequesters basic fibroblast growth factor. Cell Growth & Differ. 1997;8:471–479. [PubMed] [Google Scholar]
- Vlodavsky I, Folkman J, Sullivan R, Fridman R, Ishai-Michaeli R, Sasse J, Klagsbrun M. Endothelial cell-derived basic fibroblast growth factor: synthesis and deposition into subendothelial extracellular matrix. Proc Natl Acad Sci USA. 1987;84:2292–2296. doi: 10.1073/pnas.84.8.2292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voegel BE, S.-Lee J, Hildebrand A, Craig W, Pierschbacher MD, Wong-Staal F, Ruoslahti E. A novel integrin specificity exemplified by binding of the αvβ3 integrin to the basic domain of the HIV tat protein and vitronectin. J Cell Biol. 1993;121:461–468. doi: 10.1083/jcb.121.2.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuori K, Ruoslahti E. Activation of protein kinase C precedes α5β1 integrin-mediated cell spreading on fibronectin. J Biol Chem. 1993;268:21459–21462. [PubMed] [Google Scholar]
- Weeks BS, Desai K, Loewenstein PM, Klotman ME, PE, Klotman.Green M, Kleinman HK. Identification of a novel cell attachment domain in the HIV-1 tat protein and its 90-Kda cell surface binding protein. J Biol Chem. 1993;268:5279–5284. [PubMed] [Google Scholar]
- Williams EJ, Furness J, Walsh FS, Doherty P. Activation of the FGF receptor underlies neurite outgrowth stimulated by L1, N-CAM and N-chaderin. Neuron. 1994;13:583–594. doi: 10.1016/0896-6273(94)90027-2. [DOI] [PubMed] [Google Scholar]
- Yamada KD. Adhesive recognition sequences.J. Biol Chem. 1991;266:12809–12812. [PubMed] [Google Scholar]
- Yamada KD, Kennedy DW. Peptide inhibitors of fibronectin, laminin and other adhesion molecules: unique and shared features. J Cell Physiol. 1987;130:21–28. doi: 10.1002/jcp.1041300105. [DOI] [PubMed] [Google Scholar]