Abstract
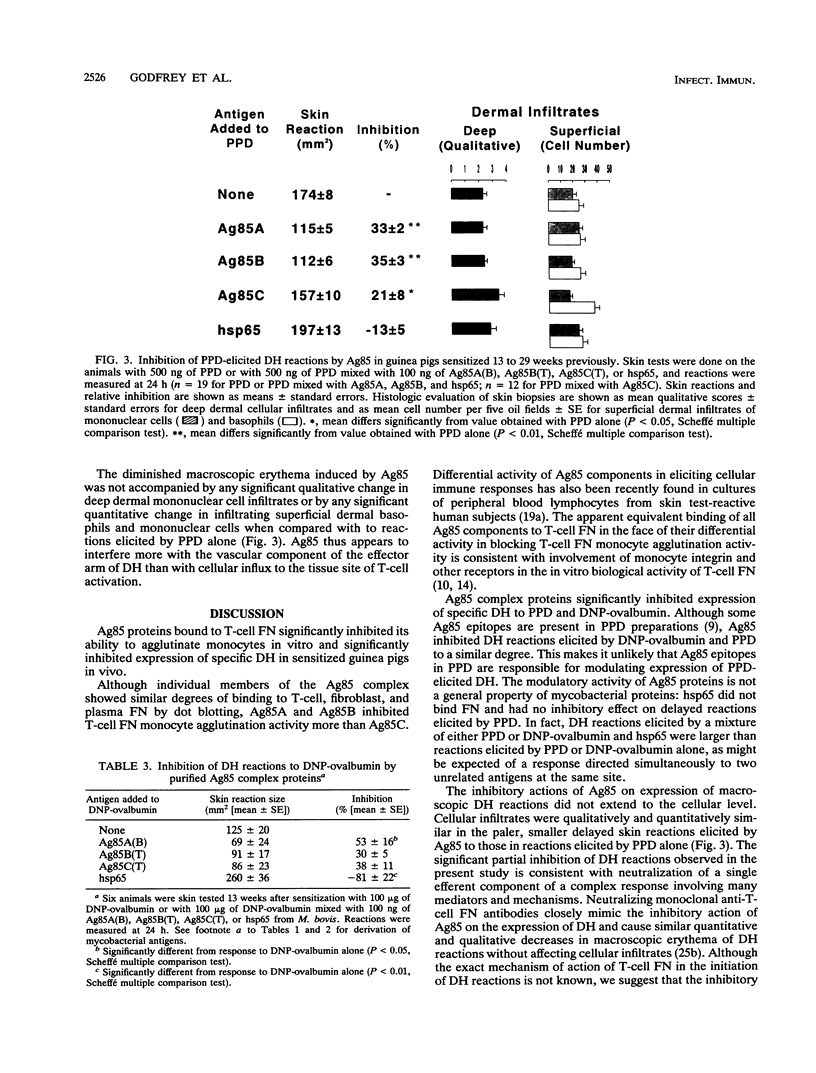
Although demonstration of delayed hypersensitivity to purified protein derivative of tuberculin (PPD) is an important element in the diagnosis of infection with Mycobacterium tuberculosis, many patients with tuberculosis are anergic. Several possible mechanisms for this specific lack of response have been described. We have now uncovered an additional one. T-cell fibronectin (FN), a lymphokine secreted by activated T cells, is closely associated with the initiation of delayed hypersensitivity reactions. Mycobacterial antigen 85 (Ag85) proteins have been shown to bind to plasma FN. The ability of Ag85 to bind to T-cell FN and modulate expression of delayed hypersensitivity was therefore studied. Purified Ag85 proteins from M. tuberculosis, Mycobacterium bovis BCG, or Mycobacterium kansasii bound to T-cell FN, fibroblast FN, and plasma FN in vitro. Purified 65-kDa heat shock protein (hsp65) from M. bovis BCG did not bind to any FN. Ag85, but not hsp65, inhibited the ability of T-cell FN to agglutinate monocytes in vitro in a dose-dependent manner. In vivo, mixtures of PPD or dinitrophenyl-ovalbumin and purified M. tuberculosis or M. bovis BCG Ag85 proteins elicited significantly smaller delayed hypersensitivity inflammatory reactions in sensitized guinea pigs than did PPD or dinitrophenyl-ovalbumin alone. Purified hsp65 did not inhibit expression of delayed hypersensitivity to PPD or dinitrophenyl-ovalbumin. We suggest that Ag85 proteins could inhibit in vivo expression of delayed hypersensitivity during mycobacterial infections because of their interaction with T-cell FN.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abou-Zeid C., Ratliff T. L., Wiker H. G., Harboe M., Bennedsen J., Rook G. A. Characterization of fibronectin-binding antigens released by Mycobacterium tuberculosis and Mycobacterium bovis BCG. Infect Immun. 1988 Dec;56(12):3046–3051. doi: 10.1128/iai.56.12.3046-3051.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abou-Zeid C., Smith I., Grange J. M., Ratliff T. L., Steele J., Rook G. A. The secreted antigens of Mycobacterium tuberculosis and their relationship to those recognized by the available antibodies. J Gen Microbiol. 1988 Feb;134(2):531–538. doi: 10.1099/00221287-134-2-531. [DOI] [PubMed] [Google Scholar]
- American Thoracic Society. Diagnostic standards and classification of tuberculosis. Am Rev Respir Dis. 1990 Sep;142(3):725–735. doi: 10.1164/ajrccm/142.3.725. [DOI] [PubMed] [Google Scholar]
- Andersen P., Askgaard D., Ljungqvist L., Bentzon M. W., Heron I. T-cell proliferative response to antigens secreted by Mycobacterium tuberculosis. Infect Immun. 1991 Apr;59(4):1558–1563. doi: 10.1128/iai.59.4.1558-1563.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes P. F., Bloch A. B., Davidson P. T., Snider D. E., Jr Tuberculosis in patients with human immunodeficiency virus infection. N Engl J Med. 1991 Jun 6;324(23):1644–1650. doi: 10.1056/NEJM199106063242307. [DOI] [PubMed] [Google Scholar]
- Barnes P. F., Mehra V., Hirschfield G. R., Fong S. J., Abou-Zeid C., Rook G. A., Hunter S. W., Brennan P. J., Modlin R. L. Characterization of T cell antigens associated with the cell wall protein-peptidoglycan complex of Mycobacterium tuberculosis. J Immunol. 1989 Oct 15;143(8):2656–2662. [PubMed] [Google Scholar]
- Daniel T. M., Oxtoby M. J., Pinto E., Moreno E. The immune spectrum in patients with pulmonary tuberculosis. Am Rev Respir Dis. 1981 May;123(5):556–559. doi: 10.1164/arrd.1981.123.5.556. [DOI] [PubMed] [Google Scholar]
- De Bruyn J., Huygen K., Bosmans R., Fauville M., Lippens R., Van Vooren J. P., Falmagne P., Weckx M., Wiker H. G., Harboe M. Purification, characterization and identification of a 32 kDa protein antigen of Mycobacterium bovis BCG. Microb Pathog. 1987 May;2(5):351–366. doi: 10.1016/0882-4010(87)90077-5. [DOI] [PubMed] [Google Scholar]
- Donson J., Mandy K., Feng Z. H., Mandy S., Brown E. J., Godfrey H. P. Role of monocyte fucose-receptors in T-cell fibronectin activity. Immunology. 1991 Nov;74(3):473–477. [PMC free article] [PubMed] [Google Scholar]
- Fukui Y., Hirai T., Uchida T., Yoneda M. Extracellular proteins of tubercle bacilli. IV. Alpha and beta antigens as major extracellular protein products and as cellular components of a strain (H37Rv) of Mycobacterium tuberculosis. Biken J. 1965 Dec;8(4):189–199. [PubMed] [Google Scholar]
- George K. L., Falkinham J. O., 3rd Identification of cytoplasmic membrane protein antigens of Mycobacterium avium, M. intracellulare, and M. scrofulaceum. Can J Microbiol. 1989 May;35(5):529–534. doi: 10.1139/m89-084. [DOI] [PubMed] [Google Scholar]
- Godfrey H. P., Canfield L. S., Haak-Frendscho M., Melancon-Kaplan J., Brown E. J., Kaplan A. P. Relationship of human macrophage agglutination factor to other fibronectins. Immunology. 1989 Jul;67(3):321–327. [PMC free article] [PubMed] [Google Scholar]
- Godfrey H. P., Phillips M. E., Askenase P. W. Histopathology of delayed-onset hypersensitivities in contact-sensitive guinea pigs. Int Arch Allergy Appl Immunol. 1983;70(1):50–58. doi: 10.1159/000233273. [DOI] [PubMed] [Google Scholar]
- Godfrey H. P. T cell fibronectin: an unexpected inflammatory lymphokine. Lymphokine Res. 1990 Fall;9(3):435–447. [PubMed] [Google Scholar]
- Harboe M., Wiker H. G., Nagai S. Protein antigens of mycobacteria studied by quantitative immunologic techniques. Clin Infect Dis. 1992 Jan;14(1):313–319. doi: 10.1093/clinids/14.1.313. [DOI] [PubMed] [Google Scholar]
- Havlir D. V., Wallis R. S., Boom W. H., Daniel T. M., Chervenak K., Ellner J. J. Human immune response to Mycobacterium tuberculosis antigens. Infect Immun. 1991 Feb;59(2):665–670. doi: 10.1128/iai.59.2.665-670.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huygen K., Palfliet K., Jurion F., Hilgers J., ten Berg R., Van Vooren J. P., De Bruyn J. H-2-linked control of in vitro gamma interferon production in response to a 32-kilodalton antigen (P32) of Mycobacterium bovis bacillus Calmette-Guérin. Infect Immun. 1988 Dec;56(12):3196–3200. doi: 10.1128/iai.56.12.3196-3200.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huygen K., Van Vooren J. P., Turneer M., Bosmans R., Dierckx P., De Bruyn J. Specific lymphoproliferation, gamma interferon production, and serum immunoglobulin G directed against a purified 32 kDa mycobacterial protein antigen (P32) in patients with active tuberculosis. Scand J Immunol. 1988 Feb;27(2):187–194. doi: 10.1111/j.1365-3083.1988.tb02338.x. [DOI] [PubMed] [Google Scholar]
- Jesty J., Godfrey H. P. Parlin, a general microcomputer program for parallel-line analysis of bioassays. Am J Clin Pathol. 1986 Apr;85(4):485–489. doi: 10.1093/ajcp/85.4.485. [DOI] [PubMed] [Google Scholar]
- Launois P., Huygen K., De Bruyn J., N'Diaye M., Diouf B., Sarthouj L., Grimaud J., Millan J. T cell response to purified filtrate antigen 85 from Mycobacterium bovis Bacilli Calmette-Guérin (BCG) in leprosy patients. Clin Exp Immunol. 1991 Nov;86(2):286–290. doi: 10.1111/j.1365-2249.1991.tb05811.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manca F., Rossi G., Valle M. T., Lantero S., Li Pira G., Fenoglio D., De Bruin J., Costantini M., Damiani G., Balbi B. Limited clonal heterogeneity of antigen-specific T cells localizing in the pleural space during mycobacterial infection. Infect Immun. 1991 Feb;59(2):503–513. doi: 10.1128/iai.59.2.503-513.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagai S., Wiker H. G., Harboe M., Kinomoto M. Isolation and partial characterization of major protein antigens in the culture fluid of Mycobacterium tuberculosis. Infect Immun. 1991 Jan;59(1):372–382. doi: 10.1128/iai.59.1.372-382.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratliff T. L., McGarr J. A., Abou-Zeid C., Rook G. A., Stanford J. L., Aslanzadeh J., Brown E. J. Attachment of mycobacteria to fibronectin-coated surfaces. J Gen Microbiol. 1988 May;134(5):1307–1313. doi: 10.1099/00221287-134-5-1307. [DOI] [PubMed] [Google Scholar]
- Salgame P., Abrams J. S., Clayberger C., Goldstein H., Convit J., Modlin R. L., Bloom B. R. Differing lymphokine profiles of functional subsets of human CD4 and CD8 T cell clones. Science. 1991 Oct 11;254(5029):279–282. doi: 10.1126/science.254.5029.279. [DOI] [PubMed] [Google Scholar]
- Tasaka H., Matsuo Y. Specificity and distribution of alpha antigens of Mycobacterium kansasii and Mycobacterium marinum. Am Rev Respir Dis. 1984 Oct;130(4):647–649. doi: 10.1164/arrd.1984.130.4.647. [DOI] [PubMed] [Google Scholar]
- Thole J. E., Keulen W. J., De Bruyn J., Kolk A. H., Groothuis D. G., Berwald L. G., Tiesjema R. H., van Embden J. D. Characterization, sequence determination, and immunogenicity of a 64-kilodalton protein of Mycobacterium bovis BCG expressed in escherichia coli K-12. Infect Immun. 1987 Jun;55(6):1466–1475. doi: 10.1128/iai.55.6.1466-1475.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toossi Z., Edmonds K. L., Tomford J. W., Ellner J. J. Suppression of purified protein derivative-induced interleukin-2 production by interaction of CD16 (Leu 11 reactive) lymphocytes and adherent mononuclear cells in tuberculosis. J Infect Dis. 1989 Feb;159(2):352–356. doi: 10.1093/infdis/159.2.352. [DOI] [PubMed] [Google Scholar]
- Wallenstein S., Zucker C. L., Fleiss J. L. Some statistical methods useful in circulation research. Circ Res. 1980 Jul;47(1):1–9. doi: 10.1161/01.res.47.1.1. [DOI] [PubMed] [Google Scholar]
- Wiker H. G., Harboe M., Lea T. E. Purification and characterization of two protein antigens from the heterogeneous BCG85 complex in Mycobacterium bovis BCG. Int Arch Allergy Appl Immunol. 1986;81(4):298–306. doi: 10.1159/000234153. [DOI] [PubMed] [Google Scholar]
- Wiker H. G., Harboe M., Nagai S., Bennedsen J. Quantitative and qualitative studies on the major extracellular antigen of Mycobacterium tuberculosis H37Rv and Mycobacterium bovis BCG. Am Rev Respir Dis. 1990 Apr;141(4 Pt 1):830–838. doi: 10.1164/ajrccm/141.4_Pt_1.830. [DOI] [PubMed] [Google Scholar]
- Wiker H. G., Harboe M., Nagai S., Patarroyo M. E., Ramirez C., Cruz N. MPB59, a widely cross-reacting protein of Mycobacterium bovis BCG. Int Arch Allergy Appl Immunol. 1986;81(4):307–314. doi: 10.1159/000234154. [DOI] [PubMed] [Google Scholar]
- Worsaae A., Ljungqvist L., Hasløv K., Heron I., Bennedsen J. Allergenic and blastogenic reactivity of three antigens from Mycobacterium tuberculosis in sensitized guinea pigs. Infect Immun. 1987 Dec;55(12):2922–2927. doi: 10.1128/iai.55.12.2922-2927.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamura M., Uyemura K., Deans R. J., Weinberg K., Rea T. H., Bloom B. R., Modlin R. L. Defining protective responses to pathogens: cytokine profiles in leprosy lesions. Science. 1991 Oct 11;254(5029):277–279. doi: 10.1126/science.254.5029.277. [DOI] [PubMed] [Google Scholar]



