Abstract
A small fraction of the CD8 T cell effector population that responds to an infection progresses through the contraction phase to the memory stage. The factors regulating the extent of contraction are poorly understood. Competition for limited resources has been widely postulated to be the cause of cell death during the contraction phase, but our data show that competition does not affect contraction kinetics. We go on to demonstrate that all effector cells present at the peak of the response have the potential to become bona fide memory cells, thus excluding selection on the basis of functionality. We propose that the fate of a CD8 effector cell is predetermined before the onset of contraction and discuss possible mechanisms of regulation.
Keywords: infection, Listeria monocytogenes, memory, effector, bim
CD8 T cells leave the thymus 4–5 days after undergoing positive selection (1) and become fully mature CD8 T cells shortly after entering the periphery (2). Once a CD8 T cell is activated by antigen recognition in the periphery, it starts to proliferate and differentiate into an effector cell. The CD8 response typically peaks ≈7 days after an infection and is followed by the contraction phase, when 90–95% of the effector cells die in the ensuing days and weeks and the remaining 5–10% become long-lived memory cells (3–6). What cues do CD8 T cells receive that trigger the onset of contraction and regulate the extent of contraction?
The inflammatory environment during the priming phase has been identified as a key factor that influences contraction. A limited amount of inflammation leads to reduced effector proliferation and lack of a contraction phase (7). Mice that lack IL-12 generate a weaker primary response than their WT littermates, but show an increase in CD8 memory cell formation (8). In a setting of inflammation induced by infection, the duration of the initial T cell receptor (TCR) priming stimulus influences the size of the effector population (the longer lasting the signal the bigger the effector pool), but it does not appear to affect the onset or extent of contraction (9). Similarly, the kinetics of pathogen clearance seem to have limited to no impact on the onset or extent of contraction (10, 11). The extent of contraction, i.e., the ratio of the number of cells at the peak of the response to the number at the memory phase, is highly reproducible and comparable within different model systems, yet the mechanisms that control the extent of contraction so tightly are based on speculation.
There are two popular hypotheses that try to explain this phenomenon. The first states that cytokine deprivation caused by having a large population of effector cells competing for limited resources is responsible for cell death during the contraction phase (12–16). Indeed, injecting IL-2 (17) or IL-15 (15) during the contraction phase enhances CD8 T cell survival and limits the extent of contraction. The second hypothesis is based on the observation that the IL7Rαhi KLRG-1lo (memory precursor cell) effector cell subset present at the peak of expansion preferentially survives contraction, whereas the IL7Rαlo KLRG-1hi (terminally differentiated effector cell) subset is more prone to cell death (18). It has been proposed that terminally differentiated effector cells cannot make functional memory cells (“decreasing potential model”) (5) and are subsequently weeded out during the contraction phase. The first hypothesis postulates that the extent of contraction is actively regulated during the contraction phase, whereas the second hypothesis predicts that the fate of the cell is determined before the contraction phase. The two hypotheses are not mutually exclusive as the subset of terminally differentiated cells might be destined to die and cells of the memory precursor subset might compete for limited resources.
Several studies examined the mechanisms causing cell death during contraction using either lymphocytic choriomeningitis virus (LCMV) or herpes simplex virus (HSV) as a model system. These data suggest that cell death during contraction is caspase independent (19) and that it cannot be rescued by overexpression of the prosurvival molecules bcl-2 or bcl-xl in CD8 T cells (20). However, CD8 effector cells that lack the proapoptotic molecule bim (21) are almost completely spared during contraction (22). We established an adoptive cotransfer system exploiting this unique feature of bim−/− CD8 T cells to test the two hypotheses of contraction. Previous studies that examined the parameters that influence the extent of contraction were limited to altering variables like antigen presentation, inflammation, CD8 recruitment, etc., before the contraction phase (7, 10). This makes it impossible to determine what aspects of contraction are controlled in the expansion phase and what aspects are regulated during the contraction phase. Using the bim−/− and WT cell cotransfer system, we overcome this limitation by keeping every parameter constant during the priming phase, leaving the extent of competition between effector cells as the only variable in the contraction phase. This allowed us to directly test the extent to which CD8 T cell contraction is regulated before or after the onset of contraction. Furthermore, we used the system to address whether there is a population of functionally unfit, terminally differentiated effector cells that is eliminated during contraction.
Results
Characterization of Bim−/− CD8 T Cells.
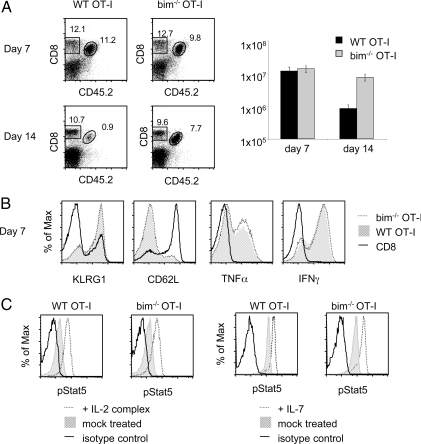
Bim−/− mice display severe signs of autoimmunity and >50% are terminally ill at 1 year of age (23). B cell and T cell numbers are increased in knockout animals (23) and it is clear that the peripheral T cell phenotype is partially the result of impaired thymic negative selection (24). To avoid complications of using potentially autoreactive polyclonal bim−/− CD8 T cells, we bred bim−/− mice to OT-I TCR transgenic mice. Previous experiments in the literature have been done in a bim−/− environment and it was not clear if the lack of CD8 T cell contraction was solely a CD8 T cell intrinsic characteristic (22, 25). To address this, we transferred 104 naïve bim−/− OT-I or WT OT-I T cells into congenic B6 hosts and infected the mice 1 day later with a priming dose of L. monocytogenes secreting ovalbumin (LM-OVA). WT and bim−/− OT-I T cells expanded to the same extent by day 7 following infection (Fig. 1A Top). On day 14, the WT population had undergone contraction as shown by the 10-fold decrease in numbers compared to the day 7 time point. However, bim−/− OT-I T cell numbers hardly changed between day 7 and day 14 (Fig. 1A). We concluded that the lack of contraction is indeed entirely CD8 T cell intrinsic and not caused by autoreactive TCRs or extrinsic stimuli from the bim−/− environment.
Fig. 1.
Comparison of adoptively transferred WT and bim−/− OT-I T cells after immunization. (A) 104 bim−/− or WT OT-I T cells were adoptively transferred into CD45.1 congenic B6 hosts and primed with LM-OVA 1 day later. Spleens were analyzed 7 days and 14 days after priming to determine extent of contraction. The number of splenic OT-I T cells on these days is shown in the histogram. (B) Phenotype (KLRG-1, CD62L) and function (TNFα and IFNγ) of host CD8 (black line), bim−/− OT-I T cells (dotted line) and WT OT-I (transparent gray) effector cells were determined 7 days after infection with LM-OVA. (C) Bim−/− and WT OT-I T cell responsiveness to cytokine stimulation were tested in vivo by IL-2 complex treatment of bim−/− OT-I or WT OT-I mice (Left) and in vitro by determining IL-7 responsiveness (Right) of splenocytes containing memory bim−/− OT-I T cells and WT OT-I T cells.
We also compared the phenotype and function of WT and bim−/− OT-I T cells on day 7 to ensure that bim−/− OT-I T cells are equivalent to WT cells until the onset of the contraction phase. The expression pattern of KLRG-1, CD62L (Fig. 1B) and other phenotypic markers such as IL-7Rα and CD27 (data not shown) is identical between bim−/− and WT effector cells. Production of IFNγ and TNFα by the two types of effector cells was indistinguishable as well (Fig. 1B), showing that bim−/− OT-I T cells are functionally equivalent to WT OT-I T cells. Finally, to ensure that bim−/− OT-I T cells are indeed competitors for cytokines we tested their ability to respond to IL-7 signals in vitro and IL-2 complex stimulation in vivo. We found no difference in the extent of Stat5 phosphorylation between the WT and bim−/− group and conclude that bim−/− OT-I T cells are viable competitors indistinguishable from their WT counterparts in their ability to bind and respond to cytokines (Fig. 1C).
CD8 T Cells Do Not Contract More in the Presence of Competitors.
After establishing that bim−/− OT-I effector cells were valid competitors, we went on to ask if the presence of these noncontracting bim−/− OT-I T cells impacts the fate of WT OT-I T cells during contraction. The experimental setup consisted of transferring 104 WT OT-I T cells into one set of B6 hosts, 104 bim−/− OT-I T cells into another set of hosts, and a mix of 5 × 103 WT OT-I T cells and 5 × 103 bim−/− OT-I T cells into a third set of hosts. The premise of the setup was that all three groups would be identical until day 7 as all three groups received a total of 104 OVA-specific T cells and the same priming dose of LM-OVA. Thus, T cell expansion and pathogen clearance should be identical, providing a scenario where the only variable parameter was the number of effector T cells, i.e., the amount of competition for resources, after the peak of the expansion.
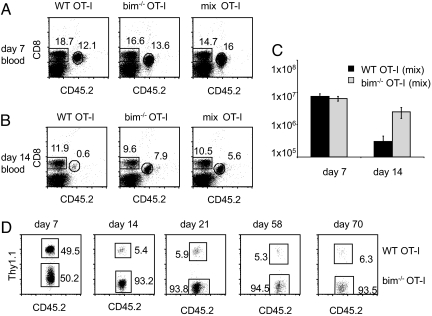
We challenged the three different groups (WT only, bim−/− only, mix) with LM-OVA and bled the mice 7 days later to confirm successful priming of all animals (Fig. 2A). All three groups had equally sized OT-I populations and bim−/− and WT OT-I T cells expanded equivalently in the mix group (Fig. 2 A and D). The mice were bled again on day 14 to determine the extent of contraction (Fig. 2B). The OT-I T cells in the bim−/−-only group showed little contraction, while the WT-only group had 10-fold fewer OT-I T cells by percentage (Fig. 2B). Mice that received a mixture of WT and bim−/− OT-I T cells (mix OT-I group) had significantly fewer OT-I T cells than the bim−/−-only group. More than 90% of the OT-I T cells in the mix group were bim−/− OT-I T cells (Fig. 2D). The percentage of WT OT-I T cells (of the total OT-I T cell pool) on day 14 ranged from 5% to 15% in different experiments (Figs. 2D, 5, 6, and data not shown). We consistently found that the remaining WT cells of the mix group were ≈2-fold lower (by percentage) compared to the WT-only group. Because the WT-only group received twice the number of WT cells transferred originally (and had twice the amount on day 7), this suggested that WT cells in the mix group were unaffected by the presence of a large cohort of competitors. To confirm that survival of effector cells was indeed unaffected by a large number of competitors during contraction, we determined absolute cell numbers in the spleen on days 7 and 14 (Fig. 2C). We found that each population expanded equally well and in proportion to its starting population, i.e., there were twice as many OT-Is present from the bim−/−-only and WT-only groups (that received 104 cells each) compared to the bim−/− and WT population in the mix group (that received 5 × 103 cells of each). Similar to the blood, WT OT-I T cells in the spleen did not contract more in the presence of a large cohort of bim−/− T cells (mix group) than they did in the WT-only group (Figs. 1A and 2C). We continued to follow the OT-I populations in the mice that received a mixture of WT and bim−/− OT-I T cells over a period of 10 weeks and found the ratio of WT to bim−/− cells remained stable (Fig. 2D).
Fig. 2.
WT OT-I T cells contract independently of the extent of competition. 104 WT (Thy1.1+) OT-I T cells (WT only), 104 bim−/− OT-I T cells (bim only) and a mix of 5 × 103 WT (Thy1.1+) and 5 × 103 bim−/− OT-I T cells (mix) were adoptively transferred into CD45.1+ congenic B6 hosts and primed with LM-OVA 1 day later. The percentage of OT-I cells in the blood was determined on days (A) 7 and (B) 14. (C) Mice were killed on day 7 and day 14 to establish OT-I T cell numbers of each group in the spleen. (D) Mice were bled on days 7, 14, 21, 58, and 70 postpriming and the ratio of WT to bim−/− OT-I cells was determined using CD45.2 (to distinguish CD45.1+ host CD8 T cells from CD45.2+ OT-I T cells) and Thy1.1 (to discriminate WT from bim−/− OT-I T cells). Data shown is gated on OT-I T cells.
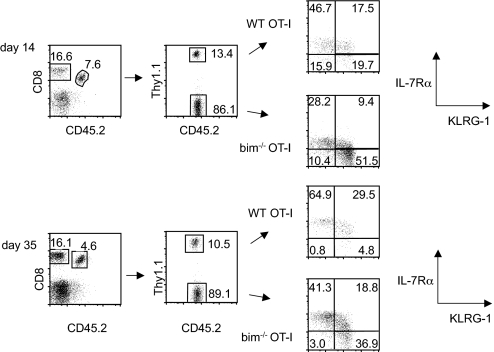
Fig. 5.
Bim−/− OT-I T cells acquire a memory phenotype over time. A mix of 5 × 103 WT (Thy1.1+) and 5 × 103 bim−/− OT-I T cells was adoptively transferred into CD45.1+ congenic B6 hosts and primed with LM-OVA 1 day later. The IL-7Rα and KLRG-1 phenotype of WT and bim−/− cells in the same host was determined on days 14 and 35 postinfection.
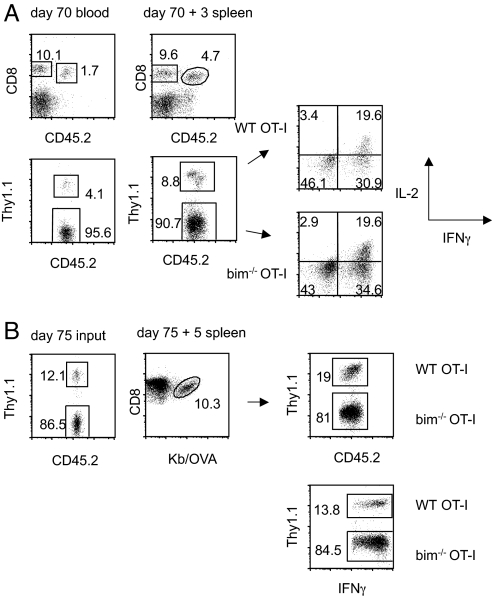
Fig. 6.
All bim−/− OT-I T cells have the ability to make functional memory cells. Mice received a mix of 5 × 103 WT (Thy1.1+) and 5 × 103 bim−/− OT-I T cells and were infected with VSV-OVA 1 day later. (A) Mice were bled on day 70 to assess the WT-to-bim−/− ratio, infected with LM-OVA, and killed 3 days later. The WT-to-bim−/− ratio and ability to make IL-2 and IFNγ were determined. (B) Memory cells (day 75 post-VSV-OVA infection) were sorted and 1 × 104 cells injected (without changing the bim-to-WT ratio) into B6 hosts. Mice were infected with LM-OVA and killed 5 days later. OT-I T cells were identified by tetramer staining or as IFNγ+ cells and bim−/− and WT cells were distinguished using Thy1.1.
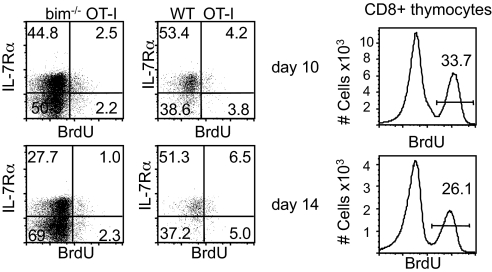
A previous study showed that polyclonal bim−/− and WT effector cells proliferated equally well between days 6 and 7, and on day 20 after priming (22). We wanted to formally exclude the possibility that the lack of bim allowed cells to proliferate during the contraction phase to outnumber the WT cells. We administered two injections of BrdU, once at 24 h and then again 12 h before analysis and found very limited BrdU incorporation in either of the two effector populations both on day 10 and day 14 postimmunization (Fig. 3), thus excluding a proliferative advantage of the bim−/− subset during the contraction phase. Thymocytes were examined as an internal standard for BrdU incorporation.
Fig. 3.
Bim−/− OT-I T cells do not proliferate more than their WT counterparts. A mix of 5 × 103 WT (Thy1.1+) and 5 × 103 bim−/− OT-I T cells (mix) was adoptively transferred into CD45.1+ congenic B6 hosts and primed with LM-OVA 1 day later. One set of mice was injected i.p. with BrdU on day 9, then again 12 h later, and analyzed for BrdU incorporation on day 10. The second set of mice was injected on day 13, then again 12 h later, and analyzed for BrdU incorporation on day 14. CD8+ thymocytes were examined as a positive control for BrdU incorporation (Right).
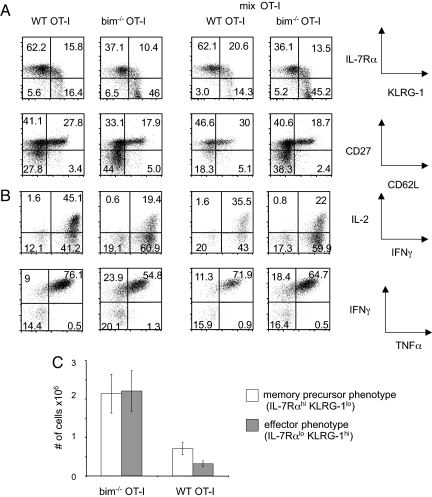
We also compared the phenotype and function of OT-I T cells in all three groups on day 14 after infection, when the contraction phase has mostly concluded. While we did not detect phenotypic or functional differences between WT and bim−/− cells on day 7 (Fig. 1B), differences became apparent by day 14 (Fig. 4A). At this point, the surviving WT OT-I cells consisted of a higher percentage of memory precursors, i.e., WT cells had a higher percentage of CD62L+CD27+ cells and fewer CD62L−CD27− cells (Fig. 4A Lower). Conversely, the bim−/− population had a higher proportion of IL7Rαlo KLRG-1hi terminally differentiated effector cells (>45%), compared to the WT population (18%) (Fig. 4A Upper). Bim−/− cells also had a smaller cohort of IL-2 producers (20% vs. 40% in the WT group), while the percentage of IFNγ producing cells (80%) did not differ (Fig. 4B Upper). The percentage of TNFα, IFNγ double-producing cells was similar between the two groups (Fig. 4B Lower). In absolute numbers the bim−/−-only group not only had more IL7Rαlo KLRG-1hi cells, but also had three times more memory precursor cells (IL-7Rαhi KLRG-1lo cells) than the WT-only group at day 14 postinfection (Fig. 4C). To confirm that bim−/− and WT OT-I T cells display the same characteristics in nonlymphoid tissue, we analyzed the lungs of animals on days 7, 10, 14, and 21 after infection (data not shown). We found that the WT-to-bim−/− ratio in the lung was equivalent to the ratio found in the spleen.
Fig. 4.
WT OT-I T cell function and phenotype are not influenced by competition during contraction. 104 WT (Thy1.1+) OT-I T cells (WT only), 104 bim−/− OT-I T cells (bim only), and a mix of 5 × 103 WT (Thy1.1+) and 5 × 103 bim−/− OT-I T cells (mix) were adoptively transferred into CD45.1+ congenic B6 hosts and primed with LM-OVA 1 day later. (A) Phenotypic and (B) functional analysis of OT-I T cells in all three groups on day 14 postinfection. (C) Number of terminally differentiated effector (IL7Rαlo KLRG-1hi) and memory precursor (IL7Rαhi KLRG-1lo) WT and bim−/− OT-I T cells in the spleen on day 14 postinfection.
To ask whether WT OT-I T cells are affected by the presence of increased competition, we compared phenotype and function of WT OT-I T cells from the WT-only group with WT cells from the mix group, but we did not observe significant differences (Fig. 4 A and B).
All Effector Cells Have the Potential to Become Fully Functional Memory Cells.
It has been speculated that terminally differentiated, IL7Rαlo KLRG-1hi CD8 effector cells are not capable of becoming functional memory cells (26). On day 14, half of the bim−/− OT-I population displays the phenotype of terminally differentiated effectors (Figs. 4A and 5), correlating with a reduction in the proportion of cells with a memory precursor, IL-2 producing phenotype (Fig. 4B). Because the lack of bim rescues terminally differentiated effector cells from death, we wanted to address whether the phenotype of these bim−/− cells was stable. We found that the percentage of IL-7Rαhi KLRG-1lo cells increases in the WT and the bim−/− OT-I population between day 14 and day 35 (Fig. 5), indicating that these terminally differentiated cells were not merely kept alive, but capable of further differentiation.
Because the lack of bim rescues terminally differentiated effector cells from death, we wanted to address whether there was a functional basis for their preferred elimination. To test whether contraction eliminates effector cells incapable of forming a fit memory population, mice that had received a mix of WT and bim−/− OT-I T cells and a priming dose of vesicular stomatitis virus (VSV)-OVA were rechallenged on day 70 with LM-OVA. Three days following the rechallenge, WT and bim−/− cells were equally capable of producing IL-2, IFNγ, and granzyme B (Fig. 6A and data not shown). Also, WT cells expanded more as they increased twofold in percentage after rechallenge (Fig. 6A). We speculated that this difference in expansion might be even more pronounced if memory cells were present at a lower frequency before rechallenge and had more time to expand. To test this, we isolated OT-I memory cells from mice that had received a mixture of WT and bim−/− OT-I T cells and had been primed with VSV-OVA 75 days prior. Ten thousand of these memory cells were transferred to new hosts and challenged with LM-OVA (Fig. 6B). At the time of transfer the OT-I memory cells consisted of a 1-to-7 ratio of WT to bim−/− (Fig. 6B Left). Five days following rechallenge, the overall number of OT-I cells increased dramatically and the ratio shifted to a 1-to-4 ratio (Fig. 6B Right). Regardless of the recall strategy, the WT cells had a slight advantage in their ability to expand and accumulate, but no functional differences were found after the rechallenge (Figs. 5, 6, and data not shown). It appears that effector cells rescued from death during contraction can differentiate into functional memory cells capable of a recall response.
Discussion
A number of recent studies have established that several aspects of peripheral T cell differentiation are programmed, i.e., a T cell is on a differentiation autopilot once it receives the appropriate activating stimuli (27–29). A brief antigenic stimulus in the context of an infection is sufficient to activate a naïve CD8 T cell, initiate and sustain its proliferation and differentiation into an effector cell, and subsequent progression to a memory cell (9). The contraction phase that connects the initial expansion phase with the memory phase has been a black box from which surviving effectors emerge as bona fide long-lived memory cells (3–5). Numerous pieces of evidence suggest that competition for limited resources is a potential cause for the massive CD8 T cell death during the contraction phase. The availability of cytokines regulates T cell homeostasis—an overabundance of cytokines can trigger proliferation and the lack of essential survival cytokines like IL-7 can lead to cell death (30). Indeed, injections of the prosurvival cytokines IL-15 (15) and IL-2 (17) during contraction can rescue effector cells and increase the size of the memory pool. Bim and puma are members of the proapoptotic BH3-only family that regulates survival vs. cell death in the absence of cytokines (31). As such, cells that lack either bim (22, 32) or puma (12, 32) progress in greater numbers to the memory stage. Thus it has been widely proposed that cytokine deprivation causes death during contraction (12–16). However, using a cotransfer system of WT and bim−/− OT-I T cells, we found that competition at a physiologically relevant level does not regulate contraction of CD8 effector cells. We kept the number of effector CD8 T cells at the peak of the response constant, but increased the number of effector T cells more than fivefold during contraction without affecting number, function, or phenotype of the OT-I WT cells (Figs. 2 and 3). While adding exogenous cytokines at the right time can spare cells from death during contraction and might have therapeutic potential, our data clearly show that it is not competition for cytokines or any other limiting resource that determines the extent of contraction of CD8 T cells in a physiological setting. This includes clonotype-specific competition, which has been shown to play a key role in CD4 T cell viability (33). If contraction is not actively regulated by competition, is cell death predetermined for functionally unfit cells that get subsequently weeded out during contraction?
We show that all bim−/− effector cells at the peak of the response can give rise to functional and fit memory cells that are maintained equivalently to their WT counterparts over the course of 10 weeks (Fig. 2D). This is in contrast to a previous study (25), probably because of the facts that our bim−/− memory cells had a defined TCR specificity and were neither autoreactive themselves nor maintained in an autoreactive environment. In agreement with the aforementioned study, we found that the lack of bim results in a higher percentage of IL7Rαlo KLRG-1hi cells at day 14 compared to the WT population (Fig. 4B). This is because IL7Rαlo KLRG-1hi cells contract more than their IL7Rαhi KLRG-1lo counterparts (18), although it is noteworthy that this is a trend rather than a rule as WT IL7Rαhi KLRG-1lo cells contract substantially as well (Fig. 4C). Bim−/− OT-I T cells do acquire a memory phenotype following the contraction phase, suggesting that the large cohort of doomed effector cells is capable of further differentiation (Fig. 5).
The WT-to-bim−/− ratio is stable over time (Fig. 2D), suggesting that the change in the phenotype of bim−/− OT-I cells is not because of a preferential loss of the IL7Rαlo KLRG-1hi bim−/− population nor of an increase in the turnover of bim−/− IL-7Rαhi KLRG-1lo cells, as this would skew the WT-to-bim−/− ratio. However, while the WT-to-bim−/− ratio remained constant, the overall percentage of OT-I T cells in the blood decreased even 30 days after infection (data not shown). This late decrease in memory CD8 T cells, which is equal in WT and bim−/− cells, may be explained by slow elimination of IL7Rαlo KLRG-1hi cells. Further experiments will be required to obtain a definitive answer.
The equivalent functional ability of the IL7Rαlo KLRG-1hi and IL7Rαhi KLRG-1lo subsets is undisputed, but the proliferative potential upon restimulation is controversial (18, 26, 34–37). Our data agree with the notion that IL7Rαlo KLRG-1hi cells are not impaired upon restimulation (Fig. 6). In fact, our data show that both the IL7Rαhi KLRG-1lo cells that preferentially survive contraction and IL7Rαlo KLRG-1hi cells that tend to be eliminated during contraction are indeed functional (Fig. 6). The presence of a large fraction of doomed, nonfunctional bim−/− OT-I T cells would have resulted in a major shift in the ratio of bim−/− to WT cells following recall, which was not observed in recall responses (Fig. 6). This suggests that every single effector T cell present at the peak of the response has the intrinsic ability to become a functional memory cell if allowed to survive contraction.
Our initial experiments demonstrated that contraction is predetermined and not influenced by the number of T cells present during contraction, but there is no specific marker that identifies surviving CD8 T cells before the contraction phase and correlates strictly with their fate during contraction. A recent study demonstrated that the KLRG-1 phenotype of effector cells is determined ≈2 days before the onset of contraction, but a thorough analysis of effector cells during the expansion phase failed to reveal another differentially regulated marker (34). Similarly, microarray characterization of the two KLRG-1 subsets did not give more clues regarding the regulation of life vs. death during contraction (34).
How could contraction be regulated? Epigenetic modifications have been shown to play a key role in CD8 T cell function and differentiation (38). They are key regulators of many biological processes that involve lineage commitment or differentiation. Cell death could be the default pathway during contraction and a combination of epigenetic modifications and transcriptional changes could allow a subset of cells to survive and progress to the memory stage. This could be done by unequal segregation of proteins in the daughter cells after mitosis (39, 40), leading to the generation of a T cell subset that is poised to survive, which could explain how the fate of a cell during contraction can be predetermined and tightly regulated. The notion of asymmetric cell division has been established in developmental processes and has recently been suggested to play a role in CD8 T cell differentiation as well (41).
Experimental Procedures
Mice.
Bim−/− mice (stock no. 004525) and C57BL/6.SJL (stock no. 002014) mice were obtained from The Jackson Laboratory (Bar Harbor, ME) and housed in specific pathogen-free conditions in the animal facilities at the University of Washington (Seattle, WA). OT-I TCR transgenic mice congenic for Thy1.1 were bred and maintained in the same facilities. OT-I TCR transgenic mice were bred to bim−/− mice to generate bim−/− OT-I TCR transgenic mice. C57BL/6.SJL (CD45.1+) host mice were infected at 8–12 weeks of age. Data shown for each experiment are from at least three independent experiments with at least three animals per group. All experiments were done in accordance with the Institutional Animal Care and Use Committee guidelines.
Adoptive Transfer and Cell Sorting.
Naïve CD44low OT-I T cells were isolated from lymph nodes using a CD8 isolation kit (Miltenyi) plus anti-CD44bio (IM-7) as previously described (42). A total number of 1 × 104 OT-I T cells (WT only, bim−/− only, or a 1:1 mix) per recipient was transferred intravenously (i.v.).
Infections.
L. monocytogenes that expresses a secreted form of OVA (LM-OVA) (43) was grown as previously described (44). For primary infections, mice were injected i.v. with 2 × 103 cfu LM-OVA 1 day after adoptive transfer of OT-I T cells. For secondary infections, mice received 2 × 105 cfu LM-OVA and were killed 3–5 days later. For priming with VSV-OVA (45) and vaccinia virus (VAC)-OVA (46) mice were injected i.v. with 1 × 106 pfu and 2 × 106 pfu, respectively.
Flow Cytometry.
Recipient mice were killed at the time points indicated and single-cell suspensions were prepared from the spleen, and in some experiments from lymph nodes and lungs after perfusion of the animal. Cells were typically stained with anti-CD8, anti-CD62L, anti-CD27, anti-KLRG-1, anti-IL-7Rα, anti-Thy1.1, anti-CD45.2 (eBioscience and BD). For intracellular staining, cells were prepared with the Cytofix/Cytoperm kit in the presence of brefeldin A (BD) and stained with anti-IFNγ, IL-2, TNF-α, anti-Thy1.1, anti-CD45.2, and anti-CD8 (eBiosciences and BD). For BrdU incorporation, 1 mg BrdU was injected i.p. 12 and 24 h before harvesting (2 mg total). Cells were stained using anti-BrdU-APC Abs according to manufacturer's protocol (BrdU Flow Kit; BD). Cells were analyzed using a FACSCanto (BD) and analyzed using FLOWJO (TreeStar) software.
Stat5 Phosphorylation.
Mice were treated with IL-2 complex for 1 h and the amount of Stat5 phosphorylation was determined as previously described (47). Briefly, splenocytes were fixed in 1.6% formaldehyde/PBS for 30 min at room temperature, followed by permeabilization with ice-cold methanol. The fixed and permeabilized cells were stained in 2% FCS/PBS with anti-phospho-STAT5 (Y694; Phosflow, BD) mAb. Mouse IgG1 mAb (BDZ) was used as the isotype control. For in vitro experiments, single-cell suspensions of spleens were prepared and resuspended in prewarmed RP10; IL-7 was added to a final concentration of 1 ng/ml and incubated for 30 min at 37 °C before analysis.
Acknowledgments.
We acknowledge B. Dere, B. Paul, and P. Xiao for technical assistance and members of the Bevan lab for discussions. This work was supported by the Howard Hughes Medical Institute and National Institutes of Health Grant AI-19335 (to M.J.B.). M.P. is a fellow of the Leukemia & Lymphoma Society.
Footnotes
The authors declare no conflict of interest.
References
- 1.McCaughtry TM, Wilken MS, Hogquist KA. Thymic emigration revisited. J Exp Med. 2007;204:2513–2520. doi: 10.1084/jem.20070601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boursalian TE, Golob J, Soper DM, Cooper CJ, Fink PJ. Continued maturation of thymic emigrants in the periphery. Nat Immunol. 2004;5:418–425. doi: 10.1038/ni1049. [DOI] [PubMed] [Google Scholar]
- 3.Williams MA, Bevan MJ. Effector and memory CTL differentiation. Annu Rev Immunol. 2007;25:171–192. doi: 10.1146/annurev.immunol.25.022106.141548. [DOI] [PubMed] [Google Scholar]
- 4.Harty JT, Badovinac VP. Shaping and reshaping CD8+ T-cell memory. Nat Rev Immunol. 2008;8:107–119. doi: 10.1038/nri2251. [DOI] [PubMed] [Google Scholar]
- 5.Joshi NS, Kaech SM. Effector CD8 T cell development: A balancing act between memory cell potential and terminal differentiation. J Immunol. 2008;180:1309–1315. doi: 10.4049/jimmunol.180.3.1309. [DOI] [PubMed] [Google Scholar]
- 6.Prlic M, Williams MA, Bevan MJ. Requirements for CD8 T-cell priming, memory generation and maintenance. Curr Opin Immunol. 2007;19:315–319. doi: 10.1016/j.coi.2007.04.010. [DOI] [PubMed] [Google Scholar]
- 7.Badovinac VP, Porter BB, Harty JT. CD8+ T cell contraction is controlled by early inflammation. Nat Immunol. 2004;5:809–817. doi: 10.1038/ni1098. [DOI] [PubMed] [Google Scholar]
- 8.Pearce EL, Shen H. Generation of CD8 T cell memory is regulated by IL-12. J Immunol. 2007;179:2074–2081. doi: 10.4049/jimmunol.179.4.2074. [DOI] [PubMed] [Google Scholar]
- 9.Prlic M, Hernandez-Hoyos G, Bevan MJ. Duration of the initial TCR stimulus controls the magnitude but not functionality of the CD8+ T cell response. J Exp Med. 2006;203:2135–2143. doi: 10.1084/jem.20060928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Badovinac VP, Porter BB, Harty JT. Programmed contraction of CD8(+) T cells after infection. Nat Immunol. 2002;3:619–626. doi: 10.1038/ni804. [DOI] [PubMed] [Google Scholar]
- 11.Porter BB, Harty JT. The onset of CD8+-T-cell contraction is influenced by the peak of Listeria monocytogenes infection and antigen display. Infect Immun. 2006;74:1528–1536. doi: 10.1128/IAI.74.3.1528-1536.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fischer SF, Belz GT, Strasser A. BH3-only protein Puma contributes to death of antigen-specific T cells during shutdown of an immune response to acute viral infection. Proc Natl Acad Sci USA. 2008;105:3035–3040. doi: 10.1073/pnas.0706913105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Strasser A, Pellegrini M. T-lymphocyte death during shutdown of an immune response. Trends Immunol. 2004;25:610–615. doi: 10.1016/j.it.2004.08.012. [DOI] [PubMed] [Google Scholar]
- 14.Vella AT, Dow S, Potter TA, Kappler J, Marrack P. Cytokine-induced survival of activated T cells in vitro and in vivo. Proc Natl Acad Sci USA. 1998;95:3810–3815. doi: 10.1073/pnas.95.7.3810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yajima T, et al. IL-15 regulates CD8+ T cell contraction during primary infection. J Immunol. 2006;176:507–515. doi: 10.4049/jimmunol.176.1.507. [DOI] [PubMed] [Google Scholar]
- 16.Hacker G, Bauer A, Villunger A. Apoptosis in activated T cells: What are the triggers, and what the signal transducers? Cell Cycle. 2006;5:2421–2424. doi: 10.4161/cc.5.21.3397. [DOI] [PubMed] [Google Scholar]
- 17.Blattman JN, et al. Therapeutic use of IL-2 to enhance antiviral T-cell responses in vivo. Nat Med. 2003;9:540–547. doi: 10.1038/nm866. [DOI] [PubMed] [Google Scholar]
- 18.Kaech SM, et al. Selective expression of the interleukin 7 receptor identifies effector CD8 T cells that give rise to long-lived memory cells. Nat Immunol. 2003;4:1191–1198. doi: 10.1038/ni1009. [DOI] [PubMed] [Google Scholar]
- 19.Nussbaum AK, Whitton JL. The contraction phase of virus-specific CD8+ T cells is unaffected by a pan-caspase inhibitor. J Immunol. 2004;173:6611–6618. doi: 10.4049/jimmunol.173.11.6611. [DOI] [PubMed] [Google Scholar]
- 20.Petschner F, et al. Constitutive expression of Bcl-xL or Bcl-2 prevents peptide antigen-induced T cell deletion but does not influence T cell homeostasis after a viral infection. Eur J Immunol. 1998;28:560–569. doi: 10.1002/(SICI)1521-4141(199802)28:02<560::AID-IMMU560>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 21.Marrack P, Kappler J. Control of T cell viability. Annu Rev Immunol. 2004;22:765–787. doi: 10.1146/annurev.immunol.22.012703.104554. [DOI] [PubMed] [Google Scholar]
- 22.Pellegrini M, Belz G, Bouillet P, Strasser A. Shutdown of an acute T cell immune response to viral infection is mediated by the proapoptotic Bcl-2 homology 3-only protein Bim. Proc Natl Acad Sci USA. 2003;100:14175–14180. doi: 10.1073/pnas.2336198100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bouillet P, et al. Proapoptotic Bcl-2 relative Bim required for certain apoptotic responses, leukocyte homeostasis, and to preclude autoimmunity. Science. 1999;286:1735–1738. doi: 10.1126/science.286.5445.1735. [DOI] [PubMed] [Google Scholar]
- 24.Bouillet P, et al. BH3-only Bcl-2 family member Bim is required for apoptosis of autoreactive thymocytes. Nature. 2002;415:922–926. doi: 10.1038/415922a. [DOI] [PubMed] [Google Scholar]
- 25.Wojciechowski S, et al. Bim mediates apoptosis of CD127(lo) effector T cells and limits T cell memory. Eur J Immunol. 2006;36:1694–1706. doi: 10.1002/eji.200635897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Joshi NS, et al. Inflammation directs memory precursor and short-lived effector CD8(+) T cell fates via the graded expression of T-bet transcription factor. Immunity. 2007;27:281–295. doi: 10.1016/j.immuni.2007.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kaech SM, Ahmed R. Memory CD8+ T cell differentiation: Initial antigen encounter triggers a developmental program in naive cells. Nat Immunol. 2001;2:415–422. doi: 10.1038/87720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van Stipdonk MJ, Lemmens EE, Schoenberger SP. Naive CTLs require a single brief period of antigenic stimulation for clonal expansion and differentiation. Nat Immunol. 2001;2:423–429. doi: 10.1038/87730. [DOI] [PubMed] [Google Scholar]
- 29.Bevan MJ, Fink PJ. The CD8 response on autopilot. Nat Immunol. 2001;2:381–382. doi: 10.1038/87676. [DOI] [PubMed] [Google Scholar]
- 30.Jameson SC. Maintaining the norm: T-cell homeostasis. Nat Rev Immunol. 2002;2:547–556. doi: 10.1038/nri853. [DOI] [PubMed] [Google Scholar]
- 31.Pellegrini M, et al. Loss of Bim increases T cell production and function in interleukin 7 receptor-deficient mice. J Exp Med. 2004;200:1189–1195. doi: 10.1084/jem.20041328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bauer A, et al. The NF-kappaB regulator Bcl-3 and the BH3-only proteins Bim and Puma control the death of activated T cells. Proc Natl Acad Sci USA. 2006;103:10979–10984. doi: 10.1073/pnas.0603625103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hataye J, Moon JJ, Khoruts A, Reilly C, Jenkins MK. Naive and memory CD4+ T cell survival controlled by clonal abundance. Science. 2006;312:114–116. doi: 10.1126/science.1124228. [DOI] [PubMed] [Google Scholar]
- 34.Sarkar S, et al. Functional and genomic profiling of effector CD8 T cell subsets with distinct memory fates. J Exp Med. 2008;205:625–640. doi: 10.1084/jem.20071641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Voehringer D, et al. Viral infections induce abundant numbers of senescent CD8 T cells. J Immunol. 2001;167:4838–4843. doi: 10.4049/jimmunol.167.9.4838. [DOI] [PubMed] [Google Scholar]
- 36.Masopust D, Ha SJ, Vezys V, Ahmed R. Stimulation history dictates memory CD8 T cell phenotype: Implications for prime-boost vaccination. J Immunol. 2006;177:831–839. doi: 10.4049/jimmunol.177.2.831. [DOI] [PubMed] [Google Scholar]
- 37.Hikono H, et al. Activation phenotype, rather than central- or effector-memory phenotype, predicts the recall efficacy of memory CD8+ T cells. J Exp Med. 2007;204:1625–1636. doi: 10.1084/jem.20070322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Northrop JK, Thomas RM, Wells AD, Shen H. Epigenetic remodeling of the IL-2 and IFN-gamma loci in memory CD8 T cells is influenced by CD4 T cells. J Immunol. 2006;177:1062–1069. doi: 10.4049/jimmunol.177.2.1062. [DOI] [PubMed] [Google Scholar]
- 39.Morrison SJ, Kimble J. Asymmetric and symmetric stem-cell divisions in development and cancer. Nature. 2006;441:1068–1074. doi: 10.1038/nature04956. [DOI] [PubMed] [Google Scholar]
- 40.Fuentealba LC, Eivers E, Geissert D, Taelman V, De Robertis EM. Asymmetric mitosis: Unequal segregation of proteins destined for degradation. Proc Natl Acad Sci USA. 2008;105:7732–7737. doi: 10.1073/pnas.0803027105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chang JT, et al. Asymmetric T lymphocyte division in the initiation of adaptive immune responses. Science. 2007;315:1687–1691. doi: 10.1126/science.1139393. [DOI] [PubMed] [Google Scholar]
- 42.Prlic M, Blazar BR, Khoruts A, Zell T, Jameson SC. Homeostatic expansion occurs independently of costimulatory signals. J Immunol. 2001;167:5664–5668. doi: 10.4049/jimmunol.167.10.5664. [DOI] [PubMed] [Google Scholar]
- 43.Pope C, et al. Organ-specific regulation of the CD8 T cell response to Listeria monocytogenes infection. J Immunol. 2001;166:3402–3409. doi: 10.4049/jimmunol.166.5.3402. [DOI] [PubMed] [Google Scholar]
- 44.Williams MA, Bevan MJ. Shortening the infectious period does not alter expansion of CD8 T cells but diminishes their capacity to differentiate into memory cells. J Immunol. 2004;173:6694–6702. doi: 10.4049/jimmunol.173.11.6694. [DOI] [PubMed] [Google Scholar]
- 45.Kim SK, et al. Generation of mucosal cytotoxic T cells against soluble protein by tissue-specific environmental and costimulatory signals. Proc Natl Acad Sci USA. 1998;95:10814–10819. doi: 10.1073/pnas.95.18.10814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Prlic M, Gibbs J, Jameson SC. Characteristics of NK cell migration early after vaccinia infection. J Immunol. 2005;175:2152–2157. doi: 10.4049/jimmunol.175.4.2152. [DOI] [PubMed] [Google Scholar]
- 47.Kamimura D, Bevan MJ. Naive CD8+ T cells differentiate into protective memory-like cells after IL-2 anti IL-2 complex treatment in vivo. J Exp Med. 2007;204:1803–1812. doi: 10.1084/jem.20070543. [DOI] [PMC free article] [PubMed] [Google Scholar]