Figure 1.
Nicole Baumgarth
Like the innate antibodies she studies, Nicole Baumgarth acts fast in the face of adversity. When she wasn't allowed to enroll in the African livestock program that she had her heart set on in college, Baumgarth switched to mice. When Reagan was elected, she opted for the post-doc in Australia. When a car hit her bicycle soon after moving to the US for a second post-doc, she didn't head home defeated. Alone in the hospital with a five-part fracture in her left leg, she had no one to call save one former lab mate. Although the injury has left her limping 11 years and two surgeries later, she hasn't missed a step.
Baumgarth came to California by way of Germany, the UK, and Australia. It was in Brisbane that she first got her hands on influenza, a virus of which she admiringly says elicits a nearly perfect immune response. In 1996 she moved to Stanford to work with Leonore Herzenberg on B cell responses to influenza (1). Now an associate professor at the University of California, Davis, Baumgarth finds herself back in a veterinary school environment. There, she takes a nontraditional approach to looking at how the body protects itself from various pathogens that plague society today. Besides influenza, that list includes Lyme disease, malaria, HPV, and HIV (2). She puts B cells above all else academic, focusing on the innate regulation of antibody production in response to infection (3–6).
NOVEMBER, TIME FOR THE FLU SHOT
With flu season upon us, I thought I might solicit a little free advice. Do you, an expert in influenza, get the flu shot? I get the shot now that I have two little kids. Before I had kids, I didn't do it. I thought, “I'm a healthy, middle-aged person, I'm not going to die from the flu.” But now I'm more worried about giving it to my kids.
So you think flu vaccines work? When the vaccine works, it works great. Last year, though, it didn't work because it wasn't perfectly matched [to the prevalent virus strain].
How might your research on influenza help create a better flu vaccine? One focus of my lab is to look at the cells that make broadly reactive antibodies to all sorts of different pathogens in a nonspecific way. We want to see if we can use the properties of these antibodies in a vaccine approach to either increase their numbers or to make them go to the site where they're needed for protection.
Taking advantage of a nonspecific, innate response sounds quite different from most of today's vaccines. What makes these natural antibodies so attractive? At Stanford we found that you need the natural antibodies to be protected from flu. If you don't have them, the virus will replicate faster; it's one of the things that can keep the initial infection in check. When we first looked at natural antibodies in the serum, nothing happened—the serum antibody levels stayed the same before and after the infection. Then when I started my own lab, I thought maybe it was because we had looked in the blood. The [influenza] infection actually occurs in the respiratory tract, not in the blood. So we looked in the respiratory tract, and we saw that the B1 cells accumulate there after infection. The interesting thing is that these cells are no more or less antigen specific than they were before infection…there is no expansion of a specific response, none of the things you associate with an adaptive immune response. We think an innate signal is driving these cells to the site of infection. We are now trying to find out the identity of the innate signal.
How might natural antibodies be used to fight the flu? Nobody has a problem with CD4, CD8, Th17, or Th2, but when people think of the B cell response they think, “Oh, they are just antibodies.” What I would like to convince the world of is that, like T cells, you have different B cell subsets that respond in different ways. B1 cells provide another level of protection. We have all this knowledge about protein-specific antibodies. But if we knew what makes an antibody broadly reactive we might not have to be so specific with vaccines.
If you have an outbreak of something and you don't know what it is, you can't just vaccinate people—what would you vaccinate with? But what if you could stimulate antibody from the innate immune system? Or stimulate B1 cells to migrate to the respiratory tract if you knew there was a respiratory infection? This wouldn't cure the disease and it might not even prevent infection, but it would buy you a few days during which your adaptive immune system could be activated. The fact that there is a B1 cell response to flu infection means we might be able to mimic that response with a vaccine or therapeutic. I'm excited about that.
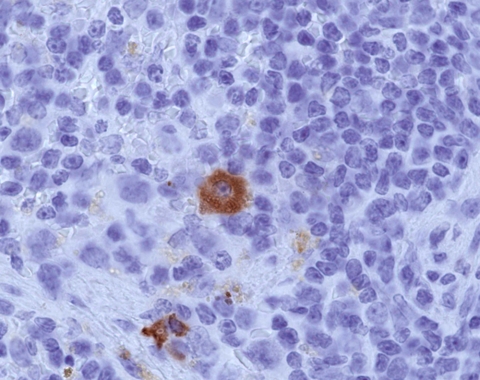
Figure 2.
A plasma B cell (brown) spotted in the lymph node of a mouse produces virus-specific antibodies seven days after influenza infection.
What has to happen before the development of a nonspecific flu vaccine can begin? The big challenge is to find ways to track them [B1 cells], because if you want to make a vaccine that stimulates these cells you need to be able to monitor them. Once we can do that we can ask questions like: Are people less protected from mutated viruses because they don't have enough of the natural antibody? That's so crucial.
AN ORGANISMAL IMMUNOLOGIST
You went to vet school in Germany, but you say you've always wanted to be in research. Has your veterinary background influenced your work today? When I started, there wasn't this focus on the larger context. But my clinical background made me want to look at the whole picture. When you look at what I've done, there is always a focus on the whole pathological process of an infection.
I wish more people would think about the immune system as a whole system. There is an emphasis on mechanisms and molecular analyses, but at the end of the day all the mechanisms work together. I get frustrated when I hear people saying that one arm of the immune system isn't important in this disease and another arm isn't important in that disease; they're all important. They all act together. In a given disease process one might be stronger, but at the end of the day they act together and that [complexity] drives a lot of what I do. Although I study B cells, I'm very aware of dendritic cells and NK cells and other [components of the immune response]. This is what I try to teach my students; it needs to fit together. If you can't make these components fit together then it's probably not going to work like that in vivo.
Is being at UC Davis, known as a bit of a farm-school, refreshing? The reason I'm here is because UC Davis has one of the best vet schools in the country. So when I was applying for jobs it was appealing to get back to where I originally came from. During my vet degree, I was always very frustrated that there was so little emphasis on original research, so I thought I could be a role model for the few students in these programs who want to do what I did. When I joined, UC Davis had just created a center for comparative medicine. The idea was that you'd have a mix of people with MDPhDs, straight PhDs and DVMPhDs that all work together in a center focused on animal models of disease.
What is your favorite molecule and why? Can it be a cell?
Sure. B cells. Nobody may agree with me. I've loved B cells for as long as I can remember. In high school in Germany, biology was one of my majors and there were four topics to choose from. I didn't know what immunology was but I chose it anyway. When I learned about antibody production…there was just something about it, I just loved it. I had to work on T cells for four years, but the moment I could, I switched back to B cells. I don't know why. Bizarre.
References
- 1.Baumgarth, N., et al. 1999. Proc. Natl. Acad. Sci. USA. 96:2250–2255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baumgarth, N., et al. 2004. Am. J. Pathol. 165:707–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baumgarth, N., et al. 2000. J. Exp. Med. 192:271–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baumgarth, N. 2000. Immunol. Rev. 176:171–180. [DOI] [PubMed] [Google Scholar]
- 5.Chang, W.L.W., et al. 2007. J. Immunol. 178:1457–1467. [DOI] [PubMed] [Google Scholar]
- 6.Coro, E.S. et al. 2006. J. Immunol. 176:4343–4351. [DOI] [PubMed] [Google Scholar]