Abstract
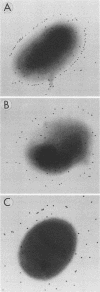
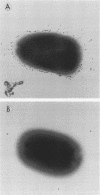
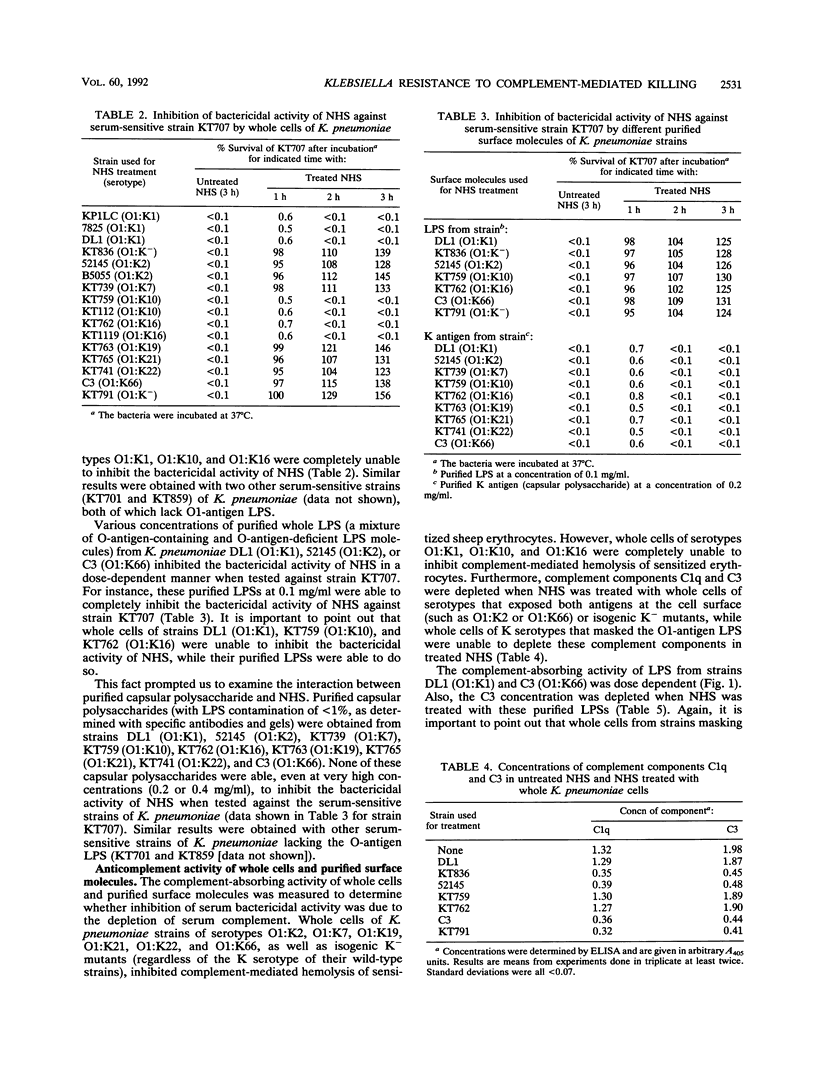
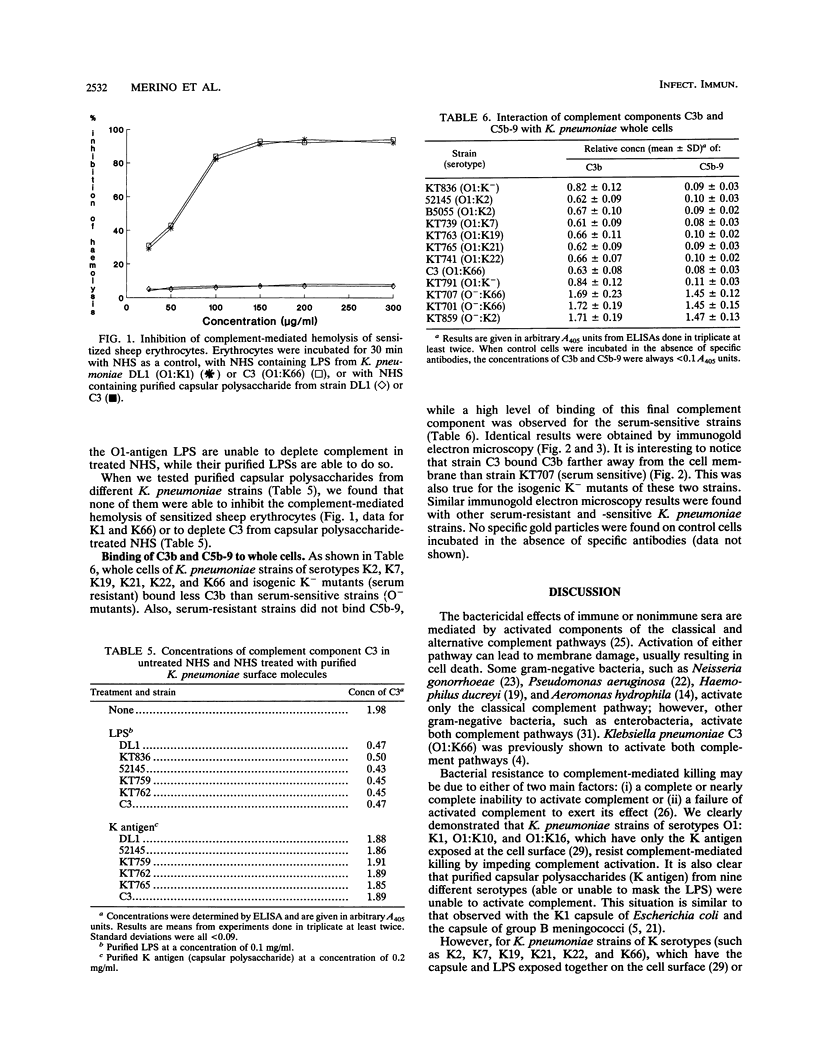
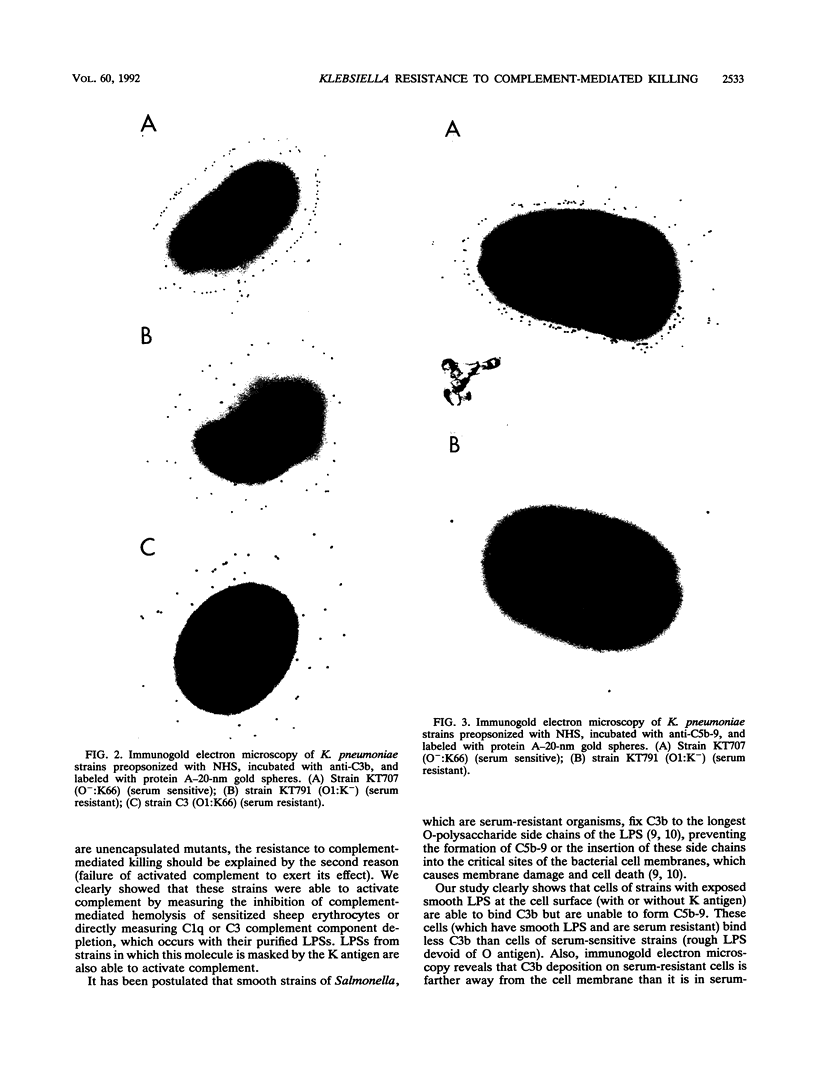
The different mechanisms of Klebsiella pneumoniae resistance to complement-mediated killing were investigated by using different strains and isogenic mutants previously characterized for their surface components. We found that strains from serotypes whose K antigen masks the lipopolysaccharide (LPS) molecules (such as serotypes K1, K10, and K16) fail to activate complement, while strains with smooth LPS exposed at the cell surface (with or without K antigen) activate complement but are resistant to complement-mediated killing. The reasons for this resistance are that C3b binds far from the cell membrane and that the lytic final complex C5b-9 (membrane attack complex) is not formed. Isogenic rough mutants (K+ or K-) are serum sensitive because they bind C3b close to the cell membrane and the lytic complex (C5b-9) is formed.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ames G. F., Spudich E. N., Nikaido H. Protein composition of the outer membrane of Salmonella typhimurium: effect of lipopolysaccharide mutations. J Bacteriol. 1974 Feb;117(2):406–416. doi: 10.1128/jb.117.2.406-416.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedí V. J., Ciurana B., Tomás J. M. Isolation and characterization of Klebsiella pneumoniae unencapsulated mutants. J Clin Microbiol. 1989 Jan;27(1):82–87. doi: 10.1128/jcm.27.1.82-87.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryan C. S., Reynolds K. L., Brenner E. R. Analysis of 1,186 episodes of gram-negative bacteremia in non-university hospitals: the effects of antimicrobial therapy. Rev Infect Dis. 1983 Jul-Aug;5(4):629–638. doi: 10.1093/clinids/5.4.629. [DOI] [PubMed] [Google Scholar]
- Ciurana B., Tomás J. M. Role of lipopolysaccharide and complement in susceptibility of Klebsiella pneumoniae to nonimmune serum. Infect Immun. 1987 Nov;55(11):2741–2746. doi: 10.1128/iai.55.11.2741-2746.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domenico P., Diedrich D. L., Straus D. C. Extracellular polysaccharide production by Klebsiella pneumoniae and its relationship to virulence. Can J Microbiol. 1985 May;31(5):472–478. doi: 10.1139/m85-088. [DOI] [PubMed] [Google Scholar]
- Filip C., Fletcher G., Wulff J. L., Earhart C. F. Solubilization of the cytoplasmic membrane of Escherichia coli by the ionic detergent sodium-lauryl sarcosinate. J Bacteriol. 1973 Sep;115(3):717–722. doi: 10.1128/jb.115.3.717-722.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hildebrandt J. F., Mayer L. W., Wang S. P., Buchanan T. M. Neisseria gonorrhoeae acquire a new principal outer-membrane protein when transformed to resistance to serum bactericidal activity. Infect Immun. 1978 Apr;20(1):267–272. doi: 10.1128/iai.20.1.267-272.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joiner K. A., Brown E. J., Frank M. M. Complement and bacteria: chemistry and biology in host defense. Annu Rev Immunol. 1984;2:461–491. doi: 10.1146/annurev.iy.02.040184.002333. [DOI] [PubMed] [Google Scholar]
- Joiner K. A., Grossman N., Schmetz M., Leive L. C3 binds preferentially to long-chain lipopolysaccharide during alternative pathway activation by Salmonella montevideo. J Immunol. 1986 Jan;136(2):710–715. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Merino S., Camprubí S., Tomás J. M. The role of lipopolysaccharide in complement-killing of Aeromonas hydrophila strains of serotype O:34. J Gen Microbiol. 1991 Jul;137(7):1583–1590. doi: 10.1099/00221287-137-7-1583. [DOI] [PubMed] [Google Scholar]
- Mizuta K., Ohta M., Mori M., Hasegawa T., Nakashima I., Kato N. Virulence for mice of Klebsiella strains belonging to the O1 group: relationship to their capsular (K) types. Infect Immun. 1983 Apr;40(1):56–61. doi: 10.1128/iai.40.1.56-61.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muschel L. H., Larsen L. J. The sensitivity of smooth and rough gram-negative bacteria to the immune bactericidal reaction. Proc Soc Exp Biol Med. 1970 Jan;133(1):345–348. doi: 10.3181/00379727-133-34472. [DOI] [PubMed] [Google Scholar]
- Nassif X., Fournier J. M., Arondel J., Sansonetti P. J. Mucoid phenotype of Klebsiella pneumoniae is a plasmid-encoded virulence factor. Infect Immun. 1989 Feb;57(2):546–552. doi: 10.1128/iai.57.2.546-552.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odumeru J. A., Wiseman G. M., Ronald A. R. Role of lipopolysaccharide and complement in susceptibility of Haemophilus ducreyi to human serum. Infect Immun. 1985 Nov;50(2):495–499. doi: 10.1128/iai.50.2.495-499.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pluschke G., Mayden J., Achtman M., Levine R. P. Role of the capsule and the O antigen in resistance of O18:K1 Escherichia coli to complement-mediated killing. Infect Immun. 1983 Dec;42(3):907–913. doi: 10.1128/iai.42.3.907-913.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiller N. L., Alazard M. J., Borowski R. S. Serum sensitivity of a Pseudomonas aeruginosa mucoid strain. Infect Immun. 1984 Sep;45(3):748–755. doi: 10.1128/iai.45.3.748-755.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafer W. M., Joiner K., Guymon L. F., Cohen M. S., Sparling P. F. Serum sensitivity of Neisseria gonorrhoeae: the role of lipopolysaccharide. J Infect Dis. 1984 Feb;149(2):175–183. doi: 10.1093/infdis/149.2.175. [DOI] [PubMed] [Google Scholar]
- Sutton A., Schneerson R., Kendall-Morris S., Robbins J. B. Differential complement resistance mediates virulence of Haemophilus influenzae type b. Infect Immun. 1982 Jan;35(1):95–104. doi: 10.1128/iai.35.1.95-104.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor P. W. Bactericidal and bacteriolytic activity of serum against gram-negative bacteria. Microbiol Rev. 1983 Mar;47(1):46–83. doi: 10.1128/mr.47.1.46-83.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomas J. M., Camprubi S., Williams P. Surface exposure of the O-antigen in Klebsiella pneumoniae O1:K1 serotype strains. Microb Pathog. 1988 Aug;5(2):141–147. doi: 10.1016/0882-4010(88)90016-2. [DOI] [PubMed] [Google Scholar]
- Tomás J. M., Benedí V. J., Ciurana B., Jofre J. Role of capsule and O antigen in resistance of Klebsiella pneumoniae to serum bactericidal activity. Infect Immun. 1986 Oct;54(1):85–89. doi: 10.1128/iai.54.1.85-89.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomás J. M., Camprubi S., Merino S., Davey M. R., Williams P. Surface exposure of O1 serotype lipopolysaccharide in Klebsiella pneumoniae strains expressing different K antigens. Infect Immun. 1991 Jun;59(6):2006–2011. doi: 10.1128/iai.59.6.2006-2011.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomás J. M., Ciurana B., Benedí V. J., Juarez A. Role of lipopolysaccharide and complement in susceptibility of Escherichia coli and Salmonella typhimurium to non-immune serum. J Gen Microbiol. 1988 Apr;134(4):1009–1016. doi: 10.1099/00221287-134-4-1009. [DOI] [PubMed] [Google Scholar]
- Tsai C. M., Frasch C. E. A sensitive silver stain for detecting lipopolysaccharides in polyacrylamide gels. Anal Biochem. 1982 Jan 1;119(1):115–119. doi: 10.1016/0003-2697(82)90673-x. [DOI] [PubMed] [Google Scholar]