Abstract
This study investigates the extent to which participants with major depression differ from healthy comparison participants in the irregularities in affective information processing, characterized by deficits in facial expression recognition, intensity categorization, and reaction time to identifying emotionally salient and neutral information. Data on diagnoses, symptom severity, and affective information processing using a facial recognition task were collected from 66 participants, male and female between ages 18 and 54 years, grouped by major depressive disorder (N = 37) or healthy nonpsychiatric (N = 29) status. Findings from MANCOVAs revealed that major depression was associated with a significantly longer reaction time to sad facial expressions compared with healthy status. Also, depressed participants demonstrated a negative bias towards interpreting neutral facial expressions as sad significantly more often than healthy participants. In turn, healthy participants interpreted neutral faces as happy significantly more often than depressed participants. No group differences were observed for facial expression recognition and intensity categorization. The observed effects suggest that depression has significant effects on the perceptivity of the intensity of negative affective stimuli, delayed speed of processing sad affective information, and biases towards interpreting neutral faces as sad.
Keywords: affective information processing, facial, mood disorders
1. INTRODUCTION
Incorrect or poorly timed processing of affective information is an important factor in determining how individuals perceive information and respond to environmental demands (Gross, 1998). Such irregularities of affective information processing are associated with a disrupted capacity for functioning, characteristic of mood disorders (Harmer et al., 2002; Phillips et al., 2003b; Rubinow & Post, 1992). Studies have examined how irregularities in affective information processing, characterized by deficits in facial expression recognition, intensity categorization, and reaction time to identifying emotionally salient and neutral information, are elicited or inhibited as a function of depression. Although certain studies indicate that adults diagnosed with DSM-based major depressive disorder (MDD) show significant misrecognition of the emotion of sadness, happiness, and anger compared to healthy controls, as well as an increased sensitivity to depression-related stimuli (sad words, sad faces) (Feinberg et al., 1986; Gur et al., 1992; Mendelwicz et al., 2005; Mikhailova et al., 1996; Persad and Polivy, 1993; Surguladze et al., 2004; Weniger et al., 2004), other studies fail to show this association (Bradley et al., 1997; Gotlib et al., 2004). With regards to standardized neutral stimuli, adults with MDD show affective information processing irregularities (Leppanen, 2004;Hale, 1998) observed in the form of preferential processing of neutral stimuli as negative (Gur et al., 1992). Collectively, this work suggests that depression symptoms facilitate the processing of negative affective information, whereby a stronger affective-motivational mood state (e.g., reflected by symptoms of depression) would increase attention to negative information, thereby facilitating processing of mood-congruent (negative) stimuli. Other results indicate that, compared with healthy controls, high severity of depressive symptoms is associated with lower ratings of perceived intensity of affective stimuli (Hale, 1998; Gur et al., 1992) and longer reaction times to identify affective stimuli (Leppanen et al., 2004). This suggests that depression may induce perception of a configuration of negative cues that contribute to withdrawal or avoidance of stimuli, thereby producing more errors in emotion recognition and longer reaction times to identify affective data. In the absence of psychopathology, emotionally salient cues may encourage approach patterns or regulated attention, thereby promoting accurate and quick recognition. The extent to which biased affective information processes are modified by affective symptoms has yet to be investigated fully (Pine et al., 2004), though it is likely that these inconsistent findings may be due, in part, to the types of affective stimuli used to evoke affective responses, the different populations examined, and the ubiquitous use of psychotropic medications among depressed research participants.
We examined the premise that major depression, an amotivational state reflecting negativism and passivity, will be associated with irregularities in affective information processing, characterized by deficits in facial expression recognition, intensity categorization, and reaction time to identifying emotionally salient and neutral information. We compared a sample of unmedicated adults reporting moderate to severe major depression on clinical and self-report measures, with a healthy (no psychiatric disorders) control group. Affective information processing was measured using a validated computer-administered task of facial expressions. We predicted that depressed participants would identify the stimuli representing sad and ‘harsh’ expressions (i.e., combined ratings from the angry, disgust, fear facial expressions) with less accuracy and less intensity compared with healthy participants. Also, we predicted that depressed participants would have a slower reaction time in recognizing negative affective faces compared to healthy participants. Finally, with regards to neutral facial expressions, we hypothesized that depressed participants would indicate a bias toward interpreting neutral facial expressions as sad and harsh more often than healthy participants.
2. Methods
Participants
We used printed advertisements to publicize the study at The University of Chicago campus, its medical center, and surrounding neighborhoods. We recruited and compared two groups: depressed participants (n = 37; 19 male, 18 female) were diagnosed with current DSM-IV unipolar major depression using the Diagnostic and Statistical Manual of Mental Disorders (4th ed.; DSM-IV; American Psychiatric Association, 1994), Beck Depression Inventory scores of at least 20 (Beck et al., 1996), and Hamilton Rating Scale for Depression (HRSD) scores of at least 14 (Hamilton, 1967). Healthy participants (n = 29; 15 male, 14 female) had no lifetime or current diagnosis of DSM-IV Axis I and Axis II conditions with BDI-II scores less than 5. All participants, ages 18 to 54 years, passed an on-site urine toxicology screen. Exclusion criteria were diagnoses of lifetime or current bipolar I or II disorders, schizophrenia, delusional disorder, organic brain disorder, primary diagnosis of another Axis I or II disorder; substance abuse or dependence in the last six months; antisocial, borderline, or schizotypal personality disorder; imminent suicide risk; current use of medications; general medical condition associated with depression (e.g., hypothyroidism); and pregnancy.
Procedure
Prospective volunteers contacted the Project Coordinator, who obtained verbal consent to conduct an eligibility screen by phone (e.g., BDI-II and review of exclusionary criteria). Upon arrival at the laboratory, volunteers signed informed consent forms. Eligible participants completed the Beck Depression Inventory-II, followed with clinical diagnostic interviews (e.g., Hamilton Rating Scale for Depression, Structured Clinical Interview for DSM-IV Axis I Disorders, and Structured Interview for DSM-IV Personality Disorders), and the computer-administered affective information processing task.
Measures
Structured Clinical Interview for the DSM-IV
Outpatient Version (SCID-I; First et al., 1997) is a semi-structured interview reviewing lifetime and current DSM-IV Axis I psychiatric disorders, assisting with the determination of inclusion or exclusion criteria and psychiatric diagnoses. Comprised of modules with questions carefully designed to map onto each specific DSM-IV diagnostic criterion, the SCID also collects data on the age of onset of each depressive episode, the number and duration of each major depressive episode, symptom profile, severity, and the number and duration of full remission intervals. Psychometric properties of the 1997 version of the SCID are documented (α= .90; Segal et al., 1994)
Structured Interview for DSM-IV Personality Disorders
(Pfohl et al., 1996) is a clinical interview that measures Axis II domains of functioning (work style, close relationships, social relations, emotions, self-perception) to measure consistent patterns of behavior and cognition.
Beck Depression Inventory-II
(BDI-II; Beck et al., 1996) is a 21-item self-report measure designed to evaluate the severity of depressive symptoms in the past two weeks. The BDI-II has demonstrated internal consistency, test-retest reliability, construct validity, and factorial validity.
Hamilton Rating Scale for Depression
(HDRS;Hamilton, 1967) is a 17-item clinician-rated measure of depression symptoms experienced in the past week. Interrater reliabilities are .84 or higher (Heglund and View, 1979) and Cronbach’s alpha coefficient for internal consistency is between 0.80 and 0.83 (Whisman et al., 1989).
Pictures of Facial Affect
(Ekman & Friesen, 1976) is a computer-administered task designed to measure perceptual processing of facial emotions using standardized affectively evocative photographs of facial expressions. Participants were presented with 110 adult facial pictures [i.e., angry (17), disgust (15), fear (15), happy (18), neutral (14), sad (17), surprise (14)] on a computer screen, and asked to identify the facial expression displayed, each for 3000 milliseconds (msec), and intensity of the expression as quickly and accurately as possible. Then the facial pictures were replaced by a picture of a keypad, and participants were asked to press an appropriate response key to identify the emotion displayed by the face. Six different options reflecting emotions (i.e., anger, disgust, fear, happiness, sadness, surprise) were given. With the neutral faces, participants were instructed to assign an emotion to an otherwise neutral facial expression to evaluate the bias towards positive or negative processing. In this paradigm, our aim was to generate a forced-choice condition, where the participants have to label neutral faces as emotional. In this regard, we use a paradigm that tests the degree to which depression, a negative mood state, elicits or influences affective information processing where there is no correct choice. In this regard, we expect that depressed individuals will engage in more careful and slower information processing that is designed to focus on forced-choice responses (Clore et al., 1994). After the participants made a response, another picture of a keypad with intensity keys was presented on the computer screen, and participants were asked to rate the intensity of the expression by pressing an appropriate response key. In keeping with the recent facial affect research, the angry, disgust, and fearful faces were combined to create a new category, ‘harsh’ faces (Stein et al., 2002). Thus, we computed the proportion of correctly identified harsh faces by computing the average correct identification of angry, disgust, and fearful faces. Likewise, the intensity rating for harsh faces was computed by averaging the intensity ratings for angry, disgust, and fearful faces. The reaction time (RT, in ms) for harsh faces was computed by averaging the speed to identify the angry, disgust, and fearful faces.
Analyses
All analyses were conducted 2-tailed at the 0.05 level of significance. For the primary analyses, Multivariate Analyses of Covariance (MANCOVAs) were performed with one between-subject factor, group (depressed vs. healthy) and four dependent variables representing the four emotions observed (or perceived, in neutral trials): harsh, sad, happy, and surprise. Subsequent univariate analyses were conducted for each dependent variable. Effect sizes are provided for primary univariate analyses using partial eta squared (ήp2). For ήp2, 0.01, 0.06, and 0.14 are considered small, medium, and large effect sizes, respectively (Cohen, 1988).
3. RESULTS
Table 1 shows demographic and clinical characteristics of the two groups, comprised of 34 men and 32 (48.5%) women, between ages 021 and 54 years (overall age M = 35.09y; SD = 9.32). In the depressed sample, BDI-II scores ranged from 20 to 61 and HRSD-17 scores ranged from 12 to 33. With regards to demographic data, a one-way ANOVA found no significant group difference of participant age [F (1, 64) = 2.76, P > .10]. Comparisons using chi-square analyses of the groups revealed no differences for gender, number of children, current employment status, marital status (all P > .10), and education (P = .09). Group differences were observed for race, χ2 (2, 66) = 20.55, P < .001. Follow-up single degree of freedom chi-square analyses identified that healthy participants had a significantly greater proportion of Caucasians to African-Americans compared to depressed participants (P < .001). Race, therefore, was included as a covariate in our analyses.
Table 1.
Demographic and Clinical Characteristics of the Sample (N=66)
| Control N = 29 |
Depressed N = 37 |
|||
|---|---|---|---|---|
| N | Mean (SD) | N | Mean (SD) | |
| Gender | ||||
| Female | 14 | 18 | ||
| Male | 15 | 19 | ||
| Age (y) | 32.97 (9.67) | 36.80 (8.82) | ||
| Ethnicity | ||||
| Caucasian | 23 | 10 | ||
| African-American | 2 | 21 | ||
| Other | 4 | 6 | ||
| Years of Education | ||||
| Part or Graduate HS | 3 | 8 | ||
| Partial College | 4 | 12 | ||
| College Graduate | 21 | 14 | ||
| Professional Graduate | 1 | 2 | ||
| Marital Status | ||||
| Married | 3 | 6 | ||
| Divorced/Separated/Wido | 3 | 7 | ||
| wed | ||||
| Never Married | 20 | 24 | ||
| Number of Children | 0.25 (0.59) | 1.29 (1.67) | ||
| BDI-II score | N/A | 32.70 (8.65) | ||
| HRSD-17 score | N/A | 20.70 (5.14) | ||
| Age of Onset of First MDD | N/A | 26.50 (11.70) | ||
| Length of Current MDE(mo) | N/A | 150.60 | ||
| (206.80) | ||||
| Lifetime MDD Episodes | N/A | 2.22 (1.29) | ||
| GAF Score | 84.10 (5.81) | 52.60 (6.88) |
HRSD-17 = Hamilton Depression Rating Scale score. BDI-II = Beck Depression Inventory, Second Edition. GAF Score = Global Assessment of Functioning Score. Age of Onset of First MDD = Age of onset of first episode of Major Depressive Disorder.
Depression Status and Affective Stimuli
A one-way MANCOVA on the number of accurately identified harsh, sad, happy, and surprised facial expressions revealed a non-significant multivariate effect of group, Wilks’ F (4, 59) = 0.13, P = .971. Subsequent univariate analyses also did not reveal significant effects of group for happy [F (1, 66) = 0.02, P = .898, ήp2 = .0003], sad [F (1, 66) = 0.04, P = .851, ήp2 = .001], surprised [F (1, 66) = 0.14, P = .713, ήp2 = .002], and harsh faces [F (1, 66) = 0.19, P = .666, ήp2 = .003].
Depression Status and Affective Intensity
A one-way MANCOVA on the perceived affective intensity of harsh, sad, happy, and surprised facial expressions revealed a non-significant multivariate effect of group, Wilks F (4, 59) = 1.07, P =.381. Subsequent univariate analyses revealed a non-significant trend of group on ratings of affective intensity for sad [F (1, 66) = 3.19, P = .079, ήp2 = .049] and harsh faces [F (1, 66) = 3.48, P = .067, ήp2 = .053]. Depressed participants rated stimuli reflecting sad (M = 3.73, SD = 0.13) and harsh facial expressions (M = 4.26, SD = 0.13) more intensely than healthy participants (Sad: M = 3.57, SD = 0.15; harsh: M = 4.11, SD = 0.15). No significant effects of group were indicated for happy [F (1, 66) = 0.25, P = .616, ήp2 = .004] and surprised faces [F (1, 66) = 1.36, P = .248, ήp2 = .021].
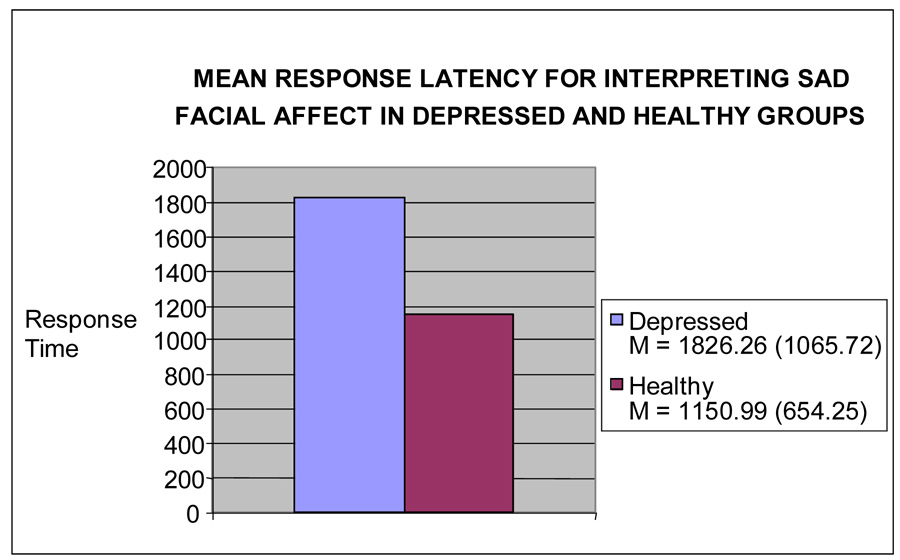
Depression Status and Response Latency
A one-way MANCOVA on the response latency to identifying harsh, sad, happy, and surprised facial expressions revealed a significant multivariate effect of group, Wilks F (4, 59) = 2.66, P < .05. Subsequent univariate analyses revealed a significant effect of group for response latency when interpreting sad faces [F (1, 66) = 10.61, P < .01, ήp2 = .15] (See Figure 1). Depressed participants showed a significantly longer reaction time (msec) to identify sad faces (M = 1922.40 msec, SD = 155.67) than healthy participants (M = 1249.70 msec, SD = 179.66). A non-significant trend was observed for surprised faces [F (1, 66) = 2.92, P = .093, ήp2 =.05] with depressed participants (M = 1629.68 msec, SD = 129.34) slower than healthy participants (M = 1393.62 msec, SD = 149.28) for surprised faces. No significant effects of group were revealed for response latency when interpreting happy [F (1, 66) = 0.17, P = .678, ήp2 = .003] or harsh faces [F (1, 66) = 1.98, P = .165, ήp2 = .031].
Figure 1.
Mean Response Latency for Interpreting Sad Facial Affect.
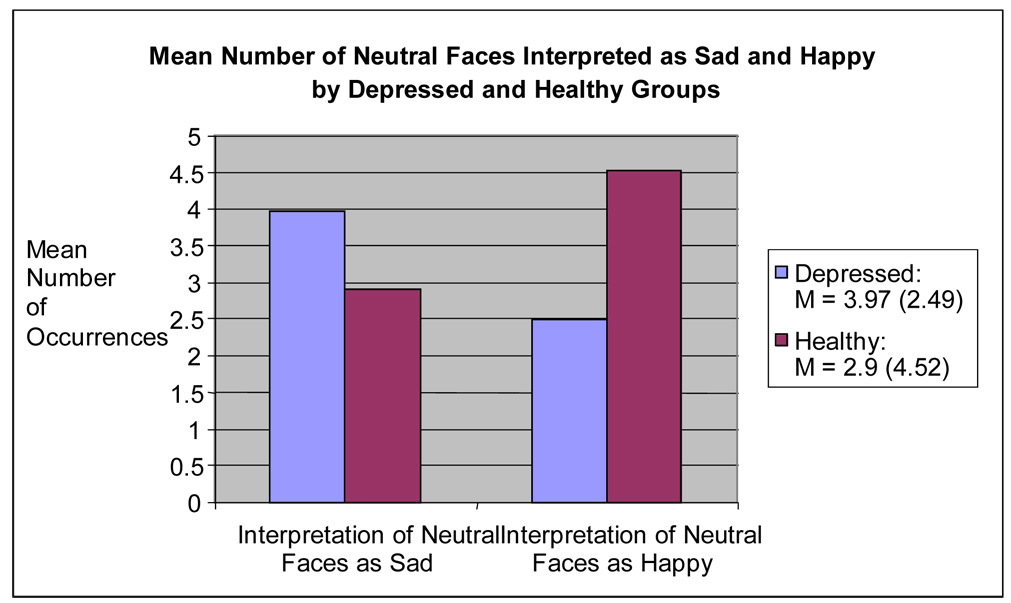
Depressive Status and Neutral Stimuli
A one-way MANCOVA on the number of neutral stimuli identified as harsh, sad, happy, and surprised revealed a non-significant multivariate effect of group, Wilks’ F (3, 60) = 2.10, P = .11. Subsequent univariate analyses revealed a significant effect of group for number of neutral faces identified as happy [F (1, 66) = 5.72, P < .05, ήp2 = .084] and sad [F (1, 66) = 4.29, P < .05, ήp2 = .065] (See Figure 2). Depressed participants identified more neutral faces as sad compared to healthy participants, while healthy participants identified more neutral faces as happy compared to depressed participants. No significant effects of group were revealed for neutral faces identified as surprised [F (1, 66) = 0.03, P = .854, ήp2 = .001] and harsh [F (1, 66) = 0.45, P = .506, ήp2 = .007].
Figure 2.
Mean Number of Neutral Faces Interpreted as Sad and Happy.
4. Discussion
This study reveals findings that illuminate how depressed participants differ from healthy participants with regards to affective information processing of emotional and neutral stimuli. We found that depressed participants were significantly slower to identify sad facial expressions compared to healthy participants. This finding supports earlier studies indicating that depressed patients show slower reaction times to processing affective information (Leppanen et al., 2004), particularly when the data is negatively-valenced (Baumeister et al., 2001). This finding is somewhat more complicated, however, when juxtaposed with existing cognitive research. Data indicates that individuals assign attentional priority to negative data where it is detected more quickly and accurately compared with neutral and positive stimuli (Baumeister et al., 2001). Additional research suggests that the differentiation of positive and negative stimuli occurs very quickly (< 120 msec), and the process of negativity bias appears to focus attention among healthy controls (Smith, Cacioppo, Larsen, & Chartrand, 2003). Taken together with our findings, it is possible that depressed individuals may make spontaneous quick inferences about positive faces, but engage in more complex and longer thinking about sad faces (Krull & Dill, 1998). Earlier research indicates that sad stimuli is commonly perceived as ‘negative’ information and it may produce long affective information processing times when the stimuli cannot be avoided (Baumeister & Cairns, 1992). Though we did not test for attention to stimuli during this task, which would reveal if avoidance is operative, this finding offers initial support for greater processing time required by depressed individuals to attend and interpret ambiguous negative data.
With regards to the interpretation of neutral facial expressions, we found that depressed participants exhibited a negative processing bias towards identifying neutral faces as sad compared with healthy participants. This is consistent with earlier research that indicates that depressed individuals are more likely to interpret neutral faces negatively (Gur et al., 1992; Leppanen et al., 2004). From an evolutionary perspective, perceptivity to potentially negative data in the environment is viewed as an adaptive function whereby an overinterpretation of negative cues serves a protective function (Pryce et al., 2005). In our data, however, depressed participants showed a heightened sensitivity of neutral data as negative. This finding may be taken as providing initial support for the processes of down-regulation of affect that may be occurring in depression, whereby the propensity to engage in an negativity bias operates inaccurately (Weary & Edwards, 1994). Also, our healthy participants identified more neutral faces as happy compared to depressed participants. This finding is consistent with research indicating a mood-congruency effect whereby nondepressed individuals are oriented towards positive information in neutral stimuli (Baumeister et al., 2001).
With regards to intensity ratings, we noted a trend in which depressed participants were more inclined to perceive sad and harsh faces as more intense than healthy participants, albeit no group differences emerged on ratings of affective intensity across disparate emotions. More research is needed to specify if the intensity ratings are due to increased attention to negative affective stimuli or distorted cognitive bias, though this trend is consistent with earlier research that suggests that depression influences perceptual processing of negative affective stimuli (Gotlib et al., 2004). Finally, we found no differences between groups in their identification of positive and negatively-valenced facial expressions, using a task that controlled for salience and type of expression. We interpret this as good news suggesting that our depressed participants, most of whom reported moderate to severe symptoms and significant global impairment of functioning, showed fundamentally no more or less errors in recognition of emotional stimuli than healthy participants. Further research in this area; however, is clearly needed. For example, it is possible that the use of a morphed facial expression paradigm, which displays emotional intensities at varying intensities, may function to generate a nuanced understanding of the variations in affective information processing between depressed and healthy individuals. Also, it would be important for future research to include prospectively designed studies with remitted and recovered depressed groups to surpass limitations that arise from comparing groups of currently depressed and healthy individuals. Such work may be useful in clarifying if these irregularities resolve with time and treatment, and potentially serve as predictors of depressive relapse.
ACKNOWLEDGEMENTS
This work was supported by grants from the American Foundation of Suicide Prevention (AFSP; J.G.) and The University of Chicago Brain Research Foundation (UC BRF; J.G., E.F.C.),National Institutes of Health MH-069764 (M.Mc.). The AFSP, UC BRF, and NIH had no further role in the study design; in the collection, analyses and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th ed. Washington, D.C.: American Psychiatric Association; 1994. [Google Scholar]
- Baumeister RF, Bratslavsky E, Finkenauer C, Vohs KD. Bad is stronger than good. Review of General Psychology. 2001;5:323–370. [Google Scholar]
- Beck AT, Steer RA, Brown GK. Manual for the BDI-II. San Antonio, TX: The Psychological Corporation; 1996. [Google Scholar]
- Bradley BP, Mogg K, Lee SC. Attentional biases for negative information in induced and naturally occurring dysphoria. Behaviour Research and Therapy. 1997;35:911–927. doi: 10.1016/s0005-7967(97)00053-3. [DOI] [PubMed] [Google Scholar]
- Bradley BP, Mogg K, Millar N, Bonham-Carter C, Fergusson E, Jenkins J, Parr M. Attentional biases for emotional faces. Cognition and Emotion. 1997;11:25–42. [Google Scholar]
- Clore GL, Schwartz N, Conway M. Affective causes and consequences of social information processing. In: Wyer R, Srull T, editors. Handbook of social cognition. 2nd ed. Hillsdale NJ: Erlbaum; 1994. pp. 323–417. [Google Scholar]
- Ekman P, Friesen WV. Pictures of facial affect. Palo Alto, CA: Consulting Psychologists Press; 1976. [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JB. Biometrics Research Department. New York: New York State Psychiatric Institute; 1996. Structured Clinical Interview for DSM-IV Axis I Disorders-Patient Edition (SCID-I/P, Version 2.0) [Google Scholar]
- Gotlib IH, Krasnoperova E, Yue DN, Joormann J. Attentional biases for negative interpersonal stimuli in clinical depression. Journal of Abnormal Psychology. 2004;113:127–135. doi: 10.1037/0021-843X.113.1.121. [DOI] [PubMed] [Google Scholar]
- Gross JJ. The emerging field of emotion regulation: An integrative review. Review of General Psychology. 1998;2:271–299. [Google Scholar]
- Gur RC, Erwin RJ, Gur RE, Zwil AS. Facial emotion discrimination: II. Behavioral findings in depression. Psychiatry Research. 1992;42:241–251. doi: 10.1016/0165-1781(92)90116-k. [DOI] [PubMed] [Google Scholar]
- Hamilton M. Development of a rating scale for primary depressive illness. British Journal of Social and Clinical Psychology. 1967;6:278–296. doi: 10.1111/j.2044-8260.1967.tb00530.x. [DOI] [PubMed] [Google Scholar]
- Harmer C, Grayson L, Goodwin G. Enhanced recognition of disgust in bipolar illness. Society of Biological Psychiatry. 2002;51:298–304. doi: 10.1016/s0006-3223(01)01249-5. [DOI] [PubMed] [Google Scholar]
- Krull DS, Dill JC. Do smiles elicit more inferences than do frowns? The effect of emotional valence on the production of spontaneous inferences. Personality and Social Psychology Bulletin. 1998;24:289–300. [Google Scholar]
- Leppanen JM, Milders M, Bell JS, Terriere E, Hietanen JK. Depression biases the recognition of emotionally neutral faces. Psychiatry Research. 2004;128:123–133. doi: 10.1016/j.psychres.2004.05.020. [DOI] [PubMed] [Google Scholar]
- Mikhailova ES, Vladimirova TV, Iznak AF, Tsusulkovskaya EJ, Sushko NV. Abnormal recognition of facial expression of emotions in depressed patients with major depression disorder and schizotypal personality disorder. Biological Psychiatry. 1996;40:697–705. doi: 10.1016/0006-3223(96)00032-7. [DOI] [PubMed] [Google Scholar]
- Pfohl B, Blum N, Zimmerman M. Structured Interview for DSM-IV Personality: SIDP-IV. Washington, DC: American Psychiatric Press; 1997. [Google Scholar]
- Pryce CR, Ruedi-Bettschen D, Dettling AC, Weston A, Russig H, Ferger B, Feldon J. Long-term effects of early-life environmental manipulations in rodents and primates: Potential animal models in depression research. Neuroscience and Biobehavioral Reviews. 2005;29:649–674. doi: 10.1016/j.neubiorev.2005.03.011. [DOI] [PubMed] [Google Scholar]
- Rubinow DR, Post RM. Impaired recognition of affect in facial expression in depressed patients. Biological Psychiatry. 1992;31:947–953. doi: 10.1016/0006-3223(92)90120-o. [DOI] [PubMed] [Google Scholar]
- Smith NK, Cacioppo JT, Larsen JT, Chartrand TL. May I have your attention please: Electrocortical responses to positive and negative stimuli. Neuropsychologia. 2003;41:171–183. doi: 10.1016/s0028-3932(02)00147-1. [DOI] [PubMed] [Google Scholar]
- Stein MB, Goldin PR, Sareen J, Zorrilla LTE, Brown GG. Increased amygdala activation to angry and contemptuous faces in generalized social phobia. Archives of General Psychiatry. 2002;59:1027–1034. doi: 10.1001/archpsyc.59.11.1027. [DOI] [PubMed] [Google Scholar]
- Surguladze SA, Young AW, Senior C, Brebion G, Travis MJ, Phillips ML. Recognition accuracy and response bias to happy and sad facial expressions in patients with major depression. Neuropsychology. 2004;18:212–218. doi: 10.1037/0894-4105.18.2.212. [DOI] [PubMed] [Google Scholar]
- Weniger G, Lange C, Ruther E, Irle E. Differential impairments of facial affect recognition in schizophrenia subtypes and major depression. Psychiatry Research. 2004;128:135–146. doi: 10.1016/j.psychres.2003.12.027. [DOI] [PubMed] [Google Scholar]