Abstract
During central nervous system (CNS) development, cell migration precedes and is key to the integration of diverse sets of cells. Mechanistically, CNS histogenesis is realized through a balanced interplay of cell-cell and cell-matrix adhesion molecules. Here, we summarize experiments that probe the developmental expression and potential significance of a set of cadherins, including M-, N and R-cadherin, for patterning of the cerebellar cortex. We established a transgenic marker that allows cerebellar granule cells to be followed from the neuroblast stage to their final, postmitotic settlement. In conjunction with flow-cytometry, this allowed us to derive a quantitative view of cadherin expression in differentiating granule cells and relate it to the expression of the same cadherins in cerebellar inhibitory interneuronal precursors. In vitro reaggregation analysis supports a role for cadherins in cell sorting and migration within the nascent cerebellar cortex that may be rationalized within the context of the differential adhesion hypothesis (Foty and Steinberg, 2005).
Keywords: cerebellum, development, cadherin, cell-adhesion, Math1, flow cytometry
Introduction
Development of the nervous system is characterized by extensive cell migrations that result in a highly ordered arrangement of neurons and glia. Moreover, neurons connect to each other and their peripheral target organs, which requires a finely tuned steering and pathfinding of neurites, and eventually synaptogenesis. The specificity of these processes is molecularly realized through the expression and activity of a host of cell-cell and cell-matrix adhesion molecules and receptors.
Cadherins constitute one major family of cell adhesion molecules critical to several aspects of nervous system development, from neurulation (e.g. Radice et al., 1997; Lele et al., 2002) to synaptogenesis (e.g., Poskanzer et al., 2003; Bozdagi et al., 2004), and possibly regeneration (Obst-Pernberg and Redies, 1999; Liu et al., 2004). Both classical and the more recently described group of protocadherins are widely expressed in the nervous system, and their expression patterns may be perceived as a reflection of molecular diversity, and possibly functional specificity within discrete central nervous circuits (e.g., Redies and Puelles, 2001; Esumi et al., 2005; Frank et al., 2005).
Yet mechanistically, differences in cell adhesivity and behavior may not only be related to expression of distinct cadherins, but also to quantitative differences in expression of a given cadherin (Duguay et al., 2003; Foty and Steinberg, 2005). Indeed, in a system where cadherins alone mediate cell adhesion, quantitative aspects may be at least equally important for proper cell sorting as molecular differences between individual cadherins (Duguay et al., 2003). This notion is supported by recent findings which relate specific developmental disturbances to quantitative disturbances of N-cadherin expression levels (Kostetskii et al., 2001; Masai et al., 2003). Still, to date, our knowledge about quantitative differences of cadherin expression in vivo, and in the nervous system in particular, is rather limited.
The cerebellar cortex is formed by a limited set of well-characterized cells, which are arranged in a highly ordered and stereotyped pattern. Histogenesis of the cerebellar cortex has been described in quite some detail (e.g., Altman and Bayer, 1997; Goldowitz and Hamre, 1998; Sotelo, 2004), and maturation and differentiation of individual cerebellar cells may be related by their expression of a host of specific molecular markers (e.g., Oberdick et al., 1998; Wang and Zoghbi, 2001). Several cadherins have been found to be expressed in the cerebellum and its anlage (e.g., Arndt et al., 1998; Luckner et al., 2001; Moore et al., 2004; Bahjaoui-Bouhaddi et al., 1997; Liu et al., 2004; Fushimi et al., 1997), and deletion of αN-catenin, which is involved in linking the classical cadherins to the neuronal cytoskeleton, shows a severe cerebellar phenotype (Park et al., 2002). Thus, the cerebellum provides a promising paradigm to further address the significance of cadherin expression for nervous system development and patterning.
The objective of the present study was to analyze the expression of a set of cadherins in the developing cerebellum. We combined genetic tagging of granule cell precursors and developing inhibitory interneurons with flow cytometric analysis to probe quantitative differences in cadherin expression of defined cellular populations. We further document that differential cadherin expression by interneuronal precursors of inhibitory interneurons and differentiating granule cells may be related to active sorting out of these cells in vitro, suggesting one mechanism to explain their strict developmental segregation in vivo.
Results
Multiple cadherins are expressed in the murine cerebellum
Previous studies (cf references in the Introduction) have reported the expression of several cadherins in the adult and developing cerebellum of various species. To assess expression of these cadherins in the murine cerebellum, we first screened cerebella obtained from newborn, early postnatal, and adult mice for the presence of mRNAs encoding N-cadherin, R-cadherin, M-cadherin, PB-cadherin, cadherin-5, -6, -8, -10, -11, -12, -14 and cadherin-20 by PCR. The data, summarized in Fig. 1, document the expression of a multitude of cadherins in the developing and adult murine cerebellum. They are fully consistent with information about the expression of this class of molecules within the cerebellum published to date. E.g., although our PCR analysis is clearly not quantitative, it reflects the developmental increase in M-cadherin expression which parallels granule cell synaptic integration (Rose et al., 1995; Bahjaoui-Bouhaddi et al., 1997). We next sought to analyze, on the level of protein expression, changes of cadherin expression in molecularly defined subsets of developing cerebellar neurons.
Figure 1.
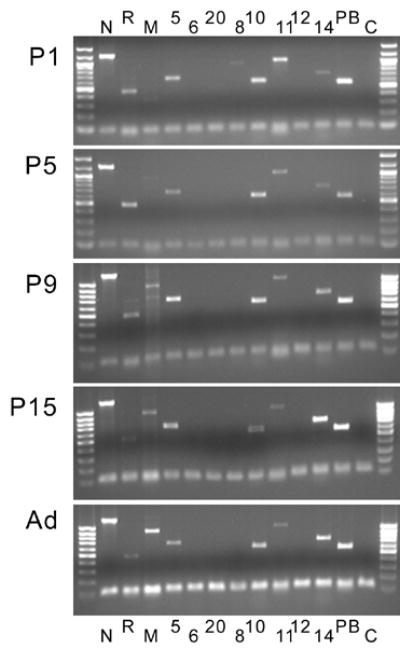
Developmental expression of mRNAs for selected cadherins in the cerebellum (postnatal days 1 (P1) to 15 (P15) and adults (Ad)). The lower band corresponds to HGPRT, which was used to monitor loading efficiency. Cadherins are indicated by their respective letters or numbers; C indicates the control lane (no cadherin primers added). Note that multiple cadherins are expressed in the cerebellar anlage. No signal was detected for cadherins-6, -12 and –20. The age-related increase in M-cadherin signal reflects the developmental up-regulation of this gene described before (Bahjaoui-Bouhaddi et al., 1997).
Math1-EGFP mice as a means to follow granule cell development
To facilitate observation and isolation of cerebellar granule cells during their transition from proliferation in the external granule cell layer (EGL) to their synaptic postmigratory integration in the (internal) granule cell layer, we generated transgenic mice expressing EGFP under the control of a Math1-derived enhancer element (Fig 2A; Helms et al., 2000). Two (called A and B) out of four independent lines that all showed high expression in the external granule cell layer were analyzed in detail. As no obvious differences in the expression pattern between the two lines were observed, we limit our description here to results obtained with line A.
Figure 2. Expression of Math1-EGFP in the newborn mouse brain.
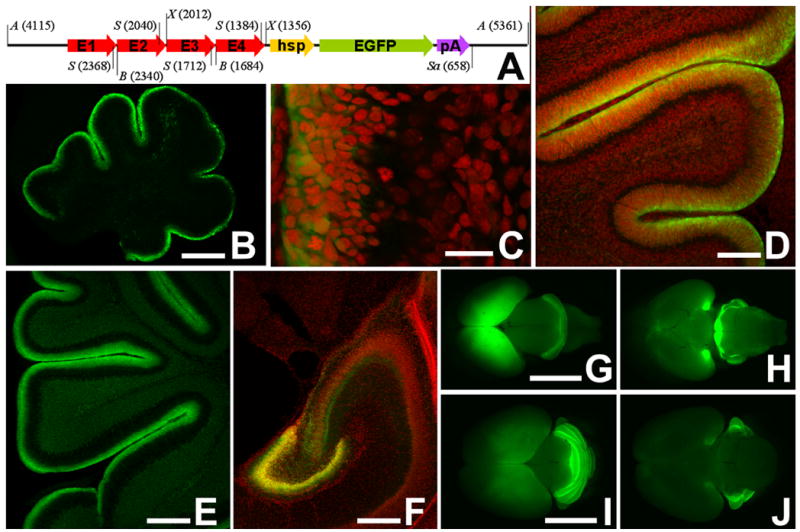
(A) Schematic diagram of the construct used to generate Math1-EGFP mice. Arrows E1 – E4 symbolize the four copies of the Math1 enhancer element B connected in series. hsp, minimal heat shock promoter; pA, pIND vector-derived polyadenylation signal. Selected restriction sites are labeled as follows: A, ApaL1; B, BlnI; S, SmaI; Sa, SacI; X, XhoI. Numerals in brackets refer to nucleotide positions relative to the origin of replication of the pBluescript base vector. Not drawn to scale.
(B) Midsagittal section through the cerebellum of a newborn (P0) Math1-EGFP transgenic mouse. EGFP-signal is confined to the external granule cell layer at the cerebellar surface.
(C) In this high power view of the cerebellum of a newborn mouse, it is evident that EGFP expression is particularly high in the outer part of the EGL (left side of the micrograph), and decreases with ongoing granule cell differentiation. Nuclei are counterstained in red with propidium iodide.
(D, E) The developmental decrease of EGFP in postmitotic and postmigratory granule cells is also evident in older animals at P3 (D) and P9 (E).
(F) Expression of Math1-EGFP in the hippocampal formation at P5.
(G–J) Whole-mount views of the newborn (P0; G, H) and P6 day old (I, J) mice seen from dorsal (G, I) and ventral (H, J). Besides cerebellar expression, expression in the hippocampal anlage (visibly fluorescent through the covering cortical anlage) and in pontine nuclei (G) is prominent at P0. Note that pontine nuclei are no longer marked at P6. Bar = 50 μm in B, E and F; 5 μm in C; 25 μm in D; 1 mm in G (for G, H); and 0.5 mm in I (for I, J).
Within the cerebellum, the Math1-EGFP transgene is strongly expressed in cells of the granule cell lineage in the external granule cell layer (Fig. 2B–E, Fig. 3A–D). Areas of Math1-EGFP expression outside the cerebellar anlage include the hippocampal formation (Fig. 2F), precerebellar pontine nuclei (Fig. 2G–J), and the spinal cord (data not shown). This pattern of expression is consistent with that observed previously in transgenic animals generated with similar constructs (Helms et al., 2000; Lumpkin et al., 2003). Significantly, just as described for the cognate Math1 gene (Ben-Arie et al., 1997; Weisheit et al., 2002), levels of EGFP are rapidly downregulated once these cells leave the proliferative pool and initiate their migration into the (internal) granule cell layer (Fig. 2C; Fig. 3A–D). To get a relative measure of EGFP levels, we related it to cell density as assessed by propidium iodide staining of cell nuclei. Representative levels of expression are shown in Fig. 3D.
Figure 3. Math1-EGFP expression levels in progressively differentiating granule cells.
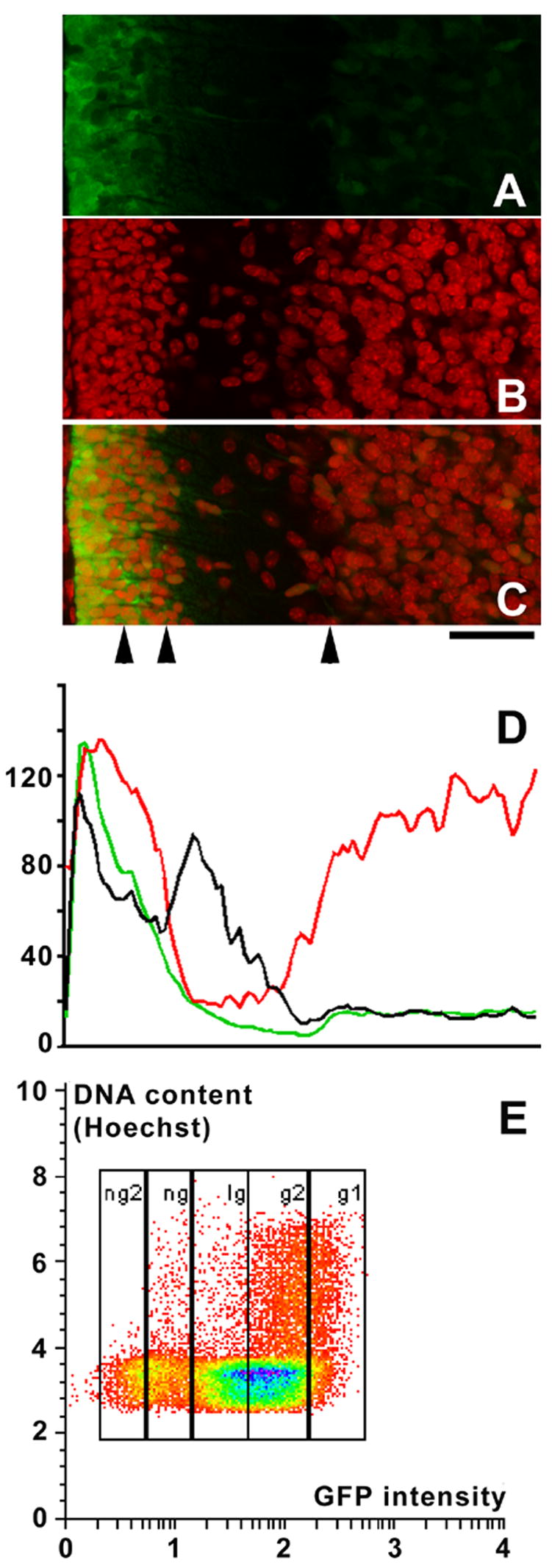
(A–C) High power view of a sagittal section spanning the cerebellar cortex of an 8 day old mouse from the meninges (left) to the internal granule cell layer. Nuclei were counterstained with propidium iodide (red signal). (A) EGFP signal alone. (B) Propidium iodide (nuclear) signal alone. (C) Overlay of both signals. Arrowheads underneath panel C indicate, from left to right, the approximate positions of the borders between the external (proliferative) part of the external granule cell layer (EGL) and its premigratory, internal part; the border between the internal part of the EGL and the molecular layer; and the Purkinje cell layer, respectively. Scale bar underneath panel C = 20 μm.
(D) Fluorescence intensity averaged over the complete area covered by panels A–C for the EGFP (green line) and the nuclear (red line) signals, and the ratio of the EGFP relative to the nuclear signal (i.e., the EGFP signal normalized to the cell density; black line). Fluorescence intensities and their ratio in arbitrary units. Note that, from the external part of the EGL to the internal granule cell layer (on the left side of the images), the relative EGFP-intensity decreases.
(E) Freshly dissociated cells from the cerebellum of an 8 day old Math1-EGFP mouse sorted according to their level of EGFP expression and their DNA content. Levels of EGFP-expression correlate with the mitotic activity of the cells: Of the intensely green cells (population g1), 50 % are in G1/G0, and 50% in S, G2 and M phase. In contrast, in the progressively less EGFP expressing populations g2, lg, ng and ng2, only 15, 4.2, 7.6 or 0.9% of all cells are in S, G2 or M phase. The presence of mitotically active cells in EGFP-negative populations reflects the presence of, e.g., proliferative cells (particularly in population ng) of other neuronal and glial lineages.
Note that GFP intensity is displayed along a logarithmic axis, that of DNA along a linear axis.
Levels of EGFP expression may also be used to delineate and characterize granule cells and their precursors by flow cytometry. As an example, we document the close correlation between levels of Math1-EGFP expression and the proliferative status of cerebellar cells (Fig. 3E). The lower the level of Math1-EGFP expression, the smaller the fraction of cells with a DNA complement above 2n (i.e., cells in S, G2, or M phases). That the latter is not null in weakly EGFP-positive or EGFP-negative cell populations reflects the fact that these comprise also a quantitatively minor complement of proliferative cells of non-granule cell lineage.
Expression of cadherin-immunoreactivity in defined subsets of cerebellar cells
Immunostaining of isolated and dissociated cerebellar cells was used to assess the expression of E-, M-, N-, P-, R-cadherin and cadherin-5 and relate it to Math1-EGFP expression. These cadherins were selected based on previous reports on their expression in the cerebellar anlage (Luckner et al., 2001; Hollnagel et al., 2002; Liu et al., 2004) and the availability of specific antibodies suitable for flow cytometric analysis. We focused on cells isolated from postnatal day 8 cerebella because they contain sizable complements of granule cells in various stages of differentiation as well as of other cerebellar cells, the developmental state of which is reasonably well characterized. The left hand column of Fig. 4 exemplifies cadherin expression as assessed by flow cytometry in cells classified based on their level of Math1-EGFP expression. In the right hand column of Fig. 4, cells are classified on the basis of their GFP expression under control of the Pax2-BAC transgene (Pfeffer et al., 2002); this allows the identification of precursors of inhibitory cerebellar interneurons, which are strongly Pax2-GFP positive (Weisheit et al., 2006; see below).
Figure 4.
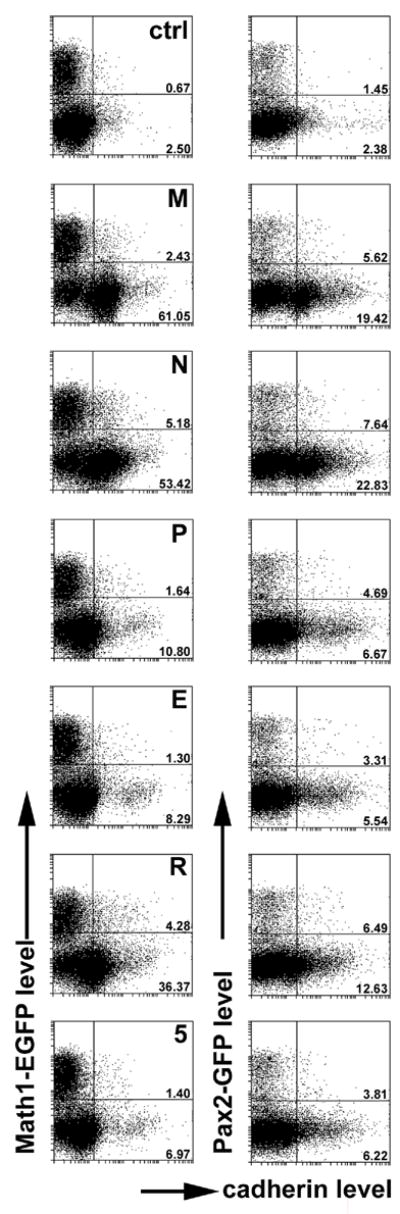
Flow cytometric analysis of cadherin expression in cerebellar cells derived from 8 day old mice expressing GFP under control of either the Math1 (left hand column) or Pax2 promoter (right hand column). We used cells incubated with secondary antibodies only (top panels; ctrl) to set up the cutoff levels for cadherin staining such that a maximum of 2.5% cells would be (erroneously) classified as positive. The cutoff between (E)GFP positive and negative cells was set based on the analysis of (E)GFP histograms. Numbers indicate the percentage of cells classified as immunopositive, separately for (E)GFP-positive and negative subsets. Particularly prominent is the expression of M-, N and R-cadherin in the Math1-negative subset of cells. For details, see text.
In cells derived from Math1-EGFP animals, staining for all cadherins tested here resulted in a clearcut immunosignal that was highly significant when compared to controls in which the primary antiserum was omitted (p < 0.001 by G-test). This was true for both strongly Math1-EGFP-positive cells (i.e., granule cell precursors) and also for cells expressing low to undetectable levels of Math1-EGFP (summarily referred to below, as Math1-GFP-negative cells). While the complement of Math1-EGFP-positive cells that may be unambiguously classified as immunopositive was in general minor (e.g. only about 1% above control for P-cadherin, E-cadherin and cadherin-5), it was much more substantial in cells classified as Math1-EGFP negative. This was particularly evident for M-cadherin (61% positive), N-cadherin (53%) and R-cadherin (36%). For P-cadherin, E-cadherin and cadherin-5, between 7–11% of all Math1-EGFP negative cells were classified immunopositive. Statistical analysis confirmed the impression that the prevalence of cells classified as immuno- (i.e., cadherin-) positive was indeed higher in the Math1-EGFP-negative subpopulation than in cells classified as Math1-EGFP-positive for all cadherins (p < 0.01), except for cadherin-5 (p = 0.095 ; tested by the Mantel-Haenszel procedure).
To further characterize Math1-EGFP-negative cells, we assessed their cell-cycle distribution (i.e., proliferative activity), separately for those classified as cadherin-negative or positive. To do so, we evaluated the ratio of cells in G1 or G0 relative to those in S, G2 or M phase based on their DNA content measured by Hoechst 33258 staining (Table 2). Cells immunopositive for E-cadherin and cadherin-5, and, to a lesser degree, those positive for P-cadherin, comprised a much greater complement of cells in S/G2/M phase (i.e. were considerably more mitotically active) than the average of all Math1-EGFP-negative cells or cells positive for M- and N-cadherin, which have previously been shown to be expressed in granule cells (Rose et al., 1995; Hollnagel et al., 2002). Cells immunoreactive for R-cadherin comprise intermediate numbers of cells in S/G2/M phase.
Table 2.
Cell cycle parameters for Math1-EGFP negative cerebellar cells classified according to their cadherin expression.
| cadherin-positive cells | cadherin-negative cells | |||
|---|---|---|---|---|
| cadherin | % in G1,G0 | % in S, G2, M | % in G1,G0 | % in S, G2, M |
| M | 91 | 9 | 93 | 7 |
| N | 90 | 10 | 93.5 | 6.5 |
| P | 84 | 16 | 92.7 | 7.3 |
| R | 87 | 13 | 93.5 | 6.5 |
| E | 79 | 21 | 93.4 | 6.6 |
| 5 | 77 | 23 | 91 | 9 |
Cells were obtained from P8 old animals. They were categorized according to Math1-EGFP expression and cadherin-immunostatus as shown in Fig 3 (left hand column). Of all Math1-EGFP negative cells, some 8.8 ± 1.0% (mean ± 1 SD; n = 7; 95% confidence interval, 6.8 – 10.8%) were classified as not in G1 or G0. Note that cells immunopositive for cadherins P, R, E and 5 comprise a fraction in S, G2 or M phase that exceeds the 95% confidence interval of controls, indicating hat they are, or include a subset of, highly proliferative cells.
Next, we sought to compare cadherin expression in cells of the granule cell lineage to that in precursors of cerebellar cortical inhibitory interneurons, which show a highly specific and developmentally regulated pattern of interaction with granule cells. To this end, we immunostained p8 cerebellar cells derived from Pax2-GFP transgenic animals (Pfeffer et al., 2002). Pax2 is a reliable marker for developing cerebellar inhibitory interneurons, as is the Pax2-GFP transgene (Maricich and Herrup, 1999; Weisheit et al., 2006). Data are summarized in the right-hand column of Fig. 4. For Pax2-GFP-positive cells, staining with all cadherin antisera resulted in an increase of cell numbers classified as immunopositive. A direct comparison of levels of cadherin expression as measured by immunofluorescence-intensity between cells classified according to their level of Math1-EGFP expression or Pax2-GFP expression is documented for M-, N-, and R-cadherin in Fig. 5. This analysis documents that, first, levels of immunoreactivity for these cadherins vary widely in all of these four populations. Second, it reveals once again the increasing levels of M-, N- and R-cadherin that parallel Math1-EGFP down-regulation (i.e., granule cell differentiation; see Discussion).
Figure 5.
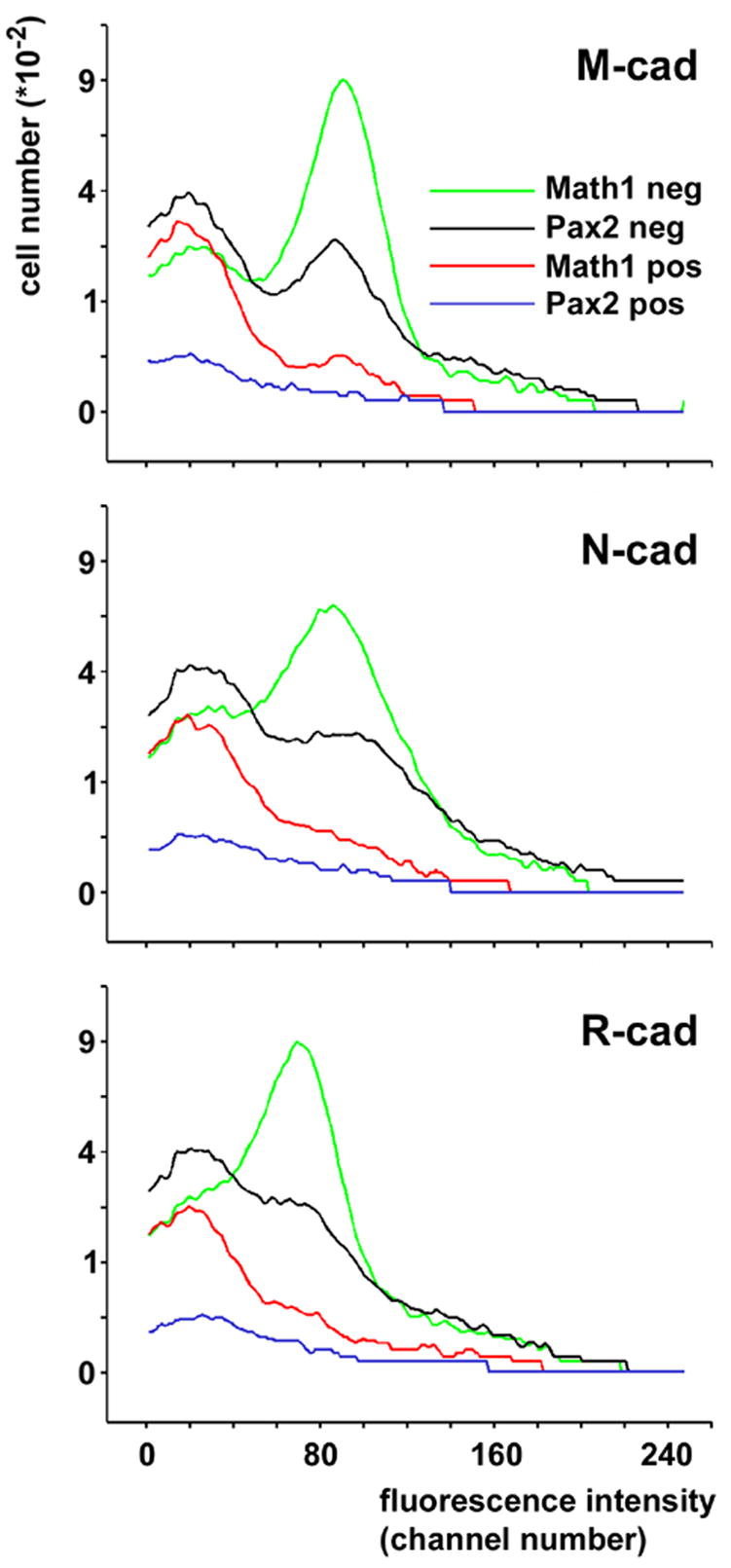
Comparison of immunoreactivity for M-, N and R-cadherin in cells classified according to their levels of Math1-EGFP expression or their levels of Pax2-GFP expression. Consistent with figure 3A–D, comparison of the curves describing Math1-EGFP-positive (green curve) and –negative cells (red curve) reveal the much higher level of immunoreactivity in the latter population. Most Pax2-GFP-positive cells (blue curve) show a level of cadherin immuno-reactivity comparable to that of Math1-EGFP positive cells. The bimodal distribution of Pax2-GFP-negative cells (black curve), which quantitatively comprises immature and maturing granule cells, closely reflects the distribution of Math1-EGFP-positive and -negative cells.
Third, and importantly, it also documents that most Pax2-GFP-positive cells display an immunosignal for M-, N- and R-cadherin that is comparable to that of Math1-EGFP-positive cells (i.e., the mode/peak of the respective frequency curves shows the same location). From a more technical point of view, it is reassuring that the position of the two modes (peaks) of the curve describing Pax2-GFP-negative cells closely mirrors those of the peaks in the curves resulting from the Math1-EGFP-positive and -negative cells, respectively. As mentioned above, Pax2-GFP-negative cells quantitatively comprise immature (Math1-EGFP-positive) and maturing (Math1-EGFP-negative) cells.
Cadherin functionality
To test functionality of cadherins expressed in developing cerebellar cells, we analyzed reaggregation of isolated, individualized cells of p3 and p8 old cerebellar anlagen in culture. We took advantage of the fact that trypsinization in the presence of calcium selectively spares (a subset of) classical cadherins (Alattia et al., 2002; Hirano et al., 1999; Shimoyama et al., 1999; Kuch et al., 1997; Kido et al., 1998). To ascertain the reliability of this approach in our hands, we first compared reaggregation of cells trypsinized in the absence of calcium (i.e., cells on which all cell adhesion proteins should have been removed; trypsinized following the TE-protocol) to that of cells trypsinized in the presence of 1 mM calcium (i.e. cells in which classical cadherins should have been spared from trypsin digestion). While reaggregation of cells trypsinized in the absence of calcium started after about three hours in vitro, at which time small aggregates became visible (Fig 6A–C), that of cells digested in the presence of calcium was evident within 15 min (Fig 6D). They rapidly increased in size (Fig 6E), and their formation was dependent on the presence of calcium in the culture medium (Fig 6F).
Figure 6. Cell segregation in cerebellar reaggregate cultures prepared from P8 cerebella.
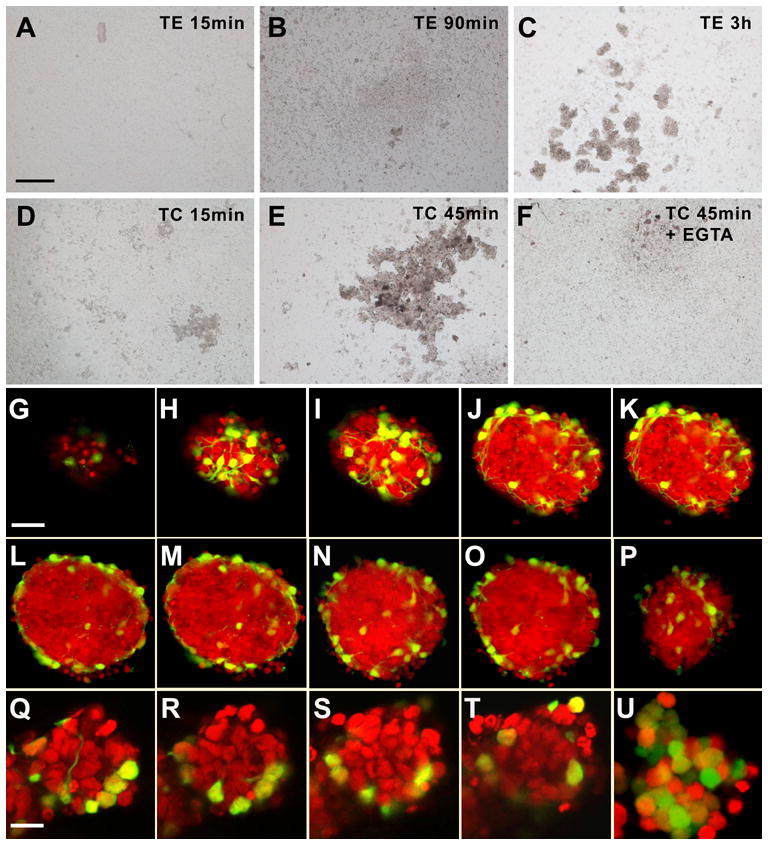
(A–F) Phase contrast images from cultures prepared with cells trypsinized under cadherin-sparing conditions (D–F) or conditions under which all surface cadherins like other surface proteins are digested (A–C).When cadherins are spared, reaggregates may be detected after 15 min or 45 min (D,E). This may be blocked by calcium withdrawal (F; Ca2+ was chelated with EGTA).When cell cadherins were destroyed during trypsination, reaggregation was not seen after 15 min or 90 min, but only after some 3 h. Bar (in A, for A–F) = 200 μm.
(G–P) Optical sections through a characteristic reaggregate culture after 48 h in vitro. After fixation and RNA digestion, nuclei were stained with propidium iodide. Optical sections were sampled every 3 μm, and every other section is shown. Pax2-GFP-positive cells are located preferentially on the surface of the aggregate. Note that G is a top view of the aggregate, and panels H and I are tangential sections close to the upper pole. P is a tangential section close to the basal pole of the aggregate. Bar = 20 μm (in G, for G–J)
(Q–T) Series of optical sections through a 90 min old culture of cells trypsinized under cadherin-sparing conditions. Optical sections were sampled every 2.5 μm, and every other section from the equatorial part of the aggregate is shown. Again, Pax2-GFP-positive cells are preferentially at the margin/surface of the aggregate.
(U) This control aggregate, obtained from a cerebellum of a Math1-EGFP transgenic mouse, documents that cells in the center of aggregates are of the granule cell lineage. Bar = 10 μm (in Q, for Q–U)
The differences in cadherin expression by immature granule cell precursors, their differentiating descendants, and Pax2-GFP-defined precursors of inhibitory interneurons suggests that cadherins might be involved in driving differential cell sorting and mixing, as predicted by the differential adhesion model (Duguay et al., 2003). Therefore, we analyzed the location of inhibitory interneuronal precursors versus that of cells of the granule cell lineage in reaggregate cultures. The availability of GFP-labeled interneuronal precursors allowed us to modify this assay such as to eliminate time consuming and artifact prone tissue processing (Vilz et al., 2005), as whole aggregates could be optically sectioned and analyzed (Fig 6G–P and Q–T). Thus, nascent or manipulated aggregates, which were too frail to withstand embedding and sectioning, became amenable to analysis. Of note, we also refrained from adding any extrinsic macromolecules, such as poly-L-lysine, which are often used to enhance aggregation and might interfere with this process (e.g., Orkand et al., 1984; Fischer and Schachner, 1988).
Analysis of aggregates by confocal microscopy revealed that Pax2-GFP-positive cells were consistently located at the surface, as documented for a 48 h old aggregate culture, prepared from P8 cerebella, in Fig 6G–P. Comparable aggregates formed in reaggregate cultures established from p3, p5 and p8 animals. Moreover, in TC-dissociated cells, segregation of Pax2-GFP-positive cells to the surface of nascent aggregates could be observed within the first hour of cultivation. An example obtained from a 90 min old aggregate is shown in Fig 6Q–T. If these cells were cultured beyond three hours, they formed aggregates even in low-calcium culture conditions (data not shown). This suggests that non-cadherins (eventually) contribute to aggregate formation and/or stabilization.
Finally, we also attempted to evaluate the contribution of single cadherins to cerebellar cell aggregation in vitro, taking advantage of the fact that function-blocking antibodies to N-cadherin and R-cadherin are available. When dissociated p8 cerebellar cells were cultured in the presence of either of these antibodies, or in a combination of both at concentrations known to block functional N- and/or R-cadherin (Van Aken et al., 2003; Guo et al., 2003; Dorrell et al., 2002; Dorrell et al., 2004), however, no appreciable interference with reaggregation could be observed after 15 min or 3 h. Moreover, no appreciable changes in the segregation of GFP-positive cells to the surface of the nascent aggregates were observed in cadherin-antibody treated aggregates.
Discussion
Here, we analyze cadherin expression in developing cerebellar granule cells and inhibitory interneurons and relate it to the dynamically changing interactions that these cells engage in during cerebellar histogenesis.
Quantitative correlation of cadherin immunoreactivity to granule cell maturation was achieved by making use of Math1-EGFP transgenic animals in which levels of EGFP in cells of the cerebellar granule cell lineage show a stringent correlation with cell position within the cerebellar cortex, and also cell proliferative status. Therefore, Math1-directed EGFP expression provides a reliable and easily quantifiable marker to assess granule cell differentiation during their critical transition from proliferation in the EGL to their settlement in the IGL.
Comparison of our cadherin data with previous results – qualitative data
Our analysis of the expression of a sizable set of cadherins in the developing cerebellum extends previous observations (e.g., Arndt et al., 1998; Luckner et al., 2001; Moore et al., 2004; Bahjaoui-Bouhaddi et al., 1997) in three substantial ways: First, multi-parameter flow cytometric analysis allowed us to derive a quantitative assessment of cadherin expression in molecularly defined cerebellar phenotypes. Second, we were able to correlate quantitative changes in cadherin expression with the developmental progression of granule cells. And third, we may relate such changes of cadherin expression to the dynamic developmental interactions of granule cells with precursors of inhibitory cerebellar interneurons.
The minor populations of cells expressing E-cadherin and cadherin-5 (also known as VE-cadherin) (most) likely represent cells of penetrating vessels, which, in contrast to meningeal cells, are not removed during cell preparation as used here. E-cadherin and cadherin-5 are established markers for vascular cells (e.g., Quadri et al., 2003; Alva et al., 2006). Further, cells expressing these cadherins are highly proliferative (table 2), as expected for cerebellar vascular endothelia which rapidly expand during the first two postnatal weeks (Acker et al., 2001). Math1-EGFP negative granule cells in contrast are postmitotic, and have previously been reported to be negative for these cadherins (Rose et al., 1995; Hollnagel et al., 2002; Alva et al., 2006). The nature of the small fraction of cells expressing P-cadherin remains unknown. Previous studies (Hollnagel et al., 2002) noted that this cadherin is not expressed in postmitotic neurons of the cerebellar granule cell layer. Consistently, we find that this set of cells comprises a substantial subset of mitotically active cells.
M-, N and R-cadherin are expressed by a major population of cells which is mostly Math1-EGFP-negative (compare the upper and lower right panels in the left of the dot plots shown in the left hand column of Fig 4). While the bulk of Math1-EGFP negative cells are maturing granule cells, this population also encompasses Purkinje cells, precursors for inhibitory interneurons, glial cells and their precursors, and cells forming the vascular system of the cerebellar anlage. It may be estimated that these (non-granule) cells account, at p8, for some 10–20% of all cerebellar cells (Schnitzer and Schachner, 1981; Fujita, 1967; Korbo et al., 1993). The presence of, e.g., glial precursors and vascular cells amongst Math1-EGFP negative cells explains why this population, and in particular the Math1-EGFP negative subpopulation positive for M-, N- and R-cadherin contain sizable fractions of mitotically active cells (see table 2). This interpretation is in keeping with the fact that N-cadherin is expressed in vascular endothelia (Luo and Radice, 2005; Navarro et al., 1998). The situation is less clear for M- (Tomi et al., 2004) and R-cadherin (Dorrell et al., 2002); the latter, however has been reported to be expressed in (retinal) astroglia (Miyawaki et al., 2004). This said, it follows from straightforward numerical considerations that most Math1-GFP negative cells expressing M-, N- and R-cadherin are indeed (postmitotic) granule cells. So is the bulk of Math1-EGFP negative cells not expressing these cadherins. This heterogeneity among postmitotic granule cells reflects that, at p8, the cerebellar anlage contains a continuum of progressively differentiated granule cells, ranging from those which have just arrived in the IGL to those which are already synaptically integrated. Such heterogeneity is also reflected by the differential expression of a set of other molecular markers in the nascent cerebellar cortex (Mellor et al., 1998; Kuhar et al., 1993; Schilling et al., 1994). The developmental increase in the expression of M-, N- and R-cadherin documented by the inverse correlation of their levels with those of Math1-EGFP is consistent with qualitative data on expression of these cadherins in the cerebellar anlage (M: Bahjaoui-Bouhaddi et al., 1997; N: Redies and Takeichi, 1993; Hollnagel et al., 2002; R: Liu et al., 2004; Arndt and Redies, 1996; Luckner et al., 2001).
Quantitative aspects of cadherin expression – cellular interactions
The classification of Math1-EGFP negative cells as either cadherin-positive or negative, as directed by cutoff-criteria derived from negative controls, is helpful for qualitative numerical comparisons. However, such a binary classification fails to convey that there is, for M-, N and R-cadherin, a gradual increase of expression, shown by the gradual shift of essentially the whole population of Math1-EGFP negative cells towards higher levels of cadherin expression (i.e., to the right; Fig 4). This is particularly evident if one compares these dot blots with those for E-cadherin and cadherin-5, where cadherin positive populations can be much better delineated from the bulk of cadherin-negative cells (cf also above).
A population-based, quantitative look also allows the developmental cadherin expression in granule cells to be related to that in precursors of cerebellar cortical inhibitory interneurons: With respect to immunoreactivity-levels of M-, N- and R-cadherin, most Pax2-GFP positive interneuronal precursors are strikingly different from differentiating (Math1-EGFP-negative) granule cells. The resulting differences in cell adhesivity may be invoked, according to the differential adhesion hypothesis (Steinberg, 1963; Foty and Steinberg, 2005) as one potential determinant of the rather unimpeded passage of the IGL by Pax2-positive inhibitory interneuronal precursors destined for the molecular layer. This interpretation is not contradicted that these latter cells, despite the fact that they express M-, N- and R-cadherin at levels comparable to Math1-EGFP positive granule cell neuroblasts, are strictly excluded from the EGL (Vilz et al., 2005; Weisheit et al., 2006). Rather, this pinpoints the fact that adhesive systems other than the cadherins under scrutiny here are clearly involved in cerebellar histogenesis.
During calcium-dependent reaggregation of cells isolated from the cerebellar anlage, those that are Pax2-GFP positive sort to the surface of reaggregates, exactly as predicted by the differential adhesion hypothesis for cells expressing low(er) levels of cadherins. This functionally confirms differences in cell adhesivity as suggested by flow cytometric analysis of M-, N- and R-cadherin. However, failure to suppress reaggregation with antibodies blocking N- and R-cadherin again point to additional cell adhesion systems which cooperate, or act in parallel with, these cadherins in the developing cerebellar anlage. Indeed, the fact that reaggregation in the presence of these antibodies occurred only in the presence of calcium, and also only if cells were trypsinized in the presence of calcium, suggests that it may indeed be mediated by cadherins (Alattia et al., 2002). As M-cadherin (Kuch et al., 1997), cadherin-6 (Shimoyama et al., 1999) and cadherin-8 (Kido et al., 1998) are not protected from trypsin digestion by Ca2+ at the concentration used here (0.04%), they seem less likely candidates. Additional aspirant cadherins known to be expressed in the cerebellar anlage include cadherin-10, -11, -14, -20, -22 and PB-cadherin (see Fig 1 and: Cadherin-11: Luckner et al., 2001; Suzuki et al., 1997); cadherin-20 (Moore et al., 2004); cadherin-22 (Saito et al., 2005) and PB-cadherin: (Kitajima et al., 1999). However, further speculation as to their role(s) will require more information about their developmental cell specificity and the development of function blocking tools.
In this context it is worthwhile to recall that even in vivo, cadherin function in the cerebellum is highly redundant. No neurological phenotype suggesting cerebellar dysfunction has been reported for several animals null for single cadherins, including R-cadherin (Dahl et al., 2002), cadherin-6 (Inoue et al., 2001), cadherin-11 (Manabe et al., 2000) and M-cadherin (Hollnagel et al., 2002). The latter mutant, indeed, exemplifies how the function of one cadherin considered highly specific based on its spatio-temporal expression pattern within the cerebellar cortex may be taken over by another (i.e., N-cadherin), much more broadly expressed cadherin. The significance of cadherins, as a class, for cerebellar development, however, is underscored by the observation that deletion of αN-catenin, which is involved in linking multiple classical cadherins to the neuronal cytoskeleton, shows a severe cerebellar phenotype (Park et al., 2002).
Some limitations of the current approach and further perspectives
A definite limitation of the present approach is that it does not allow the resolution of the differential subcellular distribution of cadherins. Their role in postmigratory synaptogenesis, (e.g. Bozdagi et al., 2004; Bamji, 2005) is thus clearly beyond its reach. In contrast, limitations arising from the fact that the current study is restricted to a subset of the multiple cell-cell and cell-substrate contact-mediating proteins differentially expressed in the cerebellar anlage (e.g., protocadherins, tetraspanins, integrins, ephrins; Hirano et al., 1999; Frank et al., 2005; Rogers et al., 1999; Juenger et al., 2005) may be overcome in the future. Likewise, the issue whether, for example, M-, N- and R-cadherin are all expressed in one homogeneous set of granule cells, or whether there exist, among differentiating granule cells, subsets distinguishable by quantitative differences in their expression of these and/or other cadherins may be approached by fully exploiting the multi-parametric potential of flow cytometry. The approach exemplified here should also permit, in developmentally characterized cells, a correlation to be made between cadherin expression and the expression/activation of cadherin signal processing pathways, known to be critical to cadherin adhesive strength (Lilien and Balsamo, 2005).
One may also speculate that differential cadherin expression of mitotically active (Math1-EGFP-positive) and terminally postmitotic cells of the granule cell lineage (which down-regulate Math1-EGFP; Fig. 2B–E, 3) contributes to the delamination of the latter from the external granule cell layer. Unfortunately, this hypothesis cannot be directly tested with our current reaggregation model, as isolated cells rapidly become postmitotic (Baader et al., 1999; Vilz et al., 2005), and small, early aggregates are for reasons of geometry and sensitivity not yet amenable to quantitative analysis of EGFP-expression patterns in a manner comparable to tissue sections. We note, however, that Vilz et al. (2005) have reported a patterned distribution, in reaggregates, of granule cells which at the time point of isolation have been in distinct phases of proliferation (and hence may be presumed to also have differed by their cadherin expression based on the present findings).
To summarize, our data define qualitative and quantitative differences of cadherin expression in molecularly defined subsets of granule cells, their precursors, and differentiating cerebellar cortical interneurons. They suggest a role for cadherins in the morphogenetic interactions of these cells, which may be interpreted within the context of the differential adhesion hypothesis (Foty and Steinberg, 2005).
Experimental Procedures
Animals
Mice expressing Pax2-GFP have been described before (Pfeffer et al., 2002). For the present experiments, we used the BAC #30 line. The transgene was kept on a C57BL/6 background, and tissues used for the present experiments were obtained from F1 offspring of parents heterozygous for the transgene.
Math1-EGFP transgenic mice
To direct EGFP-expression to cerebellar granule cell precursors, we generated a construct using an enhancer element of the Math1 gene as previously described (Helms et al., 2000; Fig 2A). This enhancer element encloses 321 bp of the Math1 enhancer B (bp position 940 to position 1261 of the published Mus musculus Atoh1 enhancer sequence, Accession number: AF218256). Four of these elements were fused in series next to a minimal heat shock promoter (derived from pIND vector, Invitrogen, Karlsruhe, Germany), followed by the coding sequence of EGFP (derived from pEGFP-N1 vector, Clontech, Mountain View, CA, USA) and a BGH polyadenylation site also derived from the pIND vector. The construct was inserted into SacI and PstI digested pBluescript vector (Stratagene, La Jolla, CA, USA). For linearization, the construct was released from the vector backbone by digestion with ApaLI, and the fragments were gel purified and eluted. Transgenic animals were made by standard pronuclear injection as previously reported (Oberdick et al., 1990). Eight positive founders were obtained, of which six produced stable lines. Four of these showed high levels of expression in the external granule cell layer. We analyzed two of them (called A and B) in detail. No obvious differences in the transgene expression pattern was observed for these two lines.
Animals were mated over night. The day after mating was taken as gestational day 0 (E0). Mice older than 30 days were considered as adults. Animal handling was done in accordance with local governmental (European Communities Council Directive 86/609/EEC) and institutional animal care regulations. All steps were taken to reduce animal suffering and to minimize the number of animals needed.
Histology and assessment of EGFP expression in tissue sections
Fifty μm sagittal vibratome sections were cut from cerebella fixed in 4% paraformaldehyde. They were stained with propidium iodide (50 μg/mL in combination with 5 U RNAse A for 10 min; cf Weisheit et al., 2006) and viewed using a confocal laser microscope (TCS SP2; Leica) operated at a pinhole size of 1 airy. To obtain a semiquantitative estimate of Math1-EGFP expression, total EGFP-based fluorescence over a given region of interest was related to propidium-iodide fluorescence over the same region. As the latter is a proportional DNA-stain, this approach provides an average estimate of EGFP expression per cell. We considered any signal emanating form the deep cerebellar mass, which is known not to contain Math1-positive granule cell precursors, as background for Math1-EGFP
Cell cultures
Cells for reaggregate cultures were obtained from cerebella of 3, 5 and 8 day old mice. Pax2-EGFP expression was verified before dissection with the aid of a dissection microscope fitted with a UV-light source and appropriate filters (MZ FLIII; Leica, Bensheim, Germany). Cerebella were isolated under sterile conditions and carefully freed of meninges. The tissue was mechanically disrupted and digested by incubation with trypsin at 37° C for 20 min. Two variants of this latter step were used. In the first, digestion was performed in phosphate buffered saline (PBS; 150 mM NaCl, 10 mM NaH2PO4) containing 0.5 mg/ml trypsin and 1 mM EDTA (TE-method). In the second approach, digestion was done in PBS containing 0.4 mg/ml trypsin and 1 mM CaCl2 (TC-method; cf Alattia et al., 2002). Trypsinization was stopped by adding an equal volume of PBS containing 8 mg/ml soybean trypsin inhibitor and 8 mg/ml bovine serum albumin (BSA; cell culture grade; Sigma). Further dissociation of TC treated cells was achieved by chelating Ca2+ with EGTA (1 mM final concentration) after the trypsin activity had been blocked. Subsequently, cells were triturated with a wide-bore plastic pipette and passed through a net of pore size 400 μm, followed by a passage through a net of 250 μm pore size. Cells were collected by centrifugation at 270 g for 8 min. The pellet was resuspended in Neurobasal medium (Invitrogen, Karlsruhe, Germany) supplemented with B-27 (2%, v/v; Invitrogen) and Glutamax (2 mM, Invitrogen). Finally, the suspension was passed eight times through a 20G needle. Effective dissociation was controlled by phase contrast microscopy. Cells were counted, and the volume was adjusted to result in a final density of 1*106 cells per ml.
Cells were transferred to 2 cm2, 4-well culture dishes (Nunc; 500 μl of cell suspension containing 5*105 cells per well) which had been coated with 1% (w/v) bovine serum albumin in PBS and shaken at 80 rpm on a gyratory shaker inside a humidified incubator (5% CO2/air). To follow reaggregation, vital cultures were photographed using phase contrast optics at the time points indicated under “Results”. For immunocytochemical and confocal analysis, cells and aggregates that had formed were fixed in 4% paraformaldehyde in PBS for 20 min. For each age analyzed, three independent preparations were performed, and a minimum of six aggregates was analyzed per experiment.
In an attempt to evaluate the significance of individual cadherins for reaggregation, we performed reaggregation assays in the presence of blocking antibodies to N-cadherin, R-cadherin, or both. Neutralizing anti-N-Cadherin was from Sigma (clone GC-4; used at a concentration of 20–50 μg/ml; cf Van Aken et al., 2003; Guo et al., 2003; neutralizing anti-R-cadherin antibodies were obtained from Santa Cruz (sc-6456 and sc7941; both used at 10–20 μg/ml; cf Dorrell et al., 2002; Dorrell et al., 2004). As controls, reaggregating cells were incubated with various antibodies to irrelevant intracellular proteins. Moreover, each experiment contained a control in which the effect of calcium chelation with EGTA (see below) was monitored. Aggregation was assessed by phase contrast microscopy after 15 min and 3 hours.
Preparation of nominally calcium-free culture medium
A working solution of EGTA (final concentration, 10 mM) in culture medium was prepared by mixing 9 parts of Neurobasal medium with one part of a neutrally buffered 100 mM EGTA stock solution. Neurobasal medium nominally contains 1.8 mM of calcium. Consequently, we mixed 18 parts of EGTA-working solution with 82 parts of Neurobasal medium to obtain nominally calcium-free medium.
PCR
For isolation of RNA, whole cerebella were dissected under RNAse free conditions in PBS. Meninges were removed and tissues were either frozen in liquid nitrogen or dissociated as described for cultures. Dissociated cells were pelleted by centrifugation (270 g for 10 min) and then shock frozen. All samples were stored at −80°C. RNA extraction was done using the Qiagen RNeasy Micro Kit and included a DNAse I digestion step as detailed in the manufacturer’s manual. RNA was quantified by spectrometry at 260 nm. One μg of RNA was used for reverse transcription with Superscript II RNase H- (Invitrogen). To monitor for potential DNA contaminations, we included appropriate controls in which reverse transcription was omitted. For PCR amplification, primers (Table 1) were used at 0.25 μM, and Taq polymerase (Fermentas) was used at a 1U/20 μl. Prior to PCR, denaturation was achieved by heating to 94°C for 3 min. For amplification, specimens were heated to 94°C for 30 s, 63°C for 30 s and 72°C for 1:30 min for 30 cyles, followed by a holding step at 4°C. The sizes of the PCR products were verified following electrophoresis on 1% agarose gels containing 0.7 μg/ml ethidium bromide.
Table 1.
Primers used for PCR
| Accession Number | FWD Primer | Reverse Primer | Product/intron spanning | |
|---|---|---|---|---|
| N-Cadherin | M31131 | caagacaaagaaacccaggaaaagtg 651–676 | gagcagtgagcgtggtcagcat 1875–1896 | 1245 / yes |
| R-Cadherin | NM_009867 | ccctctcagccgccaaatg 475–493 | ccctgctgtggactggaac 1503–1524 | 1049 / yes |
| M-Cadherin | M74541 | ccttggcctgtcctgggcag 566–586 | gggcttgaccacgcacagcac 1479–1499 | 933 / yes |
| Cadherin-5 | NM_009868 | ctggattctcggggtaaccctg 1384–1405 | gagccaccgcgcacagaat 2030–2048 | 664 / yes |
| Cadherin-6 | D82029 | ttctcgaccgggaaaccctc 1334–1352 | gaytscarsgagctsagsgag 2243–2264 | 930 / yes |
| Cadherin-8 | NM_007667 | atgcagatgatgggaagataacactg 1555–1580 | gaytscarsgagctsagsgag 2500–2521 | 966 / yes |
| Cadherin-10 | AF183946 | tccacagacatgagatgattgtaatgt 1712–1734 | gaaatcccggacatcggtg 2286–2304 | 592 / yes |
| Cadherin-11 | BC046314 | gcaggcttca ttctgacatt gactc 630–654 | gaataccttattgggctgtttgcag 1659–1684 | 1054 / yes |
| Cadherin 12 | XM_139351 | ttaaggtgatacagcagattcgccac 77–102 | tttagtgcagggacaggaattcacac 740–765 | 701 / yes |
| Cadherin-14 | AK045672 | ctgacctttccctgatgggcag 367–388 | tgcatctgtagctgtcacctgcag 1058–1081 | 714 / yes |
| Cadherin-20 | AF007116 | cgcgcgctggtcttaaaggac 24–46 | aatggcaagcactggggttgag 459–481 | 457 / yes |
| PB-Cadherin | AB019618 | gctatctcgggcatccctacg 1911–1931 | atgtcttcatccacgtctgagctc 2439–2462 | 551 / yes |
| HGPRT | BC083145 | cctaagatgagcgcaagttgaa 685–706 | ccacaggactagaacacctgctaa 746–770 | 85 / no |
HGPRT: Hypoxanthine-Guanine-Phosphoribosyl-Transferase
Flow cytometry of immunostained cells
For flow cytometry, cells were dissociated following the TC protocol as described above. To obtain sufficient numbers of dissociated cells for immunostaining, six cerebella were pooled when P3 or younger mice were used; for experiments with older animals, three to four cerebella were pooled. To analyze expression of cadherins, freshly dissociated cells were incubated with 0.1% Na-azide in PBS for 30 min. They were then reacted with one of the following monoclonal antibodies: anti-E-cadherin (clone 36); anti-M-cadherin (clone 5); anti-N-cadherin (clone 32); anti-P-cadherin (clone 56); anti-R-cadherin (clone 48); or anti-cadherin-5 (clone 75; all from BD Biosciences, Heidelberg, FRG; used 1:250, except for anti-cadherin-5, used at 1:100). The specificity and suitability for immunostaining of these antibodies has been extensively documented (eg., E: Jaksits et al., 1999; Redfield et al., 1997; M: Charrasse et al., 2006; Towler et al., 2004; Hollnagel et al., 2002; N: Nürnberger et al., 2002; Redfield et al., 1997; P: Kovacs et al., 2003; Han et al., 2000; R: Dorrell et al., 2002; 5: Corada et al., 2001). After incubation at room temperature for 1 h, cells were washed with PBS and incubated with Alexa 647-tagged secondary antibodies, again at room temperature for 20 min. Controls were incubated with secondary antibodies only. After washing with PBS, cells were fixed with 1 ml of 2% paraformaldehyde on ice for 10 min. Following addition of an equal volume of PBS, cells were centrifuged at 400g and finally resuspended, at room temperature, in 500 μl PBS containing 1.2 μg/ml Hoechst 33258 (Sigma, Deisenhofen, Germany) to stain DNA for an additional 30 min. Fluorochromes were chosen such that fluorescence could be excited and recorded individually on a three laser LSRII analytical flow cytometer (BD Biosciences, Heidelberg, Germany). Spectral spillover could therefore be excluded. Excitation and emission settings were 405 nm excitation and 440/40 band pass filter for Hoechst, 488 nm and 530/30 bandpass filter for EGFP and 635 nm laser excitation and 660/20 band pass filter for Alexa Fluor 647. Data were analyzed and presented using WEASEL analysis software (http://www.wehi.edu.au/cytometry/WEASELv2.html).
Statistics
Frequencies of data presented as RxC (2×2) tables were analyzed by the G-test procedure (Sokal and Rohlf, 1994 chapters 17.4 and 17.6). We selected the G-test over the more common Chi-square test because it shares the advantages of the latter (see, e.g., Cox et al., 1988), but is computationally simpler and has been recommended on theoretical grounds (cf (Sokal and Rohlf, 1994 for details). Frequency data (odd ratios) were compared using the Mantel-Haenszel procedure (cf Sokal and Rohlf, 1994 p763). Adjustments for multiple comparisons were made following the Benjamini-Hochberg procedure (Benjamini and Hochberg, 1995).
Acknowledgments
We are grateful to M. Busslinger (Vienna) for providing Pax2-GFP transgenic animals. We thank A. Christ, W. Langmann, S. Molly and U. Neumann for expert and diligent technical support. We also thank D. Hupfer and N. Neuhalfen for valuable help with animal husbandry. This work was supported by grants from the Deutsche Forschungsgemeinschaft (GK246/TP9) and NSF grant IBN-0138147, with additional support from NIH grant P30-NS045758.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Acker T, Beck H, Plate KH. Cell type specific expression of vascular endothelial growth factor and angiopoietin-1 and -2 suggests an important role of astrocytes in cerebellar vascularization. Mech Dev. 2001;108:45–57. doi: 10.1016/s0925-4773(01)00471-3. [DOI] [PubMed] [Google Scholar]
- Alattia JR, Tong KI, Takeichi M, Ikura M. Cadherins. Methods Mol Biol. 2002;172:199–210. doi: 10.1385/1-59259-183-3:199. [DOI] [PubMed] [Google Scholar]
- Altman J, Bayer SA. Development of the cerebellar system in relation to its evolution, structure, and functions. CRC Press; Boca Raton, FL: 1997. [Google Scholar]
- Alva JA, Zovein AC, Monvoisin A, Murphy T, Salazar A, Harvey NL, Carmeliet P, Iruela-Arispe ML. VE-Cadherin-Cre-recombinase transgenic mouse: a tool for lineage analysis and gene deletion in endothelial cells. Dev Dyn. 2006;235:759–767. doi: 10.1002/dvdy.20643. [DOI] [PubMed] [Google Scholar]
- Arndt K, Nakagawa S, Takeichi M, Redies C. Cadherin-defined segments and parasagittal cell ribbons in the developing chicken cerebellum. Molec Cell Neurosci. 1998;10:211–228. doi: 10.1006/mcne.1998.0665. [DOI] [PubMed] [Google Scholar]
- Arndt K, Redies C. Restricted expression of R-cadherin by brain nuclei and neural circuits of the developing chicken brain. J Comp Neurol. 1996;373:373–399. doi: 10.1002/(SICI)1096-9861(19960923)373:3<373::AID-CNE5>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Baader SL, Bergmann M, Mertz K, Fox PA, Gerdes J, Oberdick J, Schilling K. The differentiation of cerebellar interneurons is independent of their mitotic history. Neuroscience. 1999;90:1243–1254. doi: 10.1016/s0306-4522(98)00563-6. [DOI] [PubMed] [Google Scholar]
- Bahjaoui-Bouhaddi M, Padilla F, Nicolet M, Cifuentes-Diaz C, Fellmann D, Mege RM. Localized deposition of M-cadherin in the glomeruli of the granular layer during the postnatal development of mouse cerebellum. J Comp Neurol. 1997;378:180–195. [PubMed] [Google Scholar]
- Bamji SX. Cadherins: actin with the cytoskeleton to form synapses. Neuron. 2005;47:175–178. doi: 10.1016/j.neuron.2005.06.024. [DOI] [PubMed] [Google Scholar]
- Ben-Arie N, Bellen HJ, Armstrong DL, McCall AE, Gordatze PR, Guo Q, Matzuk MM, Zoghbi HY. Math1 is essential for genesis of cerebellar granule neurons. Nature. 1997;390:169–172. doi: 10.1038/36579. [DOI] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Statist Soc B. 1995;57:289–300. [Google Scholar]
- Bozdagi O, Valcin M, Poskanzer K, Tanaka H, Benson DL. Temporally distinct demands for classic cadherins in synapse formation and maturation. Mol Cell Neurosci. 2004;27:509–521. doi: 10.1016/j.mcn.2004.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charrasse S, Comunale F, Grumbach Y, Poulat F, Blangy A, Gauthier-Rouviere C. RhoA GTPase regulates M-cadherin activity and myoblast fusion. Mol Biol Cell. 2006;17:749–759. doi: 10.1091/mbc.E05-04-0284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corada M, Liao F, Lindgren M, Lampugnani MG, Breviario F, Frank R, Muller WA, Hicklin DJ, Bohlen P, Dejana E. Monoclonal antibodies directed to different regions of vascular endothelial cadherin extracellular domain affect adhesion and clustering of the protein and modulate endothelial permeability. Blood. 2001;97:1679–1684. doi: 10.1182/blood.v97.6.1679. [DOI] [PubMed] [Google Scholar]
- Cox C, Reeder JE, Robinson RD, Suppes SB, Wheeless LL. Comparison of frequency distributions in flow cytometry. Cytometry. 1988;9:291–298. doi: 10.1002/cyto.990090404. [DOI] [PubMed] [Google Scholar]
- Dahl U, Sjodin A, Larue L, Radice GL, Cajander S, Takeichi M, Kemler R, Semb H. Genetic dissection of cadherin function during nephrogenesis. Mol Cell Biol. 2002;22:1474–1487. doi: 10.1128/mcb.22.5.1474-1487.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorrell MI, Aguilar E, Friedlander M. Retinal vascular development is mediated by endothelial filopodia, a preexisting astrocytic template and specific R-cadherin adhesion. Invest Ophthalmol Vis Sci. 2002;43:3500–3510. [PubMed] [Google Scholar]
- Dorrell MI, Otani A, Aguilar E, Moreno SK, Friedlander M. Adult bone marrow-derived stem cells use R-cadherin to target sites of neovascularization in the developing retina. Blood. 2004;103:3420–3427. doi: 10.1182/blood-2003-09-3012. [DOI] [PubMed] [Google Scholar]
- Duguay D, Foty RA, Steinberg MS. Cadherin-mediated cell adhesion and tissue segregation: qualitative and quantitative determinants. Dev Biol. 2003;253:309–323. doi: 10.1016/s0012-1606(02)00016-7. [DOI] [PubMed] [Google Scholar]
- Esumi S, Kakazu N, Taguchi Y, Hirayama T, Sasaki A, Hirabayashi T, Koide T, Kitsukawa T, Hamada S, Yagi T. Monoallelic yet combinatorial expression of variable exons of the protocadherin-alpha gene cluster in single neurons. Nat Genet. 2005;37:171–176. doi: 10.1038/ng1500. [DOI] [PubMed] [Google Scholar]
- Fischer G, Schachner M. Characterization of Ca2+-dependent and -independent aggregation mechanisms among mouse cerebellar cells. Brain Res. 1988;471:39–47. doi: 10.1016/0165-3806(88)90151-4. [DOI] [PubMed] [Google Scholar]
- Foty RA, Steinberg MS. The differential adhesion hypothesis: a direct evaluation. Dev Biol. 2005;278:255–263. doi: 10.1016/j.ydbio.2004.11.012. [DOI] [PubMed] [Google Scholar]
- Frank M, Ebert M, Shan W, Phillips GR, Arndt K, Colman DR, Kemler R. Differential expression of individual gamma-protocadherins during mouse brain development. Mol Cell Neurosci. 2005;29:603–616. doi: 10.1016/j.mcn.2005.05.001. [DOI] [PubMed] [Google Scholar]
- Fujita S. Quantitative analysis of cell proliferation and differentiation in the cortex of the postnatal mouse cerebellum. J Cell Biol. 1967;32:277–287. doi: 10.1083/jcb.32.2.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fushimi D, Arndt K, Takeichi M, Redies C. Cloning and expression analysis of cadherin-10 in the CNS of the chicken embryo. Dev Dyn. 1997;209:269–285. doi: 10.1002/(SICI)1097-0177(199707)209:3<269::AID-AJA3>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Goldowitz D, Hamre K. The cells and molecules that make a cerebellum. TINS. 1998;21:375–382. doi: 10.1016/s0166-2236(98)01313-7. [DOI] [PubMed] [Google Scholar]
- Guo HB, Lee I, Kamar M, Pierce M. N-Acetylglucosaminyltransferase V Expression Levels Regulate Cadherin-associated Homotypic Cell-Cell Adhesion and Intracellular Signaling Pathways. J Biol Chem. 2003;278:52412–52424. doi: 10.1074/jbc.M308837200. [DOI] [PubMed] [Google Scholar]
- Han AC, Edelson MI, Peralta SA, Knudsen KA, Lifschitz-Mercer B, Czernobilsky B, Rosenblum NG, Salazar H. Cadherin expression in glandular tumors of the cervix. Cancer. 2000;89:2053–2058. doi: 10.1002/1097-0142(20001115)89:10<2053::aid-cncr4>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- Helms AW, Abney AL, Ben-Ari Y, Zoghbi HY, Johnson JE. Autoregulation and multiple enhancers control Math1 expression in the developing nervous system. Development. 2000;127:1185–1196. doi: 10.1242/dev.127.6.1185. [DOI] [PubMed] [Google Scholar]
- Hirano S, Yan Q, Suzuki ST. Expression of a novel protocadherin, OL-protocadherin, in a subset of functional systems of the developing mouse brain. J Neurosci. 1999;19:995–1005. doi: 10.1523/JNEUROSCI.19-03-00995.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollnagel A, Grund C, Franke WW, Arnold HH. The cell adhesion molecule M-cadherin is not essential for muscle development and regeneration. Mol Cell Biol. 2002;22:4760–4770. doi: 10.1128/MCB.22.13.4760-4770.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue T, Tanaka T, Takeichi M, Chisaka O, Nakamura S, Osumi N. Role of cadherins in maintaining the compartment boundary between the cortex and striatum during development. Development. 2001;128:561–569. doi: 10.1242/dev.128.4.561. [DOI] [PubMed] [Google Scholar]
- Jaksits S, Kriehuber E, Charbonnier AS, Rappersberger K, Stingl G, Maurer D. CD34+ cell-derived CD14+ precursor cells develop into Langerhans cells in a TGF-beta 1-dependent manner. J Immunol. 1999;163:4869–4877. [PubMed] [Google Scholar]
- Juenger CH, Holst MI, Duffe K, Jankowski J, Baader SL. Tetraspanin-5, Tm4sf9 mRNA expression parallels neuronal maturation in the cerebellum of normal and L7En-2 transgenic mice. J Comp Neurol. 2005;483:318–328. doi: 10.1002/cne.20439. [DOI] [PubMed] [Google Scholar]
- Kang JS, Feinleib JL, Knox S, Ketteringham MA, Krauss RS. Promyogenic members of the Ig and cadherin families associate to positively regulate differentiation. Proc Natl Acad Sci U S A. 2003;100:3989–3994. doi: 10.1073/pnas.0736565100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kido M, Obata S, Tanihara H, Rochelle JM, Seldin MF, Taketani S, Suzuki ST. Molecular properties and chromosomal location of cadherin-8. Genomics. 1998;48:186–194. doi: 10.1006/geno.1997.5152. [DOI] [PubMed] [Google Scholar]
- Kitajima K, Koshimizu U, Nakamura T. Expression of a novel type of classic cadherin, PB-cadherin in developing brain and limb buds. Dev Dyn. 1999;215:206–214. doi: 10.1002/(SICI)1097-0177(199907)215:3<206::AID-AJA3>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- Korbo L, Andersen BB, Ladefoged O, Moler A. Total numbers of various cell types in rat cerebellar cortex estimated using an unbiased stereological method. Brain Res. 1993;609:262–268. doi: 10.1016/0006-8993(93)90881-m. [DOI] [PubMed] [Google Scholar]
- Kostetskii I, Moore R, Kemler R, Radice GL. Differential adhesion leads to segregation and exclusion of N-cadherin-deficient cells in chimeric embryos. Dev Biol. 2001;234:72–79. doi: 10.1006/dbio.2001.0250. [DOI] [PubMed] [Google Scholar]
- Kovacs A, Dhillon J, Walker RA. Expression of P-cadherin, but not E-cadherin or N-cadherin, relates to pathological and functional differentiation of breast carcinomas. Mol Pathol. 2003;56:318–322. doi: 10.1136/mp.56.6.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuch C, Winnekendonk D, Butz S, Unvericht U, Kemler R, Starzinski-Powitz A. M-cadherin-mediated cell adhesion and complex formation with the catenins in myogenic mouse cells. Exp Cell Res. 1997;232:331–338. doi: 10.1006/excr.1997.3519. [DOI] [PubMed] [Google Scholar]
- Kuhar SG, Feng L, Vidan S, Ross ME, Hatten ME, Heintz N. Changing patterns of gene expression define four stages of cerebellar granule neuron differentiation. Development. 1993;117:97–104. doi: 10.1242/dev.117.1.97. [DOI] [PubMed] [Google Scholar]
- Lele Z, Folchert A, Concha M, Rauch GJ, Geisler R, Rosa F, Wilson SW, Hammerschmidt M, Bally-Cuif L. parachute/n-cadherin is required for morphogenesis and maintained integrity of the zebrafish neural tube. Development. 2002;129:3281–3294. doi: 10.1242/dev.129.14.3281. [DOI] [PubMed] [Google Scholar]
- Lilien J, Balsamo J. The regulation of cadherin-mediated adhesion by tyrosine phosphorylation/dephosphorylation of beta-catenin. Curr Opin Cell Biol. 2005;17:459–465. doi: 10.1016/j.ceb.2005.08.009. [DOI] [PubMed] [Google Scholar]
- Liu Q, Azodi E, Kerstetter AE, Wilson AL. Cadherin-2 and cadherin-4 in developing, adult and regenerating zebrafish cerebellum. Brain Res Dev Brain Res. 2004;150:63–71. doi: 10.1016/j.devbrainres.2004.03.002. [DOI] [PubMed] [Google Scholar]
- Luckner R, Obst-Pernberg K, Hirano S, Suzuki ST, Redies C. Granule cell raphes in the developing mouse cerebellum. Cell Tissue Res. 2001;303:159–172. doi: 10.1007/s004410000292. [DOI] [PubMed] [Google Scholar]
- Lumpkin EA, Collisson T, Parab P, Omer-Abdalla A, Haeberle H, Chen P, Doetzlhofer A, White P, Groves A, Segil N, Johnson JE. Math1-driven GFP expression in the developing nervous system of transgenic mice. Gene Expr Patterns. 2003;3:389–395. doi: 10.1016/s1567-133x(03)00089-9. [DOI] [PubMed] [Google Scholar]
- Luo Y, Radice GL. N-cadherin acts upstream of VE-cadherin in controlling vascular morphogenesis. J Cell Biol. 2005;169:29–34. doi: 10.1083/jcb.200411127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manabe T, Togashi H, Uchida N, Suzuki SC, Hayakawa Y, Yamamoto M, Yoda H, Miyakawa T, Takeichi M, Chisaka O. Loss of cadherin-11 adhesion receptor enhances plastic changes in hippocampal synapses and modifies behavioral responses. Mol Cell Neurosci. 2000;15:534–546. doi: 10.1006/mcne.2000.0849. [DOI] [PubMed] [Google Scholar]
- Maricich SM, Herrup K. Pax-2 expression defines a subset of GABAergic interneurons and their precursors in the developing murine cerebellum. J Neurobiol. 1999;41:281–294. doi: 10.1002/(sici)1097-4695(19991105)41:2<281::aid-neu10>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- Masai I, Lele Z, Yamaguchi M, Komori A, Nakata A, Nishiwaki Y, Wada H, Tanaka H, Nojima Y, Hammerschmidt M, Wilson SW, Okamoto H. N-cadherin mediates retinal lamination, maintenance of forebrain compartments and patterning of retinal neurites. Development. 2003;130:2479–2494. doi: 10.1242/dev.00465. [DOI] [PubMed] [Google Scholar]
- Mellor JR, Merlo D, Jones A, Wisden W, Randall AD. Mouse cerebellar granule cell differentiation: electrical activity regulates GABAA receptor α6 subunit gene. J Neurosci. 1998;18:2822–2833. doi: 10.1523/JNEUROSCI.18-08-02822.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyawaki T, Uemura A, Dezawa M, Yu RT, Ide C, Nishikawa S, Honda Y, Tanabe Y, Tanabe T. Tlx, an orphan nuclear receptor, regulates cell numbers and astrocyte development in the developing retina. J Neurosci. 2004;24:8124–8134. doi: 10.1523/JNEUROSCI.2235-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore R, Champeval D, Denat L, Tan SS, Faure F, Julien-Grille S, Larue L. Involvement of cadherins 7 and 20 in mouse embryogenesis and melanocyte transformation. Oncogene. 2004;23:6726–6735. doi: 10.1038/sj.onc.1207675. [DOI] [PubMed] [Google Scholar]
- Navarro P, Ruco L, Dejana E. Differential localization of VE- and N-cadherins in human endothelial cells: VE-cadherin competes with N-cadherin for junctional localization. J Cell Biol. 1998;140:1475–1484. doi: 10.1083/jcb.140.6.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nürnberger J, Bacallao RL, Phillips CL. Inversin forms a complex with catenins and N-cadherin in polarized epithelial cells. Mol Biol Cell. 2002;13:3096–3106. doi: 10.1091/mbc.E02-04-0195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberdick J, Baader SL, Schilling K. From zebra stripes to postal zones: deciphering patterns of gene expression in the cerebellum. TINS. 1998;21:383–391. doi: 10.1016/s0166-2236(98)01325-3. [DOI] [PubMed] [Google Scholar]
- Oberdick J, Smeyne RJ, Mann JR, Zackson S, Morgan JI. A promoter that drives transgene expression in cerebellar Purkinje and retinal bipolar neurons. Science. 1990;248:223–226. doi: 10.1126/science.2109351. [DOI] [PubMed] [Google Scholar]
- Obst-Pernberg K, Redies C. Cadherins and synaptic specificity. J Neurosci Res. 1999;58:130–138. [PubMed] [Google Scholar]
- Orkand PM, Lindner J, Schachner M. Specificity of histiotypic organization and synaptogenesis in reaggregating cell cultures of mouse cerebellum. Brain Res. 1984;318:119–134. doi: 10.1016/0165-3806(84)90067-1. [DOI] [PubMed] [Google Scholar]
- Park C, Falls W, Finger JH, Longo-Guess CM, Ackerman SL. Deletion in Catna2, encoding alphaN-catenin, causes cerebellar and hippocampal lamination defects and impaired startle modulation. Nat Genet. 2002;31:279–284. doi: 10.1038/ng908. [DOI] [PubMed] [Google Scholar]
- Pfeffer PL, Payer B, Reim G, di Magliano MP, Busslinger M. The activation and maintenance of Pax2 expression at the mid-hindbrain boundary is controlled by separate enhancers. Development. 2002;129:307–318. doi: 10.1242/dev.129.2.307. [DOI] [PubMed] [Google Scholar]
- Poskanzer K, Needleman LA, Bozdagi O, Huntley GW. N-cadherin regulates ingrowth and laminar targeting of thalamocortical axons. J Neurosci. 2003;23:2294–2305. doi: 10.1523/JNEUROSCI.23-06-02294.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quadri SK, Bhattacharjee M, Parthasarathi K, Tanita T, Bhattacharya J. Endothelial barrier strengthening by activation of focal adhesion kinase. J Biol Chem. 2003;278:13342–13349. doi: 10.1074/jbc.M209922200. [DOI] [PubMed] [Google Scholar]
- Radice GL, Rayburn H, Matsunami H, Knudsen KA, Takeichi M, Hynes RO. Developmental defects in mouse embryos lacking N-cadherin. Dev Biol. 1997;181:64–78. doi: 10.1006/dbio.1996.8443. [DOI] [PubMed] [Google Scholar]
- Redfield A, Nieman MT, Knudsen KA. Cadherins promote skeletal muscle differentiation in three-dimensional cultures. J Cell Biol. 1997;138:1323–1331. doi: 10.1083/jcb.138.6.1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redies C, Puelles L. Modularity in vertebrate brain development and evolution. Bioessays. 2001;23:1100–1111. doi: 10.1002/bies.10014. [DOI] [PubMed] [Google Scholar]
- Redies C, Takeichi M. Expression of N-cadherin mRNA during development of the mouse brain. Dev Dyn. 1993;197:26–39. doi: 10.1002/aja.1001970104. [DOI] [PubMed] [Google Scholar]
- Rogers JH, Ciossek T, Menzel P, Pasquale EB. Eph receptors and ephrins demarcate cerebellar lobules before and during their formation. Mech Dev. 1999;87:119–128. doi: 10.1016/s0925-4773(99)00154-9. [DOI] [PubMed] [Google Scholar]
- Rose O, Grund C, Reinhardt S, Starzinski-Powitz A, Franke WW. Contactus adherens, a special type of plaque-bearing adhering junction containing M-cadherin, in the granule cell layer of the cerebellar glomerulus. Proc Natl Acad Sci U S A. 1995;92:6022–6026. doi: 10.1073/pnas.92.13.6022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito S, Honma K, Kita-Matsuo H, Ochiya T, Kato K. Gene expression profiling of cerebellar development with high-throughput functional analysis. Physiol Genomics. 2005;22:8–13. doi: 10.1152/physiolgenomics.00142.2004. [DOI] [PubMed] [Google Scholar]
- Schilling K, Schmidt HHHW, Baader SL. Nitric oxide synthase expression reveals compartments of cerebellar granule cells and suggests a role for mossy fibers in their development. Neuroscience. 1994;59:893–903. doi: 10.1016/0306-4522(94)90293-3. [DOI] [PubMed] [Google Scholar]
- Schnitzer J, Schachner M. Characterization of isolated mouse cerebellar cell populations in vitro. J Neuroimmunol. 1981;1:457–470. doi: 10.1016/0165-5728(81)90023-0. [DOI] [PubMed] [Google Scholar]
- Shimoyama Y, Takeda H, Yoshihara S, Kitajima M, Hirohashi S. Biochemical characterization and functional analysis of two type II classic cadherins, cadherin-6 and -14, and comparison with E-cadherin. J Biol Chem. 1999;274:11987–11994. doi: 10.1074/jbc.274.17.11987. [DOI] [PubMed] [Google Scholar]
- Sokal RR, Rohlf FJ. Biometry. New York: W.H.Freemann and Company; 1994. [Google Scholar]
- Sotelo C. Cellular and genetic regulation of the development of the cerebellar system. Prog Neurobiol. 2004;72:295–339. doi: 10.1016/j.pneurobio.2004.03.004. [DOI] [PubMed] [Google Scholar]
- Steinberg MS. Reconstruction of tissues by dissociated cells. Science. 1963;141:401–408. doi: 10.1126/science.141.3579.401. [DOI] [PubMed] [Google Scholar]
- Suzuki SC, Inoue T, Kimura Y, Tanaka T, Takeichi M. Neuronal circuits are subdivided by differential expression of Type-II classic cadherins in postnatal mouse brain. Molec Cell Neurosci. 1997;9:433–447. doi: 10.1006/mcne.1997.0626. [DOI] [PubMed] [Google Scholar]
- Tomi M, Abukawa H, Nagai Y, Hata T, Takanaga H, Ohtsuki S, Terasaki T, Hosoya K. Retinal selectivity of gene expression in rat retinal versus brain capillary endothelial cell lines by differential display analysis. Mol Vis. 2004;10:537–43. 537–543. [PubMed] [Google Scholar]
- Towler MC, Gleeson PA, Hoshino S, Rahkila P, Manalo V, Ohkoshi N, Ordahl C, Parton RG, Brodsky FM. Clathrin isoform CHC22, a component of neuromuscular and myotendinous junctions, binds sorting nexin 5 and has increased expression during myogenesis and muscle regeneration. Mol Biol Cell. 2004;15:3181–3195. doi: 10.1091/mbc.E04-03-0249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Aken EH, De Wever O, Van Hoorde L, Bruyneel E, De Laey JJ, Mareel MM. Invasion of retinal pigment epithelial cells: N-cadherin, hepatocyte growth factor, and focal adhesion kinase. Invest Ophthalmol Vis Sci. 2003;44:463–472. doi: 10.1167/iovs.01-1096. [DOI] [PubMed] [Google Scholar]
- Vilz TO, Moepps B, Engele J, Molly S, Littman DR, Schilling K. The SDF-1/CXCR4 pathway and the development of the cerebellar system. Eur J Neurosci. 2005;22:1831–1839. doi: 10.1111/j.1460-9568.2005.04378.x. [DOI] [PubMed] [Google Scholar]
- Wang VY, Zoghbi HY. Genetic regulation of cerebellar development. Nat Rev Neurosci. 2001;2:484–491. doi: 10.1038/35081558. [DOI] [PubMed] [Google Scholar]
- Weisheit G, Gliem M, Endl E, Pfeffer PL, Busslinger M, Schilling K. Postnatal development of the murine cerebellar cortex: formation and early dispersal of basket, stellate and Golgi neurons. Eur J Neurosci. 2006;24:466–478. doi: 10.1111/j.1460-9568.2006.04915.x. [DOI] [PubMed] [Google Scholar]
- Weisheit G, Mertz D, Schilling K, Viebahn C. An efficient in situ hybridization protocol for multiple tissue sections and probes on miniaturized slides. Dev Genes Evol. 2002;212:403–406. doi: 10.1007/s00427-002-0258-8. [DOI] [PubMed] [Google Scholar]


