Abstract
Recent evidence suggests that substance P (SP) is upregulated in primary sensory neurons following axotomy, and that this change occurs in larger neurons that do not usually produce SP. If so, this upregulation may allow normally neighboring, uninjured, and non-nociceptive dorsal root ganglion (DRG) neurons to become effective in activating pain pathways. Using immunohistochemistry, we performed a unilateral L5 spinal nerve transection upon male Wistar rats, and measured SP expression in ipsilateral L4 and L5 DRGs and contralateral L5 DRGs, at 1 to 14 days postoperatively (dpo), and in control and sham operated rats. In normal and sham operated DRGs, SP was detectable almost exclusively in small neurons (≤ 800 μm2). Following surgery, the mean size of SP-positive neurons from the axotomized L5 ganglia was greater at 2, 4, 7 and 14 dpo. Among large neurons (> 800 μm2) from the axotomized L5, the percentage of SP-positive neurons increased at 2, 4, 7, and 14 dpo. Among small neurons from the axotomized L5, the percentage of SP-positive neurons was increased at 1 and 3 dpo, but was decreased at 7 and 14 dpo. Thus, SP expression is affected by axonal damage, and the time course of the expression is different between large and small DRG neurons. These data support a role of SP-producing, large DRG neurons in persistent sensory changes due to nerve injury.
INTRODUCTION
Nerve injuries that partially denervate an extremity often lead to persistent spontaneous pain and sensory hypersensitivities (Mantyh, 1991; Woolf and Mannion, 1999). The mechanisms underlying these changes remain unclear. Substance P (SP) has been implicated as an important pain-signaling peptide at the first central synapse in the spinal cord and in the brainstem (reviewed by DeVane, 2001). In normal animals SP is expressed almost exclusively in smaller dorsal root ganglion (DRG) neurons, but typically not in larger neurons (Lee et al., 1985; Szucs et al., 1999). Substance P is released in the dorsal horn following noxious peripheral stimulation (Abbadie et al., 1996; Allen et al., 1999; Schicho et al., 2005), and activates neurokinin-1 (NK-1) receptors on nociceptive spinal neurons, thereby transmitting nociceptive signals. Furthermore, spinal neurons bearing the SP receptor are important for triggering and maintaining central sensitization (Khasabov et al., 2002), and hence they may contribute to the sensory hypersensitivities that occur in partially denervated areas, as well as surrounding areas (Nichols et al., 1999).
There is considerable evidence implicating the activity of Aβ fibers, which are from large DRG neurons, in the type of tactile hypersensitivity termed allodynia (reviewed by Gracely, 1999), and that large DRG cellular SP may play a role. Tactile hypersensitivity in rodents occurs as early as 1 day after nerve injury (Sorkin and Doom, 2000; Chacur et al., 2001; Wallas et al., 2003), the same time that substantial ectopic firing begins in Aβ fibers (Sun et al., 2005). Large DRG neurons express preprotachykinin, the precursor of SP, after nerve injury and inflammation (Noguchi et al., 1994; Marchand et al., 1994; Kawakami et al., 1994; Noguchi et al., 1995; Neumann et al., 1996; Ma and Bisby, 1998b). While exogenous SP has little effect upon large DRG neurons from normal rats, it is excitatory to large DRG neurons following axotomy (Abdulla et al., 2001). Also, following sciatic nerve transection, stimulation of low threshold Aβ afferents axons can cause SP release in the spinal cord (Malcangio et al., 2000; Meyer-Tuve et al., 2001). These data support a mechanism for the involvement of larger DRG neurons in tactile hypersensitivity following nerve injury.
Although there are numerous reports that chronicle sensory changes following nerve injury, there are no studies addressing the modulation of SP in DRG neurons in the first few days following nerve injury. In this study, we have used immunocytochemical labeling to evaluate the time course of SP expression in the DRG, before and following a spinal nerve transection in rats. Here we report that the percentage of DRG neurons expressing SP is increased in the first few days following a spinal nerve lesion. We also report that for large neurons this increase is maintained, while for smaller neurons the percentage of SP-positive neurons decreases at 1 and 2 weeks following the nerve injury.
MATERIALS AND METHODS
Animals
Experiments were carried out using 42 adult male Wistar rats weighing 250-300g. The protocol was approved by the Institutional Animal Care and Use Committee of Beth Israel Deaconess Medical Center and was in accordance with the guidelines of the International Association for the Study of Pain.
Surgery
Anesthesia was induced using 2.5-3% isoflurane in oxygen, and maintained with 1.5 - 2% isoflurane in oxygen. Tight ligation and transection of the left fifth lumbar spinal nerve (L5) was performed, using technical measures aimed at minimizing tissue damage. Using strict aseptic technique, the skin over the lower lumbar transverse processes was cut over L3 - L6, and the paraspinal muscles were retracted following natural fascial planes. A specially designed retractor was made with shaped blades, and was used to maintain a small window that revealed the left L6 transverse process. The superior portion of this process was removed to reveal the ventral ramus of the left L5 spinal nerve. This nerve was freed from the surrounding tissue, tightly ligated using 7-0 prolene ∼4 mm distal to the DRG, and transected just distal to the ligature. The procedure unavoidably destroyed the L5 dorsal ramus; thus all sensory, motor, and sympathetic axons from this level were cut. The incision was closed in layers using 4-0 prolene. After surgery, the animals were randomly assigned to one of the survival time groups of 1, 2, 3, 4, 7, or 14 days postoperative (dpo; 4 rats per group). Four rats that did not undergo any procedures were used as controls. Twelve rats underwent sham surgery, which included all procedures except for the nerve ligation, to control for possible effects of the surgical procedure. These animals were randomly assigned to survival times of 1, 4, and 7 dpo (4 per group).
Immunohistochemistry
Animals were anesthetized with an overdose of sodium pentobarbital (200 mg/kg, i.p.), perfused transcardially with heparinized 0.1 M phosphate buffered saline (pH 7.4), and fixed with 300 ml of a 4% paraformaldehyde, 4% sucrose solution over 15 minutes. The axotomized L5, ipsilateral unoperated L4, and the contralateral L5 DRGs were removed from all animals and placed in the same fixative for 1 hour. They were then transferred to 30% sucrose in 0.1 M phosphate buffered saline overnight for cryoprotection. Ganglia were mounted for sectioning upon their ventral surface, as they appeared during tissue harvest; thus, frontal sections were made. Eight μm sections were cut using a cryostat and sequentially thaw-mounted on glass slides. Sections were washed in Tris buffer (pH 7.6), blocked in 4% normal goat serum with buffer (60 minutes) and incubated in anti-SP primary antibody (ImmunoStar, USA; PN 20064; 1:3000 dilution) overnight at 4°C. This polyclonal antibody was raised in rabbit against SP coupled to a carbodiimide/keyhole limpet hemocyanin conjugate. The manufacturer reports that preadsorption with SP abolished staining, and that preadsorption with neurokinin A, neurokinin B, somatostatin, and neuropeptide K did not affect staining. We performed control studies using the same antibody dilution after preadsorption with 100 μM substance P (Fisher, USA, catalog #AP70-1-10) at 37°C for 2 hours. We controlled for secondary antiserum specificity through omission of the primary antibody.
Sections were washed and reacted with biotin-conjugated goat anti-rabbit IgG (Jackson Labs; 1:300) for 90 minutes. For product visualization we used the ABC Elite Kit (Vector Labs, USA) in Tris buffer for 90 minutes, followed by DAB/H2O2/buffer mixed according to manufacturer recommendations (Vector Labs), for 30 minutes. Finally, sections were rinsed in distilled water for 10 minutes, counterstained with cresyl violet, dehydrated with ascending concentrations of alcohol, defatted with xylene, and coverslipped with DPX (Biochemika, Switzerland).
Data Collection
All slides were relabeled to blind the investigator as to the identity of the sections being analyzed. At least 2 randomly selected sections were analyzed. Reasons for rejection of a section for analysis included processing artifacts such as folding of the section, lack of adhesion to the slide, or irregular counterstaining. Each second section selected was at least 32 μm away (4 sections) from the first.
Using a digital camera (SPOT, USA), the entire section was photographed piecemeal at 200X and images were combined to form a montage. The same section was observed microscopically at 400X and 600X to identify neurons containing a visible nucleolus and to judge whether such neurons had SP-like immunoreactivity. The montage was used for record keeping; to minimize bias, all neurons with visible nucleoli from each chosen section, whether SP-positive or SP-negative, were marked. Because many neurons were elliptical, measurements of the long and short axes (AL, AS) of each nucleolated cell were made (Farel, 2002) using the SPOT software, after calibration with a precision reticle. Areas were estimated from these long and short axes (see below). The images were resized and adjusted for appropriate resolution using Adobe Photoshop, assembled using Adobe Illustrator, but not otherwise modified (e.g., contrast or color).
Our a priori target was to count a minimum of 100 neurons per ganglion, in at least 2 representative sections per ganglion. If this number was not reached in the 2 sections chosen, a 3rd section was counted, also in its entirety. However, because identification and counting continued even after the minimum 100 neurons were obtained, this method inevitably led to the counting of many more than 100 neurons per DRG (mean = 230 ± 55 neurons/DRG).
Statistical Analysis
Cell measurements were expressed as cross-sectional areas calculated using the long and short axes (AL and AS, respectively) and the formula Area = π AL AS / 4. The data were compared using ANOVA for each of SP-positive neurons and SP-negative neurons, using Fisher’s LSD, post-hoc. P-values of 0.05 or less were considered significant differences.
Proportions of SP-positive neurons were expressed as Log odds, LN(SP-positive/SP-negative), fit by a Generalized Linear Model (McCullagh and Nelder, 1989) with dispersed binomial variance, and with model effects of ganglion (ipsilateral L5 and L4, and contralateral L5), treatment (unoperated; operated 1, 2, 3, 4, 7 and 14 dpo; sham 1, 4, and 7 dpo), and the interaction of ganglion X treatment. Parameters and their standard errors were estimated by maximum likelihood fitting. The differences of interest were unoperated control vs postoperative day, and postoperative day vs sham control (i.e.; data from lesion studies were compared to unoperated control data, and also to sham control data from matching days). Differences were deemed significant when calculated t-values on N - p degrees of freedom (119 - 29 = 90 df) yielded p-values 0.05 or less. In the results, values were expressed as group means with their standard errors.
RESULTS
Animals
Surgery was performed upon 42 animals. Of these, 2 became moribund following surgery (2 and 3 days), and were euthanized. Necropsy showed no gross abnormality except for, in both rats, a highly distended and hyperemic bladder. The 40 other rats recovered without complication until euthanized. Most rats limped following the surgery, and the posture of the foot on the operated side was usually inverted and extended until sacrifice. Such posture is consistent with denervation of the muscles that cause ankle eversion and flexion and, potentially, the presence of pain in the limb. In all rats, the level and adequacy of the nerve lesion was visually confirmed during tissue harvest.
Substance P Expression
We evaluated 27,620 neurons from 119 DRGs for cross-sectional area and the presence of SP-like immunoreactivity. SP-like immunoreactivity was prominent in small neurons (≤ 800 μm2; see below) in all ganglia (Fig. 1). In the axotomized L5 ganglia, SP was also observed in large neurons (>800 μm2; Fig. 1 C-D). SP-like immunoreactivity was only very rarely observed in large neurons in any ganglia from control or sham animals (Fig. 1A-B), or from the contralateral L5 or ipsilateral L4 DRGs (data not shown). There was no specific staining in control ganglia, to which primary antibody was not applied, or in sections reacted with antibody preadsorbed to 100 μM SP.
Figure 1.
Substance P (SP) expression in intact and axotomized dorsal root ganglia. A. Sections from unoperated animals showed SP staining (brown) limited to smaller neurons. B. Sham surgery led to staining similar to unoperated sections. Labeling in sections from 4 (C) and 7 (D) days postoperatively demonstrated SP in larger neurons (arrows). Scale bar = 25 μm.
The mean areas of SP-positive neurons in axotomized ganglia were significantly higher at 2, 4, 7, and 14 dpo (437 ± 64 μm2, p = 0.04; 505 ± 100 μm2, p < 0.001; 515 ± 99 μm2, p = 0.001; 526 ± 45 μm2, p < 0.001, respectively) when compared to control (320 ± 7 μm2) and shams from matching days (4 dpo: 325 ± 50 μm2, p = 0.003; 7 dpo: 348 ± 49 μm2, p = 0.004; Fig 2A). There were no other significant differences between the other groups of SP-positive neurons (from the ipsilateral L4 or the contralateral L5 ganglia, Fig. 2 B-C). There were no significant size differences between SP-negative neurons from any ganglion at any time point (data not shown).
Figure 2.
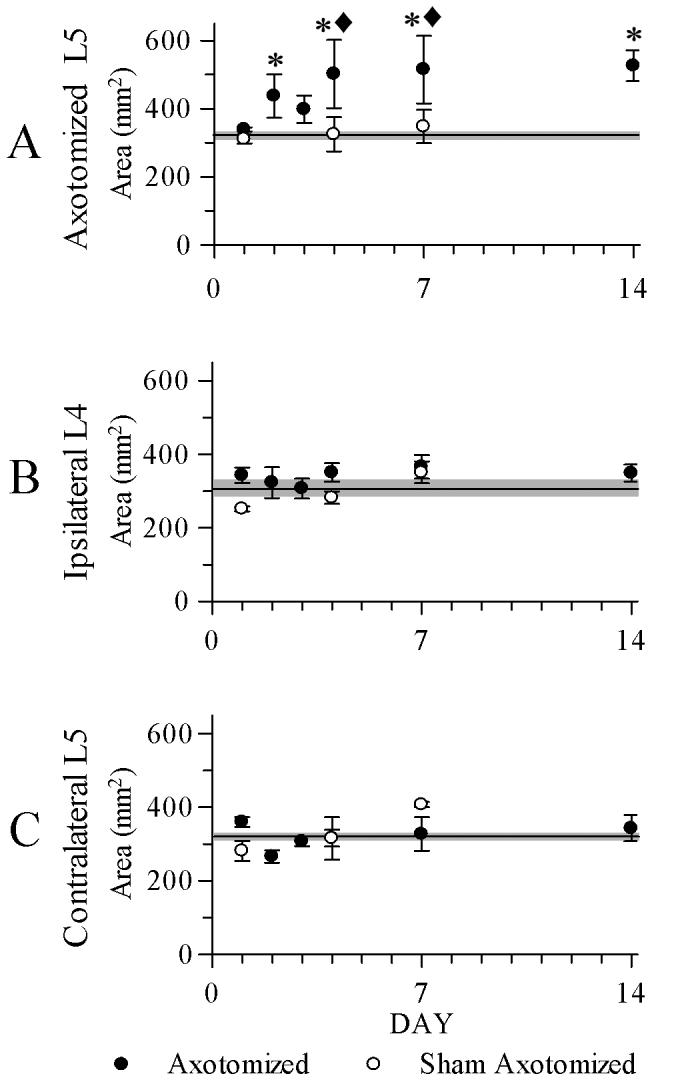
A plot of mean areas of SP - positive dorsal root ganglion neurons showing changes following axotomy. Unoperated control means are indicated in all graphs by a horizontal line, with gray shaded zones indicating the SEM of these means. A. Neuron size was significantly increased in axotomized L5 ganglia at 2, 4, 7, and 14 days postoperatively (dpo), compared to control (*) and to day-matched 4 dpo and 7 dpo sham ganglia (◆). B, C. There were no significant differences with similar comparisons of the ipsilateral L4 or contralateral L5 ganglia. N = 4 in all groups except n = 3 for ipsilateral L4 sham, 1 dpo. Error bars = SEM.
The choice of where to divide DRG neurons into large and small neurons was based upon the cell sizes taken from 10,729 neurons from the contralateral L5 ganglia (n = 40; there were no significant differences among the cell characteristics between any of these ganglia). The size distribution was highly skewed, but when plotted as a frequency histogram with 0.1 log area binning a clear bimodality appeared (Fig 3). The two peaks supported the presence of 2 overlapping populations by size (Lawson, 1979). The SP-positive neurons seemed to be associated with the first peak, as has been previously reported (Lawson, 1992). Fitting the 2 size distributions by maximum likelihood placed the intersection at approximately 800 μm2. This number was consistent with previous reports (Lawson, 1992) and was used as justification to separate the neurons from other ganglia into two subsets, small (≤ 800 μm2) and large (>800 μm2) for further analysis.
Figure 3.
Size distributions of intact L5 dorsal root ganglion neurons separated by SP content. All counted neurons (n = 10,729) from the intact L5 ganglia (n = 40) were separated by presence (open bars) or absence (shaded bars) of SP and binned by area in 0.1 natural log increments. The 2 distributions were found to intersect at 800 μm2 (grey line).
Frequency histograms of the axotomized, control, and sham L5 neurons were plotted (Fig 4). After axotomy, the SP-positive neurons expressed a rightward shift in frequency, towards larger neurons. The rightward shift could also be appreciated in the SP-negative neurons. Combined, these findings suggest a relative loss of smaller neurons following surgery, though our methods did not allow a quantitative confirmation of this possibility. Similar histograms made using the ipsilateral L4 and contralateral L5 data did not reveal such differences (data not shown).
Figure 4.
Size distributions of axotomized L5 dorsal root ganglion neurons separated by SP content, over time. Neurons from control and axotomized L5 ganglia were separated by presence (open bars) or absence (shaded bars) of SP and binned by area in 0.2 natural log increments. A greater number of larger neurons positive for SP was present starting at 2 dpo. A smaller number of SP-positive neurons was seen at 7 and 14 dpo. The number of neurons used for the histograms is in parentheses.
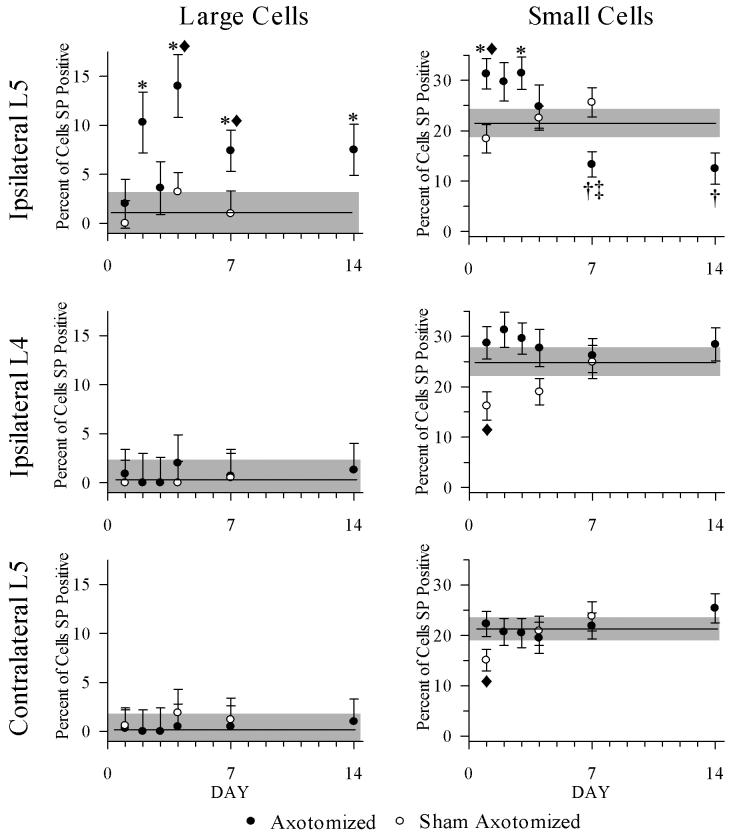
Percentages of SP-positive neurons by day were calculated after separation into two groups, small neurons (≤ 800 μm2) and large neurons (>800 μm2), and statistically and graphically analyzed (Fig 5). Data from each postoperative day were compared to their controls, and also to day-matched shams as appropriate.
Figure 5.
Percentages of SP-positive neurons >800 μm2 (left column) and ≤ 800 μm2 (right column) graphed by day. Unoperated control means are indicated in all graphs by horizontal line, with gray shaded zones indicating the SEM of these means. Left column: for large neurons from L5, there were higher percentages of SP positive neurons >800 μm2 compared to control L5 ganglia at 2, 4, 7, and 14 dpo, (* = p < 0.05), and compared to sham ganglia at days 4 and 7 (◆ = p < 0.05). There were no changes in L4 or contralateral L5. Right column: for small neurons from L5, there were higher percentages of SP positive neurons ≤ 800 μm2 compared to intact ganglia at 1 and 3 dpo (* = p < 0.05) and compared to sham ganglia at day 1 (◆ = p < 0.05). At 7 and 14 dpo, the percentages of SP positive neurons were less than control (†) and at 7 dpo was less than the day matched sham ganglia (‡). Proportions from intact L4 and L5 ganglia were different from sham ganglia at 1 dpo (◆ = p < 0.05). Error bars = SEM.
Large cell analysis
Compared to large neurons in control ipsilateral L5 ganglia, the percentages of SP-positive neurons from axotomized L5 ganglia were increased at 2, 4, 7, and 14 dpo [1.1% ± 2.1 (control) vs 10.3% ± 3.1 (2 dpo), p = 0.004; vs 14% ± 3.2 (4 dpo), p = 0.001; vs 7.4% ± 2.1 (7 dpo), p = 0.017; and vs 7.5% ± 2.6 (14 dpo), p < 0.001]. At 4 dpo and 7 dpo the percentages of large neurons were higher compared to day-matched sham ganglia [4 dpo: 10.3% ± 3.1 vs 3.2% ± 2 (sham), p = 0.001; 7 dpo: 14% ± 3.2 vs. 1% ± 2.3 (sham), p = 0.01]. There were no significant differences among ipsilateral L4 or contralateral L5 large neurons.
Small cell analysis
Compared to small neurons in control ipsilateral L5 ganglia, the percentages of SP-positive neurons from axotomized L5 ganglia were increased at 1 and 3 dpo [21.4% ± 2.6 (control) vs 31.3% ± 3, p = 0.014 (1 dpo) and vs 31.4% ± 3.2, p = 0.017 (3 dpo)]. At 1 dpo the percentage of small neurons was increased compared to neurons from day-matched sham ganglia (31.3% ± 3, p = 0.014 vs 18.4% ± 2.8 (sham), p = 0.003). However, at 7 and 14 dpo, the percentages of SP-positive neurons were significantly less than control [21.4% ± 2.6 (control) vs 13.3% ± 2.5 (7 dpo), p = 0.035 and vs 12.5% ± 3.1 (14 dpo), p = 0.048]. At 7 dpo, the percentages of SP-positive small neurons were also less than in day-matched sham ganglia (13.3% ± 2.5 vs 25.6% ± 2.9 (sham), p = 0.004). The only differences between the ipsilateral L4 and contralateral L5 ganglia were for 1 dpo versus their matched shams (1 dpo ipsilateral L4 vs sham: 28% ± 3.2 vs 16% ± 2.8, p = 0.03; 1 dpo ipsilateral L5 vs sham: 31.3% ± 3 vs 18.8% ± 2.8, p = 0.005).
DISCUSSION
We have shown that during the first few days following spinal nerve transection close to the DRG, the size spectrum of SP-positive neurons and the percentage of SP-positive neurons increase. These changes occurred in both small and large neurons, but with different time courses. The smaller neurons responded to the axotomy sooner after injury (1 dpo), but this increased SP expression was not maintained, and in fact decreased at 7 and 14 dpo. The expression of SP in larger neurons was not observed until 2 dpo, but remained significantly increased compared to unoperated and sham ganglia until 14 dpo.
Two possible explanations for the size increase of SP-positive neurons following axonal transection were considered: cell swelling due to injury, and de novo synthesis of SP, by neurons that normally do not produce SP, including larger neurons. Cell swelling could account for the appearance of large SP-positive neurons. However, cell shrinkage rather than swelling is the typical response of DRG neurons to axotomy (Vestergaard et al., 1997; Degn et al., 1999). Moreover, blinded measurements from our own material failed to reveal any statistical indication of axotomy-induced swelling, as reflected in cell size histograms of neurons from all ganglia, considering both SP-positive and SP-negative neurons (data not shown). As expected, however, some neurons showed chromatolytic changes, including eccentric nuclei and cytoplasmic pallor, especially at longer survival times. It is therefore more likely that the increased size spectrum and percentage of positive neurons were the result of de novo synthesis of SP by larger neurons.
Following axotomy of primary sensory neurons there are profound and persistent changes in the content of neuropeptides (for review see Hokfelt et al., 1994). Substance P is a neuromodulator present in 6 - 20% of the DRG neuronal population, usually limited to small sensory neurons (our findings and Otsuka and Yoshioka, 1993). Peptide content and gene expression products in DRG neurons, axons, and projections into the dorsal horn are generally down-regulated when assayed a week or more after peripheral nerve transection or tight ligation (Ahmed et al., 1995; Zhang et al., 1995a; Zhang et al., 1995b; Ji et al., 1996; Rydh-Rinder et al., 1996; Sterne et al., 1998; Ma and Bisby, 1998a; Mohiuddin et al., 1999; Antunes Bras et al., 1999; Sondell et al., 1999; Honore et al., 2000; Siri et al., 2001; Xiao et al., 2002; White and Kocsis, 2002; Wang et al., 2002; Partata et al., 2003; Hofmann et al., 2003; Valder et al., 2003; Sanderson et al., 2004; Swamydas et al., 2004); also see Hokfelt et al., 1994) for earlier studies). Nerve compression also modulates the content of SP and gene expression in primary sensory neurons (Bisby and Keen, 1986; Cameron et al., 1997; Sondell et al., 1999; Lee et al., 2001; Wong and Tan, 2002; Kobayashi et al., 2004; Swamydas et al., 2004). However, there are also reports of no changes in SP metabolism following nerve injury (Murphy et al., 1999; Macdonald et al., 2001).
In contrast, a number of studies of primary somatic afferent neurons examined shortly (up to 10 days) after nerve injury have shown increases in SP or products of gene expression for SP (Noguchi et al., 1994; Marchand et al., 1994; Noguchi et al., 1995; Neumann et al., 1996; Ma and Bisby, 1998b). Following nerve section and using in situ hybrization to detect PPT mRNA, a dramatic de novo expression of PPT mRNA was found in large and moderate sized DRG neurons but not in small DRG neurons at 7 dpo (Noguchi et al., 1994; Noguchi et al., 1995). Masseter nerve section also resulted in a upregulation of PPT mRNA in the mesencephalic trigeminal nucleus at 7 dpo (Umemoto et al., 1994).
Data regarding changes in SP at time points earlier than 1 week dpo are more limited. An apparent phenotypic switch to producing SP was also observed 2 days following the peripheral injection of turpentine to produce an inflammatory response (Neumann et al., 1996). In this study the upregulation of SP was not exclusive to large primary sensory neurons; increases also were observed in small diameter Aδ and C fibers, and enhanced SP-excitation of deep dorsal horn neurons elicited by the activation of Aδ and C fibers was demonstrated (Neumann et al., 1996). In the chronic constriction injury model of neuropathic pain, upregulation of PPT mRNA was observed in all cell size classes at 2, 5, and 10 dpo (Marchand et al., 1994). Similar patterns of up-regulation of PPT mRNA were observed following partial nerve injury and chronic constriction injury (Ma and Bisby, 1998a). The present results are consistent with these studies, as we observed an increased number of SP-positive neurons in all size classes of DRG neurons. Our study extends the observational resolution in the first week, showing that smaller neurons appear to be affected as early as 1 dpo and large neurons affected as early as 2 dpo. Of significant note is that the present methods involved confirmed axotomy of every axon of the operated spinal nerve, whereas other models involved variable levels of axotomy. This could account for some differences in the various reports.
It is uncertain what factors are responsible for the increase in SP expression we and others have observed in the first week following nerve injury, but such factors are likely to be at the injury site, and related to the inflammatory response. While there are little supportive data for this concept, local inflammation of the rear paw causes the PPT gene to be induced in Aβ fibers (Neumann et al., 1996), and SP is expressed de novo in large diameter neurons innervating the lung that have been exposed to an allergen (Myers et al., 2002; Chuaychoo et al., 2005). Nerve growth factor (NGF) may also be involved. Axotomy such as performed in our experiments reduces the transport of NGF from peripheral tissue (Hendry et al., 1974; Campenot and MacInnis, 2004). Nerve growth factor is important for long term maintenance of SP expression following peripheral nerve injury (Verge et al., 1995; Csillik et al., 2003); thus the relative reduction of SP-positive neurons after 1 week may follow reduced NGF. Also, transgenic mice that over express NGF develop ectopic fibers that express SP (Ma et al., 1995; Ribeiro-da-Silva et al., 2000) and these mice show allodynia and hyperalgesia (McLeod et al., 1999).
Alterations in the amount of SP and the type of neurons expressing it following nerve injury may alter the way the organism processes nociception in response to somatosensory stimuli. In the spinal cord, SP depolarizes and activates dorsal horn neurons bearing NK-1 receptor (Radhakrishnan et al., 1998), and may trigger and maintain spinal central sensitization (McLeod et al., 1999; Cahill and Coderre, 2002; Khasabov et al., 2002). Furthermore, dorsal horn wide dynamic range neurons in mice devoid of the gene for the NK-1 receptor do not develop “wind up” as do normal wide dynamic range neurons (Weng et al., 2001), although PPT-A knockout mice do not show sensitization of wide dynamic range neurons subsequent to the peripheral application of mustard oil (Martin et al., 2004). When sensory axons are injured, spontaneous activity ensues in axons of all sizes, though there is no consensus regarding the time course of the ongoing activity (Tal et al., 1999; Lee et al., 1999; Michaelis et al., 2000; Boucher et al., 2000; Gorodetskaya et al., 2003); for review see Abdulla et al., 2003). Such activity releases SP from primary afferent neurons in the spinal cord (Schaible et al., 1990; Duggan et al., 1995). We have confirmed that SP, usually produced only in small DRG neurons, is produced in larger DRG neurons following axotomy, and in fewer smaller neurons at later time points. Therefore, it is reasonable to presume that spinal cord SP levels will increase following axotomy due to increased SP release from spontaneously active primary afferent neurons. At earlier time points DRG neurons of all sizes may participate, but because at later time points there are fewer smaller SP-positive neurons, it is more likely that larger neurons are more critically involved.
It is possible that the increase in SP in larger neurons may increase the area of spinal cord exposed to SP, which may affect secondary nociceptive and somatosensory spinal cord neurons. Through this mechanism, increased levels of SP could be expected to affect spinal cord levels beyond the lesioned segment. Upon entering the spinal cord, primary afferent axons branch rostrally and caudally to terminate at many levels of the dorsal horn (Wall and Werman, 1976; Bullitt, 1991; Ling et al., 2003; Woodbury and Koerber, 2003). This arborization was reflected by increased SP immunoreactivity in the dorsal horn 3 segments rostrally and 1 segment caudally following nerve transection (Abbadie et al., 1996). Thus, SP released from axotomized neurons, especially those with Aβ axons, which have more elaborate ramifications, could have widespread effects on NK1-receptor bearing nociceptive neurons over spinal segments that continue to receive normal input from their respective segmental primary afferent neurons. Sensitized wide dynamic range neurons that receive inputs from both nociceptive and non-nociceptive neurons are the logical sites for cross-modality sensory confusion (i.e., touch versus pain). Once sensitized, these neurons could be driven by normal function of intact low threshold mechanoreceptors, potentially resulting in activation of nociceptive pathways via normally non-noxious stimuli.
We have shown that more DRG neurons begin to express SP as soon as one day after total spinal nerve transection, and that large neurons but not small neurons maintain this expression 1 and 2 weeks following the lesion. The pattern of increased SP expression seems to parallel numerous reports of increased mechanical sensitivity of the hindpaw following similar nerve injuries (e.g., Kim and Chung, 1992; Ringkamp et al., 1999; Hammond et al., 2004; Sun et al., 2005). Augmented spinal release of SP from spontaneously firing, damaged primary afferent neurons, including larger DRG neurons, may trigger central sensitization and thus facilitate pain mediated by normal activity in neighboring, intact receptors.
Acknowledgements
We wish to thank Drs. Alan Light, Andrew Strassman, and Paul Farel for valuable discussions during the preparation of this manuscript.
Supported by National Institutes of Health AR048925 (NIAMS)
References
- Abbadie C, Brown JL, Mantyh PW, Basbaum AI. Spinal cord substance P receptor immunoreactivity increases in both inflammatory and nerve injury models of persistent pain. Neuroscience. 1996;70:201–9. doi: 10.1016/0306-4522(95)00343-h. [DOI] [PubMed] [Google Scholar]
- Abdulla FA, Moran TD, Balasubramanyan S, Smith PA. Effects and consequences of nerve injury on the electrical properties of sensory neurons. Can J Physiol Pharmacol. 2003;81:663–82. doi: 10.1139/y03-064. [DOI] [PubMed] [Google Scholar]
- Abdulla FA, Stebbing MJ, Smith PA. Effects of substance P on excitability and ionic currents of normal and axotomized rat dorsal root ganglion neurons. Eur J Neurosci. 2001;13:545–52. doi: 10.1046/j.0953-816x.2000.01429.x. [DOI] [PubMed] [Google Scholar]
- Ahmed M, Srinivasan GR, Theodorsson E, Schultzberg M, Kreicbergs A. Effects of surgical denervation on substance P and calcitonin gene-related peptide in adjuvant arthritis. Peptides. 1995;16:569–79. doi: 10.1016/0196-9781(95)00025-f. [DOI] [PubMed] [Google Scholar]
- Allen BJ, Li J, Menning PM, Rogers SD, Ghilardi J, Mantyh PW, Simone DA. Primary afferent fibers that contribute to increased substance P receptor internalization in the spinal cord after injury. J Neurophysiol. 1999;81:1379–90. doi: 10.1152/jn.1999.81.3.1379. [DOI] [PubMed] [Google Scholar]
- Antunes Bras JM, Laporte AM, Benoliel JJ, Bourgoin S, Mauborgne A, Hamon M, Cesselin F, Pohl M. Effects of peripheral axotomy on cholecystokinin neurotransmission in the rat spinal cord. J Neurochem. 1999;72:858–67. doi: 10.1046/j.1471-4159.1999.720858.x. [DOI] [PubMed] [Google Scholar]
- Bisby MA, Keen P. Regeneration of primary afferent neurons containing substance P-like immunoreactivity. Brain Res. 1986;365:85–95. doi: 10.1016/0006-8993(86)90725-0. [DOI] [PubMed] [Google Scholar]
- Boucher TJ, Okuse K, Bennett DLH, Munson JB, Wood JN, McMahon SB. Potent analgesic effects of GDNF in neuropathic pain states. Science. 2000;290:124–7. doi: 10.1126/science.290.5489.124. [DOI] [PubMed] [Google Scholar]
- Bullitt E. Somatotopy of spinal nociceptive processing. J Comp Neurol. 1991;312:279–90. doi: 10.1002/cne.903120210. [DOI] [PubMed] [Google Scholar]
- Cahill CM, Coderre TJ. Attenuation of hyperalgesia in a rat model of neuropathic pain after intrathecal pre- or post-treatment with a neurokinin-1 antagonist. Pain. 2002;95:277–85. doi: 10.1016/S0304-3959(01)00410-9. [DOI] [PubMed] [Google Scholar]
- Cameron AA, Cliffer KD, Dougherty PM, Garrison CJ, Willis WD, Carlton SM. Time course of degenerative and regenerative changes in the dorsal horn in a rat model of peripheral neuropathy. J Comp Neurol. 1997;379:428–42. doi: 10.1002/(sici)1096-9861(19970317)379:3<428::aid-cne8>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- Campenot RB, MacInnis BL. Retrograde transport of neurotrophins: fact and function. J Neurobiol. 2004;58:217–29. doi: 10.1002/neu.10322. [DOI] [PubMed] [Google Scholar]
- Chacur M, Milligan ED, Gazda LS, Armstrong C, Wang HC, Tracey KJ, Maier SF, Watkins LR. A new model of sciatic inflammatory neuritis (SIN): induction of unilateral and bilateral mechanical allodynia following acute unilateral peri-sciatic immune activation in rats. Pain. 2001;94:231–44. doi: 10.1016/S0304-3959(01)00354-2. [DOI] [PubMed] [Google Scholar]
- Chuaychoo B, Hunter DD, Myers AC, Kollarik M, Undem BJ. Allergen-induced substance P synthesis in large-diameter sensory neurons innervating the lungs. J Allergy Clin Immunol. 2005;116:325–31. doi: 10.1016/j.jaci.2005.04.005. [DOI] [PubMed] [Google Scholar]
- Csillik B, Janka Z, Boncz I, Kalman J, Mihaly A, Vecsei L, Knyihar E. Molecular plasticity of primary nociceptive neurons: relations of the NGF-c-jun system to neurotomy and chronic pain. Ann Anat. 2003;185:303–14. doi: 10.1016/S0940-9602(03)80050-X. [DOI] [PubMed] [Google Scholar]
- Degn J, Tandrup T, Jakobsen J. Effect of nerve crush on perikaryal number and volume of neurons in adult rat dorsal root ganglion. J Comp Neurol. 1999;412:186–92. doi: 10.1002/(sici)1096-9861(19990913)412:1<186::aid-cne14>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- DeVane CL. Substance P: a new era, a new role. Pharmacotherapy. 2001;21:1061–9. doi: 10.1592/phco.21.13.1061.34612. [DOI] [PubMed] [Google Scholar]
- Duggan AW, Riley RC, Mark MA, MacMillan SJ, Schaible HG. Afferent volley patterns and the spinal release of immunoreactive substance P in the dorsal horn of the anaesthetized spinal cat. Neuroscience. 1995;65:849–58. doi: 10.1016/0306-4522(94)00541-c. [DOI] [PubMed] [Google Scholar]
- Farel PB. Sensory neuron addition in juvenile rat: time course and specificity. J Comp Neurol. 2002;449:158–65. doi: 10.1002/cne.10274. [DOI] [PubMed] [Google Scholar]
- Gorodetskaya N, Constantin C, Janig W. Ectopic activity in cutaneous regenerating afferent nerve fibers following nerve lesion in the rat. Eur J Neurosci. 2003;18:2487–97. doi: 10.1046/j.1460-9568.2003.02974.x. [DOI] [PubMed] [Google Scholar]
- Gracely RH. Pain measurement. Acta Anaesthesiol Scand. 1999;43:897–908. doi: 10.1034/j.1399-6576.1999.430907.x. [DOI] [PubMed] [Google Scholar]
- Hammond DL, Ackerman L, Holdsworth R, Elzey B. Effects of spinal nerve ligation on immunohistochemically identified neurons in the L4 and L5 dorsal root ganglia of the rat. J Comp Neurol. 2004;475:575–89. doi: 10.1002/cne.20209. [DOI] [PubMed] [Google Scholar]
- Hendry IA, Stockel K, Thoenen H, Iversen LL. The retrograde axonal transport of nerve growth factor. Brain Res. 1974;68:103–21. doi: 10.1016/0006-8993(74)90536-8. [DOI] [PubMed] [Google Scholar]
- Hofmann HA, De Vry J, Siegling A, Spreyer P, Denzer D. Pharmacological sensitivity and gene expression analysis of the tibial nerve injury model of neuropathic pain. Eur J Pharmacol. 2003;470:17–25. doi: 10.1016/s0014-2999(03)01753-9. [DOI] [PubMed] [Google Scholar]
- Hokfelt T, Zhang X, Wiesenfeld-Hallin Z. Messenger plasticity in primary sensory neurons following axotomy and its functional implications. Trends Neurosci. 1994;17:22–30. doi: 10.1016/0166-2236(94)90031-0. [DOI] [PubMed] [Google Scholar]
- Honore P, Rogers SD, Schwei MJ, Salak-Johnson JL, Luger NM, Sabino MC, Clohisy DR, Mantyh PW. Murine models of inflammatory, neuropathic and cancer pain each generates a unique set of neurochemical changes in the spinal cord and sensory neurons. Neuroscience. 2000;98:585–98. doi: 10.1016/s0306-4522(00)00110-x. [DOI] [PubMed] [Google Scholar]
- Ji RR, Zhang Q, Pettersson RF, Hokfelt T. aFGF, bFGF and NGF differentially regulate neuropeptide expression in dorsal root ganglia after axotomy and induce autotomy. Regul Pept. 1996;66:179–89. doi: 10.1016/S0167-0115(96)00101-2. [DOI] [PubMed] [Google Scholar]
- Kawakami M, Weinstein JN, Chatani K, Spratt KF, Meller ST, Gebhart GF. Experimental lumbar radiculopathy. Behavioral and histologic changes in a model of radicular pain after spinal nerve root irritation with chromic gut ligatures in the rat. Spine. 1994;19:1795–802. doi: 10.1097/00007632-199408150-00002. [DOI] [PubMed] [Google Scholar]
- Khasabov SG, Rogers SD, Ghilardi JR, Peters CM, Mantyh PW, Simone DA. Spinal neurons that possess the substance P receptor are required for the development of central sensitization. J Neurosci. 2002;22:9086–98. doi: 10.1523/JNEUROSCI.22-20-09086.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SH, Chung JM. An experimental model for peripheral neuropathy produced by segmental spinal nerve ligation in the rat. Pain. 1992;50:355–63. doi: 10.1016/0304-3959(92)90041-9. [DOI] [PubMed] [Google Scholar]
- Kobayashi S, Yoshizawa H, Yamada S. Pathology of lumbar nerve root compression. Part 2: morphological and immunohistochemical changes of dorsal root ganglion. J Orthop Res. 2004;22:180–8. doi: 10.1016/S0736-0266(03)00132-3. [DOI] [PubMed] [Google Scholar]
- Lawson SN. The postnatal development of large light and small dark neurons in mouse dorsal root ganglia: a statistical analysis of cell numbers and size. J Neurocytol. 1979;8:275–94. doi: 10.1007/BF01236123. [DOI] [PubMed] [Google Scholar]
- Lawson SN. Morphological and biochemical cell types of sensory neurones. In: Scott SA, editor. Sensory neurones: Diversity, Development and Plasticity. Oxford University Press; New York: 1992. pp. 27–59. [Google Scholar]
- Lee DH, Liu XZ, Kim HT, Chung KS, Chung JM. Receptor subtype mediating the adrenergic sensitivity of pain behavior and ectopic discharges in neuropathic Lewis rats. J Neurophysiol. 1999;81:2226–33. doi: 10.1152/jn.1999.81.5.2226. [DOI] [PubMed] [Google Scholar]
- Lee WT, Sohn MK, Park SH, Ahn SK, Lee JE, Park KA. Studies on the changes of c-fos protein in spinal cord and neurotransmitter in dorsal root ganglion of the rat with an experimental peripheral neuropathy. Yonsei Med J. 2001;42:30–40. doi: 10.3349/ymj.2001.42.1.30. [DOI] [PubMed] [Google Scholar]
- Lee Y, Takami K, Kawai Y, Girgis S, Hillyard CJ, MacIntyre I, Emson PC, Tohyama M. Distribution of calcitonin gene-related peptide in the rat peripheral nervous system with reference to its coexistence with substance P. Neuroscience. 1985;15:1227–37. doi: 10.1016/0306-4522(85)90265-9. [DOI] [PubMed] [Google Scholar]
- Ling LJ, Honda T, Shimada Y, Ozaki N, Shiraishi Y, Sugiura Y. Central projection of unmyelinated (C) primary afferent fibers from gastrocnemius muscle in the guinea pig. J Comp Neurol. 2003;461:140–50. doi: 10.1002/cne.10619. [DOI] [PubMed] [Google Scholar]
- Ma W, Bisby MA. Partial and complete sciatic nerve injuries induce similar increases of neuropeptide Y and vasoactive intestinal peptide immunoreactivities in primary sensory neurons and their central projections. Neuroscience. 1998a;86:1217–34. doi: 10.1016/s0306-4522(98)00068-2. [DOI] [PubMed] [Google Scholar]
- Ma W, Ribeiro-da-Silva A, Noel G, Julien JP, Cuello AC. Ectopic substance P and calcitonin gene-related peptide immunoreactive fibres in the spinal cord of transgenic mice over-expressing nerve growth factor. Eur J Neurosci. 1995;7:2021–35. doi: 10.1111/j.1460-9568.1995.tb00625.x. [DOI] [PubMed] [Google Scholar]
- Ma WY, Bisby MA. Increase of preprotachykinin mRNA and substance P immunoreactivity in spared dorsal root ganglion neurons following partial sciatic nerve injury. Eur J Neurosci. 1998b;10:2388–99. doi: 10.1046/j.1460-9568.1998.00249.x. [DOI] [PubMed] [Google Scholar]
- Macdonald R, Bingham S, Bond BC, Parsons AA, Philpott KL. Determination of changes in mRNA expression in a rat model of neuropathic pain by Taqman (TM) quantitative RT-PCR. Mol Brain Res. 2001;90:48–56. doi: 10.1016/s0169-328x(01)00086-9. [DOI] [PubMed] [Google Scholar]
- Malcangio M, Ramer MS, Jones MG, McMahon SB. Abnormal substance P release from the spinal cord following injury to primary sensory neurons. Eur J Neurosci. 2000;12:397–9. doi: 10.1046/j.1460-9568.2000.00946.x. [DOI] [PubMed] [Google Scholar]
- Mantyh PW. Substance P and the inflammatory and immune response. Ann N Y Acad Sci. 1991;632:263–71. doi: 10.1111/j.1749-6632.1991.tb33114.x. [DOI] [PubMed] [Google Scholar]
- Marchand JE, Wurm WH, Kato T, Kream RM. Altered tachykinin expression by dorsal root ganglion neurons in a rat model of neuropathic pain. Pain. 1994;58:219–31. doi: 10.1016/0304-3959(94)90202-X. [DOI] [PubMed] [Google Scholar]
- Martin WJ, Cao Y, Basbaum AI. Characterization of wide dynamic range neurons in the deep dorsal horn of the spinal cord in preprotachykinin-a null mice in vivo. J Neurophysiol. 2004;91:1945–54. doi: 10.1152/jn.00945.2003. [DOI] [PubMed] [Google Scholar]
- McCullagh P, Nelder JA. Generalized linear models. 2 ed. Chapman and Hall; London: 1989. [Google Scholar]
- McLeod AL, Ritchie J, Cuello AC, Julien JP, Ribeiro-da-Silva A, Henry JL. Transgenic mice over-expressing substance P exhibit allodynia and hyperalgesia which are reversed by substance P and N-methyl-D-aspartate receptor antagonists. Neuroscience. 1999;89:891–9. doi: 10.1016/s0306-4522(98)00365-0. [DOI] [PubMed] [Google Scholar]
- Meyer-Tuve A, Malcangio M, Ebersberger A, Mazario J, Schaible HG. Effect of brain-derived neurotrophic factor on the release of substance P from rat spinal cord. NeuroReport. 2001;12:21–4. doi: 10.1097/00001756-200101220-00012. [DOI] [PubMed] [Google Scholar]
- Michaelis M, Liu XG, Janig W. Axotomized and intact muscle afferents but no skin afferents develop ongoing discharges of dorsal root ganglion origin after peripheral nerve lesion. J Neurosci. 2000;20:2742–8. doi: 10.1523/JNEUROSCI.20-07-02742.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohiuddin L, Delcroix JD, Fernyhough P, Tomlinson DR. Focally administered nerve growth factor suppresses molecular regenerative responses of axotomized peripheral afferents in rats. Neuroscience. 1999;91:265–71. doi: 10.1016/s0306-4522(98)00582-x. [DOI] [PubMed] [Google Scholar]
- Murphy PG, Ramer MS, Borthwick L, Gauldie J, Richardson PM, Bisby MA. Endogenous interleukin-6 contributes to hypersensitivity to cutaneous stimuli and changes in neuropeptides associated with chronic nerve constriction in mice. Eur J Neurosci. 1999;11:2243–53. doi: 10.1046/j.1460-9568.1999.00641.x. [DOI] [PubMed] [Google Scholar]
- Myers AC, Kajekar R, Undem BJ. Allergic inflammation-induced neuropeptide production in rapidly adapting afferent nerves in guinea pig airways. Am J Physiol Lung Cell Mol Physiol. 2002;282:L775–L781. doi: 10.1152/ajplung.00353.2001. [DOI] [PubMed] [Google Scholar]
- Neumann S, Doubell TP, Leslie T, Woolf CJ. Inflammatory pain hypersensitivity mediated by phenotypic switch in myelinated primary sensory neurons. Nature. 1996;384:360–4. doi: 10.1038/384360a0. [DOI] [PubMed] [Google Scholar]
- Nichols ML, Allen BJ, Rogers SD, Ghilardi JR, Honore P, Luger NM, Finke MP, Li J, Lappi DA, Simone DA, Mantyh PW. Transmission of chronic nociception by spinal neurons expressing the substance P receptor. Science. 1999;286:1558–61. doi: 10.1126/science.286.5444.1558. [DOI] [PubMed] [Google Scholar]
- Noguchi K, Dubner R, De Leon M, Senba E, Ruda MA. Axotomy induces preprotachykinin gene expression in a subpopulation of dorsal root ganglion neurons. J Neurosci Res. 1994;37:596–603. doi: 10.1002/jnr.490370506. [DOI] [PubMed] [Google Scholar]
- Noguchi K, Kawai Y, Fukuoka T, Senba E, Miki K. Substance P induced by peripheral nerve injury in primary afferent sensory neurons and its effect on dorsal column nucleus neurons. J Neurosci. 1995;15:7633–43. doi: 10.1523/JNEUROSCI.15-11-07633.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otsuka M, Yoshioka K. Neurotransmitter functions of mammalian tachykinins. Physiol Rev. 1993;73:229–308. doi: 10.1152/physrev.1993.73.2.229. [DOI] [PubMed] [Google Scholar]
- Partata WA, Krepsky AMR, Xavier LL, Marques M, Achaval M. Substance P immunoreactivity in the lumbar spinal cord of the turtle Trachemys dorbigni following peripheral nerve injury. BRAZILIAN JOURNAL OF MEDICAL AND BIOLOGICAL RESEARCH. 2003;36:515–20. doi: 10.1590/s0100-879x2003000400015. [DOI] [PubMed] [Google Scholar]
- Radhakrishnan V, Iyengar S, Henry JL. The nonpeptide NK-1 receptor antagonists LY303870 and LY306740 block the responses of spinal dorsal horn neurons to substance P and to peripheral noxious stimuli. Neuroscience. 1998;83:1251–60. doi: 10.1016/s0306-4522(97)00313-8. [DOI] [PubMed] [Google Scholar]
- Ribeiro-da-Silva A, Cuello AC, Henry JL. NGF over-expression during development leads to permanent alterations in innervation in the spinal cord and in behavioural responses to sensory stimuli. Neuropeptides. 2000;34:281–91. doi: 10.1054/npep.2000.0822. [DOI] [PubMed] [Google Scholar]
- Ringkamp M, Grethel EJ, Choi Y, Meyer RA, Raja SN. Mechanical hyperalgesia after spinal nerve ligation in rat is not reversed by intraplantar or systemic administration of adrenergic antagonists. Pain. 1999;79:135–41. doi: 10.1016/s0304-3959(98)00185-7. [DOI] [PubMed] [Google Scholar]
- Rydh-Rinder M, Holmberg K, Elfvin LG, Wiesenfeld-Hallin Z, Hokfelt T. Effects of peripheral axotomy on neuropeptides and nitric oxide synthase in dorsal root ganglia and spinal cord of the guinea pig: an immunohistochemical study. Brain Res. 1996;707:180–8. doi: 10.1016/0006-8993(95)01231-1. [DOI] [PubMed] [Google Scholar]
- Sanderson NK, Skinner K, Julius D, Basbaum AI. Co-localization of endomorphin-2 and substance P in primary afferent nociceptors and effects of injury: a light and electron microscopic study in the rat. Eur J Neurosci. 2004;19:1789–99. doi: 10.1111/j.1460-9568.2004.03284.x. [DOI] [PubMed] [Google Scholar]
- Schaible HG, Jarrott B, Hope PJ, Duggan AW. Release of immunoreactive substance P in the spinal cord during development of acute arthritis in the knee joint of the cat: a study with antibody microprobes. Brain Res. 1990;529:214–23. doi: 10.1016/0006-8993(90)90830-5. [DOI] [PubMed] [Google Scholar]
- Schicho R, Donnerer J, Liebmann I, Lippe IT. Nociceptive transmitter release in the dorsal spinal cord by capsaicin-sensitive fibers after noxious gastric stimulation. Brain Res. 2005;1039:108–15. doi: 10.1016/j.brainres.2005.01.050. [DOI] [PubMed] [Google Scholar]
- Siri CR, Shortland PJ, Grant G, Olivius NP. Delayed administration of NGF reverses nerve injury induced central alterations of primary afferents. NeuroReport. 2001;12:1899–902. doi: 10.1097/00001756-200107030-00026. [DOI] [PubMed] [Google Scholar]
- Sondell M, Lundborg G, Kanje M. Vascular endothelial growth factor stimulates Schwann cell invasion and neovascularization of acellular nerve grafts. Brain Res. 1999;846:219–28. doi: 10.1016/s0006-8993(99)02056-9. [DOI] [PubMed] [Google Scholar]
- Sorkin LS, Doom CM. Epineurial application of TNF elicits an acute mechanical hyperalgesia in the awake rat. J Peripher Nerv Syst. 2000;5:96–100. doi: 10.1046/j.1529-8027.2000.00012.x. [DOI] [PubMed] [Google Scholar]
- Sterne GD, Brown RA, Green CJ, Terenghi G. NT-3 modulates NPY expression in primary sensory neurons following peripheral nerve injury. J Anat. 1998;193:273–81. doi: 10.1046/j.1469-7580.1998.19320273.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Q, Tu H, Xing GG, Han JS, Wan Y. Ectopic discharges from injured nerve fibers are highly correlated with tactile allodynia only in early, but not late, stage in rats with spinal nerve ligation. Exp Neurol. 2005;191:128–36. doi: 10.1016/j.expneurol.2004.09.008. [DOI] [PubMed] [Google Scholar]
- Swamydas M, Skoff AM, Adler JE. Partial sciatic nerve transection causes redistribution of pain-related peptides and lowers withdrawal threshold. Exp Neurol. 2004;188:444–51. doi: 10.1016/j.expneurol.2004.04.018. [DOI] [PubMed] [Google Scholar]
- Szucs P, Polgar E, Spigelman I, Porszasz R, Nagy I. Neurokinin-1 receptor expression in dorsal root ganglion neurons of young rats. J Peripher Nerv Syst. 1999;4:270–8. [PubMed] [Google Scholar]
- Tal M, Wall PD, Devor M. Myelinated afferent fiber types that become spontaneously active and mechanosensitive following nerve transection in the rat. Brain Res. 1999;824:218–23. doi: 10.1016/s0006-8993(99)01190-7. [DOI] [PubMed] [Google Scholar]
- Umemoto S, Noguchi K, Kawai Y, Senba E. The expression of neuropeptides and their mRNAs in the trigeminal mesencephalic nucleus following masseteric nerve transection. Brain Res Mol Brain Res. 1994;23:93–9. doi: 10.1016/0169-328x(94)90215-1. [DOI] [PubMed] [Google Scholar]
- Valder CR, Liu JJ, Song YH, Luo ZD. Coupling gene chip analyses and rat genetic variances in identifying potential target genes that may contribute to neuropathic allodynia development. J Neurochem. 2003;87:560–73. doi: 10.1046/j.1471-4159.2003.02016.x. [DOI] [PubMed] [Google Scholar]
- Verge VM, Richardson PM, Wiesenfeld-Hallin Z, Hokfelt T. Differential influence of nerve growth factor on neuropeptide expression in vivo: a novel role in peptide suppression in adult sensory neurons. J Neurosci. 1995;15:2081–96. doi: 10.1523/JNEUROSCI.15-03-02081.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vestergaard S, Tandrup T, Jakobsen J. Effect of permanent axotomy on number and volume of dorsal root ganglion cell bodies. J Comp Neurol. 1997;388:307–12. [PubMed] [Google Scholar]
- Wall PD, Werman R. The physiology and anatomy of long ranging afferent fibres within the spinal cord. J Physiol (Lond) 1976;255:321–34. doi: 10.1113/jphysiol.1976.sp011282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallas TR, Winterson BJ, Ransil BJ, Bove GM. Paw withdrawal thresholds and persistent hindlimb flexion in experimental mononeuropathies. J Pain. 2003;4:222–30. doi: 10.1016/s1526-5900(03)00619-9. [DOI] [PubMed] [Google Scholar]
- Wang H, Sun H, Della PK, Benz RJ, Xu J, Gerhold DL, Holder DJ, Koblan KS. Chronic neuropathic pain is accompanied by global changes in gene expression and shares pathobiology with neurodegenerative diseases. Neuroscience. 2002;114:529–46. doi: 10.1016/s0306-4522(02)00341-x. [DOI] [PubMed] [Google Scholar]
- Weng HR, Mansikka H, Winchurch R, Raja SN, Dougherty PM. Sensory processing in the deep spinal dorsal horn of neurokinin-1 receptor knockout mice. Anesthesiology. 2001;94:1105–12. doi: 10.1097/00000542-200106000-00027. [DOI] [PubMed] [Google Scholar]
- White FA, Kocsis JD. A-fiber sprouting in spinal cord dorsal horn is attenuated by proximal nerve stump encapsulation. Exp Neurol. 2002;177:385–95. doi: 10.1006/exnr.2002.7996. [DOI] [PubMed] [Google Scholar]
- Wong HK, Tan KJ. Effects of corticosteroids on nerve root recovery after spinal nerve root compression. Clin Orthop. 2002:248–52. doi: 10.1097/00003086-200210000-00036. [DOI] [PubMed] [Google Scholar]
- Woodbury CJ, Koerber HR. Widespread projections from myelinated nociceptors throughout the substantia gelatinosa provide novel insights into neonatal hypersensitivity. J Neurosci. 2003;23:601–10. doi: 10.1523/JNEUROSCI.23-02-00601.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolf CJ, Mannion RJ. Neuropathic pain: aetiology, symptoms, mechanisms, and management. Lancet. 1999;353:1959–64. doi: 10.1016/S0140-6736(99)01307-0. [DOI] [PubMed] [Google Scholar]
- Xiao HS, Huang QH, Zhang FX, Bao L, Lu YJ, Guo C, Yang L, Huang WJ, Fu G, Xu SH, Cheng XP, Yan Q, Zhu ZD, Zhang X, Chen Z, Han ZG. Identification of gene expression profile of dorsal root ganglion in the rat peripheral axotomy model of neuropathic pain. Proc Natl Acad Sci U S A. 2002;99:8360–5. doi: 10.1073/pnas.122231899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q, Ji RR, Lindsay R, Hokfelt T. Effect of growth factors on substance P mRNA expression in axotomized dorsal root ganglia. NeuroReport. 1995a;6:1309–12. doi: 10.1097/00001756-199506090-00020. [DOI] [PubMed] [Google Scholar]
- Zhang X, Aman K, Hokfelt T. Secretory pathways of neuropeptides in rat lumbar dorsal root ganglion neurons and effects of peripheral axotomy. J Comp Neurol. 1995b;352:481–500. doi: 10.1002/cne.903520402. [DOI] [PubMed] [Google Scholar]