Abstract
Traditional herbal formulations, such as Juzen-taiho-to (TJ-48), are used extensively in medical practice in Asia even though their mechanism of action remains elusive. This study tested a hypothesis that TJ-48 is protective against hepatocarcinogenesis by impeding Kupffer cell-induced oxidative stress. Forty-eight patients were randomly assigned to receive TJ-48 (n = 10), or no supplementation (n = 38) for up to 6 years after surgical treatment for hepatocellular carcinoma (HCC). In addition, to investigate the mechanism of protective action of TJ-48, diethylnitrosamine-containing water was administered for 22 weeks to male mice that were fed regular chow or TJ-48-containing diet. Liver tumor incidence, cell proliferation, number of 8-hydroxy-2′-deoxyguanosine-or F4/80-positive cells, and cytokine expression were evaluated. Although most of the patients experienced recurrence of HCC, a significantly longer intrahepatic recurrence-free survival was observed in the TJ-48 group. In mice, TJ-48 inhibited the development of liver tumors, reduced oxidative DNA damage, inflammatory cell infiltration and cytokine expression. Administration of TJ-48 improves intrahepatic recurrence-free survival after surgical treatment of hepatocellular carcinoma. On the basis of animal experiments, we reason that the protective mechanism of TJ-48 involves inhibition of Kupffer cells. This leads to lower levels of pro-inflammatory cytokines and oxidants in liver which may slow down the process of hepatocarcinogenesis and improves hepatic recurrence-free survival in patients with HCC.
Keywords: TJ-48, hepatocellular carcinoma, Kupffer cell, intrahepatic recurrence-free survival
Hepatocellular carcinoma (HCC) is one of the world’s most common cancers and it is commonly associated with liver cirrhosis due to alcohol and chronic viral infection (hepatitis virus B and C), aflatoxin B1 exposure, and a variety of metabolic liver diseases.1,2 Chronic inflammation is thought to be tightly linked to the mechanisms of HCC through increased production of free radicals from macrophages and neutrophils at the site of inflammation.3,4 Indeed, markers of oxidative stress, such as 8-hydroxy-deoxyguanosine (8-OHdG), 4-hydroxynonenal and malondialdehyde, are commonly elevated in the liver of patients with chronic viral hepatitis infection and correlate well with the degree of viral infection and inflammation, known risk factors for HCC.5–7 Recently, we evaluated the relationship between inflammation, intrahepatic oxidative stress, oxidative DNA damage and the progression of carcinogenesis in HCV-infected human liver and showed that patients with greater intra-hepatic oxidative stress exhibited much faster HCC recurrence.8
Because a strong correlation exists between inflammatory cells in liver, oxidative stress and cancer, chronic inflammation is considered to be one of the prime targets for therapeutic intervention to prevent both progression of chronic liver disease into cancer, and relapse of HCC. Currently available anti-viral therapies, which are partially directed towards mitigation of intra-hepatic inflammation, are the only means of interrupting the progression from chronic hepatitis to HCC.9 However, complementary and alternative medicines are becoming popular among patients with liver disease, particularly chronic hepatitis C and alcohol-induced liver disease.10 Silymarin, glycyrrhizin, Chinese traditional medicine, herbal medicine 861, CH-100, TJ-9, TJ-108, and Phyllanthus amarus are the herbs or blends commonly used for hepatic disorders around the world.11,12
Juzen-taiho-to (TJ-48) is a traditional Japanese herbal medicine formula which is composed of 10 different herbs and the content of this preparation is tightly regulated.13 It is used in medical practice in Japan for treatment of patients with chronic disease. Interestingly, several recent studies have shown that TJ-48 has an anti-tumor effect in animal models.14–16 Although the active ingredient(s) of this or other traditional herbal medicines have not been determined and the mechanisms of their protective action remains obscure, some constituents of TJ-48 have been shown to possess antioxidant activities17,18 and act as immunomodulators.19,20
Although there is a growing body of evidence that herbal preparations may prove beneficial in management of chronic liver diseases, caution has been urged as only a few clinical trials have been conducted. This study, an extension of our previous observation that higher intrahepatic inflammation correlates with shorter disease-free survival after removal of the primary HCC,8 tested if administration of TJ-48 will have a beneficial effect in patients who underwent liver tumor resection. Although relatively small, the current study shows considerable improvement in intrahepatic recurrence-free survival in patients on TJ-48 supplementation. Next, we tested a hypothesis that TJ-48 exerts its beneficial effects against liver carcinogenesis by impeding Kupffer cell-induced oxidative stress. In a mouse model of liver carcinogenesis induced by diethylnitrosamine (DEN), TJ-48 inhibited the development of liver tumors, as well as reduced the number of inflammatory cells, expression of proinflammatory cytokines, and oxidative DNA damage in liver. In cultured mouse Kupffer cells, TJ-48 significantly blunted DEN-induced production of proinflammatory cytokines and reactive oxygen species. Thus, we suggest that treatment with TJ-48 may be beneficial as a chronic adjuvant therapy in patients who undergo curative HCC removal and that it acts by blunting inflammation in liver.
Material and methods
Patients and study design
This is a retrospective single-center, open-label study. From 2000 to 2005, consecutive patients in the University of Yamanashi Hospital (Yamanashi Prefecture, Japan) who (i) were diagnosed as having HCC with no vascular invasion and (ii) underwent curative surgery (resection or intra-operative microwave coagulation therapy (MCT)), were enrolled in this study. Confirmation of the complete removal of the tumor(s) in patients who underwent MCT was obtained by means of computed tomography. After surgery, patients were assigned to receive TJ-48 (n = 10, 7.5 g daily oral dose starting 1 month after surgery and continuing for up to 6 years), or not (control group, n = 38). TJ-48, supplied by Tsumura (Tokyo, Japan), is a mixture of 10 plants (Table I). The mixture (28.5 g) was added to 285 ml of water, extracted at 100°C for 1 hr, filtered and spray-dried (TJ-48, 2.3 g). No adverse effects because of the administration of TJ-48 were observed. All patients returned to the ambulatory care clinic for additional tests monthly. Serum α-fetoprotein levels were measured every month. In addition, ultrasonography and computed tomography of the liver were performed every 3 and 6 months, respectively. Informed consent was obtained from each patient included in the study and the study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki as reflected in a priori approval by the Institutional Board on Ethics for Human Science at the University of Yamanashi. All clinical data are summarized in Table II.
TABLE I.
COMPOSITION OF JUZEN-TAIHO-TO (TJ-48)
| Crude drug | Botanical origin | Ratio (g) |
|---|---|---|
| Astragali radix | Root of Astragalus membranaceus Bunge | 3.0 |
| Cinnamomi cortex | Bark of Cinnamomum cassia Blume | 3.0 |
| Rehmanniae radix | Root of Rehmannia glutinosa Libosch var. purpurea Makino | 3.0 |
| Paeoniae radix | Rhizome of Paeonia lactiflora Pall | 3.0 |
| Cnidii rhizoma | Rhizome of Cnidium officinale Makino | 3.0 |
| Atractylodis lanceae rhizoma | Rhizome of Atractylodes lancea DC | 3.0 |
| Angelicae radix | Root of Angelica acutiloba Kitagawa | 3.0 |
| Ginseng radix | Root of Panax ginseng C.A. Meyer | 3.0 |
| Hoelen | Fungus of Poria cocos Wolf | 3.0 |
| Glycyrrhizae radix | Root of Glycyrrhiza uralensis Fisch. and DC | 1.5 |
TABLE II.
BASELINE CHARACTERISTICS OF THE STUDY POPULATION
| Control group (n = 38) | TJ-8 group (n = 10) | p Value | ||
|---|---|---|---|---|
| Age1 | 66.7 ± 7.0 | 66.9 ± 7.6 | 0.9242 | |
| Gender | M | 32 (84.2%) | 6 (60.0%) | 0.1833 |
| F | 6 (15.8%) | 4 (40.0%) | ||
| Viral infection status | HBV | 1 (2.6%) | 1 (10.0%) | 0.9603 |
| HCV | 35 (92.1%) | 8 (80.0%) | ||
| No virus | 2 (5.3%) | 1 (10.0%) | ||
| Platelet (104/μl) | 12.5 ± 4.6 | 10.5 ± 3.5 | 0.1952 | |
| Alanine aminotransferase (IU/l)1 | 58.1 ± 38.5 | 50.6 ± 34.7 | 0.5792 | |
| Total bilirubin (mg/dl)1 | 0.86 ± 0.35 | 0.83 ± 0.29 | 0.8022 | |
| Prothrombin time (%)1 | 80.8 ± 14.3 | 74.7 ± 8.8 | 0.2062 | |
| Indocyanine green (%)1 | 19.9 ± 8.4 | 27.9 ± 12.0 | 0.0222 | |
| Alpha-fetoprotein (ng/ml)1 | 543.5 ± 1417.8 | 161.1 ± 350.4 | 0.4052 | |
| Tumor diameter | ≥20 mm | 11 (28.9%) | 6 (60.0%) | 0.1463 |
| >20 mm | 27 (71.1%) | 4 (40.0%) | ||
| Number of tumors | Solitary | 21 (55.3%) | 5 (50.0%) | 0.9533 |
| Multiple | 17 (44.7%) | 5 (50.0%) | ||
| Tumor stage | I | 6 (15.8%) | 2 (20.0%) | 0.8323 |
| II | 20 (52.6%) | 7 (70.0%) | ||
| III | 12 (31.6%) | 1 (10.0%) | ||
| IV | 0 (0%) | 0 (0%) | ||
| Tumor differentiation | Well | 12 (31.6%) | 2 (20.0%) | 0.7013 |
| Moderate or poor | 26 (68.4%) | 8 (80.0%) | ||
| Liver cirrhosis | No cirrhosis | 17 (44.7%) | 2 (20%) | 0.2763 |
| Cirrhosis | 21 (55.3%) | 8 (80.0%) |
The data shown are mean ± standard deviation.
Statistical analysis was performed by two-tailed student t-test.
Statistical analysis was performed by m × n chi square test.
Animals and treatments
Male mice (C57BL/6N and C3H/HeN) (8 weeks old) were obtained from Charles River (Yokohama, Japan). All animals used for this study were housed in sterilized cages in a facility with a 12-hr night/day cycle. Temperature and relative humidity were held at (23 ± 2)°C and (50 ± 10)%, respectively. The University of Yamanashi staff maintained these animal facilities, and veterinarians were always available to ensure animal health. All animals were given humane care in compliance with governmental regulations and institutional guidelines and studies were performed according to protocols approved by the appropriate institutional review board. Before experimenting, animals were randomly assigned into one of 3 groups (Fig. 1). Control group received tap water and a standard lab chow (Oriental Yeast, Tokyo, Japan) ad libitum throughout the study (up to 22 weeks). DEN group was given DEN (Sigma, St. Louis, MO, 25 mg/l)-containing water for 10 weeks followed by normal tap water for 12 weeks and was maintained on a standard lab chow ad libitum. DEN + TJ-48 group was similar to the DEN group except that mice were maintained on a lab chow supplemented with TJ-48 (1.6% w/w). All animals had free access to food and water and the health status of the animals was monitored every other day. Before killing, mice were anesthetized with diethyl ether and following exsanguination livers were removed, weighed and examined for presence of tumors. Sections from the right median and left lateral lobe were fixed in 10% formalin. The remaining tissue was snap frozen in liquid nitrogen. The samples were stored at −80°C until assayed.
Figure 1.
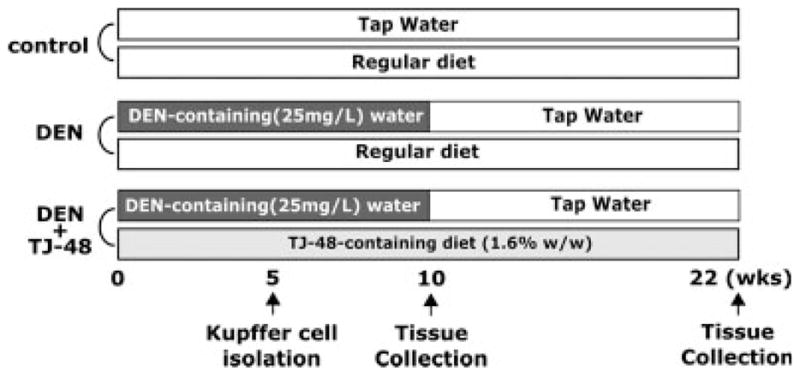
Schematic representation of the treatments used in the mouse model of DEN-induced liver carcinogenesis. See Material and methods section for experimental details.
Immunohistochemical detection of 8-hydroxy-2-deoxyguanosine (8-OHdG), proliferating cell nuclear antigen (PCNA) and F4/80 antigen
Formalin-fixed, paraffin-embedded sections (5 μm) of mouse liver were mounted on glass slides and stained immunohistochemically (overnight at 4°C) with anti-8-OH-dG (1:200, Fitzgerald, Concord, MA), anti-PCNA (1:250, Abcam, Cambridge, MA), or anti-F4/80 (1:100, Serotec, Oxford, England) antibodies. Before addition of the primary antibody, sections were deparaffinized and placed in phosphate-buffered saline with 1% Tween 20. Biotinylated secondary antibody conjugated with avidin-biotin-horseradish peroxidase (DAKO Envison system HRP, DAKO Cytomation, Carpinteria, CA) and 3′,3′-diaminobenzidine tetrahydrochloride were used for standard avidin-biotinylated peroxidase detection (8-OH-dG and PCNA). ABC Elite kit (Vector Laboratories, Burlingame, CA) was used as the means of secondary detection of F4/80-positive cells. All slides were counterstained with hematoxylin. To ensure the quantitative measurement of each immunoreaction, all sections from each animal and group to be compared were processed in parallel. Cells that stained positive for each primary antibody were counted in at least 10 different random fields at ×200 magnification (at least 1,000 cells in each slide) using BIOQUANT software (BIOQUANT Image Analysis, Nashville, TN) and the percentage of positive cells was calculated.
RNA isolation and real-time PCR
Although frozen, ~30 mg of tissue was removed for each mouse liver sample and homogenized in 600 μl RLT buffer (Qiagen, Valencia, CA) containing 1% β-mercaptoethanol. The lysate was centrifuged for 3 min at 13,000 rpm. RNA was isolated using an RNeasy kit (Qiagen) as per the manufacturer’s protocol. RNA integrity and quantification were assessed using RNA 6000 Nano Assay LabChips (Agilent Technologies, Santa Clara, CA) and analyzed on a 2100 Bioanalyzer (Agilent Technologies) as per the manufacturer’s protocol. Total RNA (10 μg) was reverse transcribed using random primers and the high capacity cDNA archive kit (Applied Biosystems, Foster City, CA) according to the manufacturer’s protocol. The resulting cDNA was stored at −20°C. The following Taqman (Applied Biosystems) gene expression assays were used for quantitative real-time PCR: Tnfα (Mm00443258_ml), IL-1β (Mm00434228_m1), and Gus-b (Mm00446953_m1). Reactions were performed in a 96-well assay format. Each plate contained one experimental gene and a housekeeping (Gus-b) gene. Each reaction contained 2 μl of cDNA template, 20.5 μl of RNase-free H2O, 2.5 μl of TaqMan gene expression assay primer and 25 μl of TaqMan universal PCR master mix (Applied Biosystems). Reactions were processed for 1 cycle of 2 min at 50°C and 10 min at 95°C, followed by 40 cycles of 15 sec at 95°C and 1 min at 60°C on the Mx3000P QPCR instrument (Stratagene, La Jolla, CA). All reactions were carried out in triplicate. The cycle threshold (Ct) for each sample was determined from the linear region of the amplification plot. The ΔCt value for Tnfα and IL-1β relative to the control gene Gus-b was determined. The ΔΔCt values were calculated using treated group means relative to control group means. Fold change data were calculated from the ΔΔCt values.
Kupffer cell isolation and assessment of cytokine production
Two experiments were conducted to evaluate the effects of TJ-48 on the production of inflammatory cytokines by Kupffer cells in vivo and in vitro. In the first experiment, 8-week-old male C57BL/6N mice were randomly divided into control (normal chow, tap water), DEN (normal chow, 25 mg/l in drinking water), and DEN (25 mg/l in drinking water) + TJ-48 (1.6% w/w in the diet) groups as detailed earlier. Kupffer cells were isolated after 5 weeks of treatment (Fig. 1) as detailed elsewhere21 with minor modifications. Briefly, mice were anesthetized with sodium pentobarbital (80 mg/kg body weight, i.p.), the abdomen was opened and the portal vein was cannulated. The liver was perfused in situ for 5 min with Ca2+/Mg2+-free liver perfusion medium (LPM-1: 8,000 mg/l NaCl, 400 mg/l KCl, 88.17 mg/l NaH2PO4-2H2O, 120.45 mg/l Na2HPO4, 2380 mg/l HEPES, 350 mg/l NaHCO3, 190 mg/l EDTA, 900 mg/l glucose, 6 mg/l Phenol red; pH 7.4, 37°C), and was then perfused with complete liver perfusion medium (LPM-2: same as LPM-1 except without EDTA and glucose, but with 560 mg/l CaCl2-2H2O; pH 7.4, 37°C) containing 0.06% collagenase type IV (Sigma, St. Louis, MO) for additional 15 min. After perfusion, the liver was removed, cut into small pieces, and homogenized. After passing through a gauze filter (mesh size ~60 m), cells were washed twice with warm Gey’s balanced salt solution (GBSS-B: 370 mg/l KCl, 210 mg/l MgCl2-6H2O, 70 mg/l MgSO4-7H2O, 150 mg/l NaH2PO4-2H2O, 30 mg/l KH2PO4, 1,090 mg/l glucose, 227 mg/l NaHCO3, 225 mg/l CaCl2-2H2O, 6 mg/l phenol red, 8,000 mg/l NaCl, 100 U/l streptomycin, 105 U/l penicillin G; pH 7.4) and centrifuged over 16% (wt/vol) Nycodenz (Axis-Shields, Oslo, Norway) gradient for 20 min at 1,900g at 4°C. Kupffer cells were collected from under the interface, washed with GBSS-B and resuspended at a concentration of 1 × 106 cells/ml in D-MEM media (Invitrogen, Carlsbad, CA). Isolated Kupffer cells were seeded (106 per well) in 24-well dishes and cultured in D-MEM media for 12 hr. After changing the media to remove the nonadherent cells, cultures were maintained for additional 24 hr and the media was collected and stored at −80°C.
In the second experiment, 8-week-old male C57BL/6N mice were maintained on a standard lab chow and received either tap water, or DEN (25 mg/l) in drinking water for 5 weeks and Kupffer cells were isolated as detailed earlier, seeded into 24-well dishes (5 ×105/well), and cultured for the first 12 hr as detailed earlier. Then, cells were cultured in D-MEM containing TJ-48 (0–1,000 μg/ml) for 24 hr. After changing the media to remove the TJ-48, cells were cultured for additional 24 hr and the media was collected and stored at −80°C.
Viability of the cells was confirmed by trypan blue exclusion and was >80% in all experiments. Purity of Kupffer cell isolates was confirmed by phagocytosis of 1 μm FITC-labeled latex beads (Polysciences, Warrington, PA) as detailed elsewhere22 and was >90% in all experiments. Five to twenty million Kupffer cells were obtained from each mouse. In vivo treatment with DEN had no effect on either yield or viability of Kupffer cells (data not shown). Tumor necrosis factor (TNF)-α, interleukins (IL)-1β and IL-6 production from Kupffer cells into the medium were assayed using enzyme-linked immunosorbent assay kits according to the manufacturer’s protocol (Biosource, Camarillo, CA). In all experiments Kupffer cells were isolated from 3 different mice in each group.
Assessment of oxidant production by Kupffer cells
Two experiments were conducted to assess the effects of TJ-48 on production of oxidants by Kupffer cells in vivo and in vitro. In the first experiment, 8-week-old male C57BL/6N mice were randomly divided into control (normal chow) and TJ-48 (1.6% w/w) groups. The mice of both groups received tap water and after 5 weeks of treatment, Kupffer cells were isolated as detailed above and seeded into 96-well tissue culture plates at the density of 2 × 105 cells/well. Cells were incubated in D-MEM media for 24 hr, washed with warm HBSS, and 2′,7′-dichlorofluorescein diacetate (DCF-DA, 50 μM, Sigma) was added into each well. The plate was covered and incubated for 30 min at 37°C in the dark. Then, cells were washed with fresh HBSS and D-MEM media (control), or media with DEN (2.5 mg/ml) was added and cells incubated for additional 24 hs. In the second experiment, Kupffer cells were isolated (as detailed above) from untreated C57BL/6N mice and seeded into 96-well tissue culture plates at the density of 2 × 105 cells/well. Cells were incubated in D-MEM media for 12 hr, washed and fresh D-MEM media (control), or media with TJ-48 (1,000 μg/ml) were added and cells incubated for 24 hr. After loading cells with DCF-DA as detailed above, fresh D-MEM media, or with media with DEN (2.5 mg/ml) was added and cells were incubated for additional 24 hr. Oxidant production was assessed from fluorescence (485 nm excitation and 535 nm emission) of oxidized DCF-DA as previously described23 using a microplate spectrofluorometer (SpectraMax GEMINI EM, Molecular Devices, Sunnyvale, CA).
Statistical analysis
The JMP software (ver. 6.0, SAS institute, Cary, NC) was used for statistical analysis. Intrahepatic recurrence-free survival curves were calculated using Kaplan–Meier method and were compared by means of the log-rank test. Cox proportional hazard model was used for univariate and multivariate analysis. One-way ANOVA with Newman-Keul’s post-hoc test was used for the determination of significance as appropriate. A p value less than 0.05 was selected before the study as the level of significance.
Results
TJ-48 improves intrahepatic recurrence-free survival after removal of the primary HCC
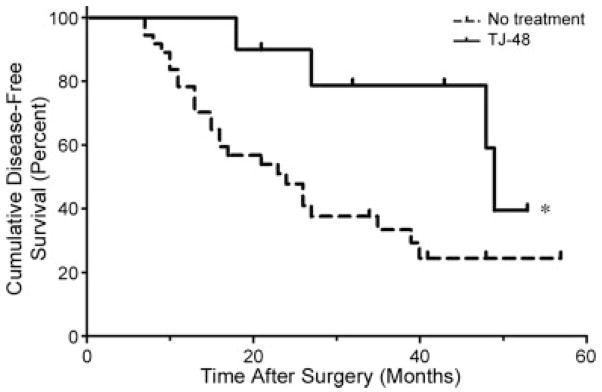
In this study, a total of 48 consecutive patients were enrolled and randomly assigned to the TJ-48 group (n = 10), or the control group (n = 38). Patients were followed up for up to 6 years after surgical treatment of primary HCC by monthly ambulatory screening for recurrence of HCC. Baseline clinical characteristics of patients in each group are provided in Table II. There were no significant differences between groups in all parameters traditionally evaluated in patients with HCC with the exception of indocyanine green retention which was significantly greater in the TJ-48 group as compared with the controls. At the time of the final analysis of the data (December 2005) a mean follow-up period of each patient was 25.8 month, and 4 patients in the TJ-48 group and 26 patients in the control group experienced recurrence of HCC. The median intrahepatic recurrence-free survival time was 49 months in the TJ-48 group and 24 months in the control group (p = 0.023, Hazard ratio = 0.391, 95%CI = 0.173–0.879, Log rank test, Fig. 2).
Figure 2.
Administration of TJ-48 to patients who underwent surgical treatment of hepatocellular carcinoma (HCC) improves the length of intrahepatic recurrence-free survival. Kaplan–Meier method was used to plot hepatic recurrence-free survival in a total of 48 consecutive patients who were randomly assigned to receive TJ-48 (7.5 g daily, n = 10, solid line), or no treatment (n = 38, dashed line). Asterisk (*) denotes statistical significant (p <0.05, Log-rank test) difference between groups.
In the univariate analysis (Cox proportional hazard model, Table III) of intrahepatic recurrence-free survival of the patients in this study, tumor stage was found to be a significant variable associated with poor prognosis, whereas the treatment with TJ-48 was a variable associated with significantly improved clinical outcome. No other clinical measurement collected in this study achieved statistical significance in predicting the length of intrahepatic recurrence-free survival in all 48 patients. When multivariate analysis was performed using variables that had a p value of less than 0.2 in the univariate analysis (gender, tumor multiplicity, tumor stage, evidence of liver cirrhosis and TJ-48 treatment), TJ-48 administration was identified as the only independent factor associated with improved intrahepatic recurrence-free survival after the surgery in patients with primary HCC (Table III).
TABLE III.
UNIVARIATE AND MULTIVARIATE ANALYSIS OF PROGNOSTIC MARKERS ASSOCIATED WITH INTRAHEPATIC RECURRENCE-FREE SURVIVAL IN PATIENTS WITH LIVER TUMORS
| Variable | Hazard ratio | 95% CI | p Value |
|---|---|---|---|
| Univariate analysis | |||
| Age | 1.003 | 0.953–1.056 | 0.912 |
| Gender (male/female) | 1.508 | 0.938–2.782 | 0.095 |
| HBV (infected/non-infected) | 0.694 | 0.095–2.744 | 0.506 |
| HCV (infected/non-infected) | 1.654 | 0.739–5.589 | |
| Platelets count | 1.033 | 0.951–1.120 | 0.435 |
| Alanine aminotransferase | 0.998 | 0.987–1.008 | 0.761 |
| Total bilirubin | 1.129 | 0.360–3.107 | 0.826 |
| Prothrombin time | 1.003 | 0.974–1.033 | 0.840 |
| Indocyanine green | 0.999 | 0.960–1.034 | 0.958 |
| Alpha-fetoprotein | 1.000 | 0.999–1.000 | 0.588 |
| Tumor diameter (≤20 mm/>20 mm) | 1.274 | 0.878–1.913 | 0.207 |
| Tumor multiplicity (multiple/solitary) | 1.431 | 0.991–2.077 | 0.056 |
| Tumor stage | 1.810 | 1.021–3.257 | 0.042 |
| Tumor differentiation (moderate-poor/well) | 1.111 | 0.755–1.720 | 0.607 |
| Liver cirrhosis (cirrhosis/no cirrhosis) | 0.765 | 0.529–1.109 | 0.154 |
| TJ-48 (treated/not treated) | 0.545 | 0.292–0.889 | 0.013 |
| Multivariate analysis | |||
| Gender | 1.187 | 0.699–2.263 | 0.546 |
| Tumor multiplicity | 1.742 | 0.967–3.164 | 0.064 |
| Tumor stage | 0.936 | 0.408–2.244 | 0.878 |
| Liver cirrhosis | 0.784 | 0.523–1.176 | 0.236 |
| TJ-48 treatment | 0.512 | 0.261–0.890 | 0.016 |
The results from Cox proportional hazard analysis are shown for all clinical variables available in this study. For multivariate analysis, only those variables that have a p-value less than 0.2 in the univariate analysis were selected.
It should be noted that control and TJ-48 groups in our human population are not completely balanced with respect to the tumor diameter even though this difference was not statistically significant. Nonetheless, to address the issue of potential bias in patient distribution with respect to tumor diameter and to test whether this may have affected the findings on the protective action of TJ-48, we conducted additional statistical analyses. First, we determined if there is statistically significant interaction between administration of TJ-48 and tumor diameter in the Cox proportional hazard model. There was no significant difference between TJ-48 risk ratio in patients with tumors >20 mm and that in patients with tumors ≤20 mm. Second, TJ-48 was still significant in the Cox proportional hazard model when adjusted for tumor diameter.
TJ-48 abrogates DEN-induced hepatocarcinogenesis in the mouse
To test the mechanism of protective action of TJ-48, we used a mouse model of hepatocarcinogenesis caused by DEN.24 We chose two mouse inbred strains for these experiments, C57BL/6N and C3H/HeN, because the former is known to be resistant to spontaneous hepatocarcinogenesis, whereas the latter is prone to liver tumors.25 Mice were given DEN (25 mg/l) in drinking water for 10 and 22 weeks (Fig. 1). No liver tumors were observed in mice after 10 weeks of treatment of DEN in either strain. After 22 weeks of DEN administration, ~22% of C57BL/6N and ~63% of C3H/HeN mice developed hepatocellular carcinomas (Table IV). Interestingly, when mice were coadministered TJ-48 in the feed (1.6% w/w), the DEN-associated hepatocarcinogenesis was abolished completely in C57BL/6N strain, whereas tumor incidence was reduced to ~41% in C3H/HeN mice.
TABLE IV.
EFFECT OF TJ-48 ON DEVELOPMENT OF MOUSE LIVER TUMORS, AND 8-OHDG- AND PCNA- POSITIVE HEPATOCYTES
| Strain | Group | Weeks | Tumor development (%) | 8-OHdG-positive hepatocytes (%) | PCNA-positive hepatocytes (%) |
|---|---|---|---|---|---|
| C57BL/6N | Control (N = 5) | 10 | 0 (0/5) | 28.7 ± 5.9 | 36.5 ± 5.6 |
| DEN (N = 5) | 0 (0/5) | 53.4 ± 1.51 | 55.6 ± 2.71 | ||
| DEN + TJ-48 (N = 5) | 0 (0/5) | 40.2 ± 2.42 | 55.2 ± 4.41 | ||
| Control (N = 5) | 22 | 0 (0/5) | 26.0 ± 5.7 | N/D | |
| DEN (N = 27) | 22.23 (6/27) | 67.0 ± 2.11 | N/D | ||
| DEN + TJ-48 (N = 20) | 0 (0/20) | 24.9 ± 3.52 | N/D | ||
| C3H/HeN | Control (N = 5) | 10 | 0 (0/5) | 31.8 ± 1.2 | 25.3 ± 3.1 |
| DEN (N = 5) | 0 (0/5) | 59.2 ± 2.31 | 42.0 ± 3.01 | ||
| DEN + TJ-48 (N = 5) | 0 (0/5) | 36.1 ± 3.72 | 40.9 ± 3.31 | ||
| Control (N = 5) | 22 | 0 (0/5) | 25.8 ± 4.3 | N/D | |
| DEN (N = 19) | 63.2 (12/19) | 37.1 ± 2.6 | N/D | ||
| DEN + TJ-48 (N = 17) | 41.2 (7/17) | 26.8 ± 5.0 | N/D |
Data are expressed as mean ± SEM. N/D, not determined.
p < 0.05, compared to control group at the corresponding time point (one-way ANOVA with Newman-Keul’s post-hoc test).
p < 0.05, compared to DEN group at the corresponding time point (one-way ANOVA with Newman-Keul’s post-hoc test).
p < 0.05, compared to DEN + TJ-48 group at the corresponding time point (Yates 2×2 Chi square test).
TJ-48 blocks DEN-induced inflammation and oxidative stress in mouse liver
Even though DEN is a well-established DNA-damaging agent, the mechanisms of DEN-induced carcinogenesis also are known to involve hepatocyte death, inflammation, oxidative stress, and increased cell proliferation.26 Therefore, we evaluated the effects of TJ-48 on cell proliferation, infiltration of the inflammatory cells, and oxidative DNA damage caused by DEN. In both mouse strains, treatment with DEN for 10 weeks resulted in increased cell proliferation in liver (as measured by the number of PCNA-positive cells, Table IV), the outcome that was not affected by TJ-48.
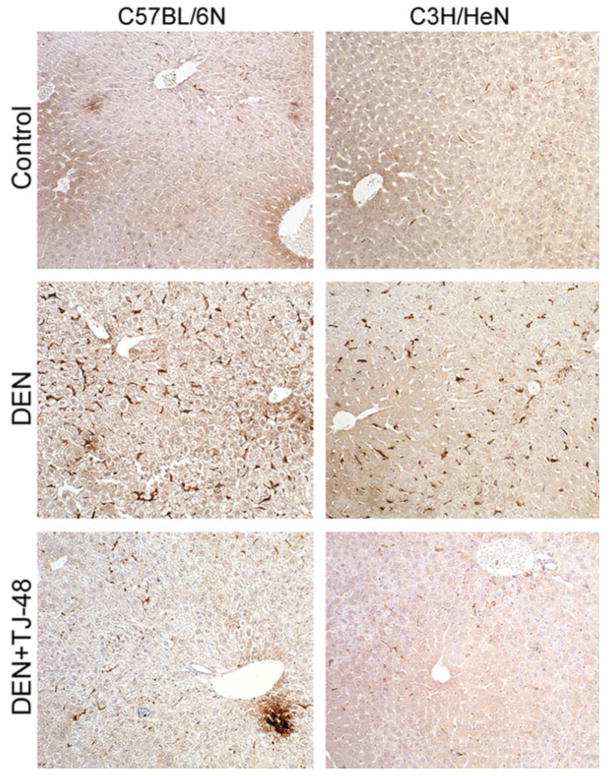
Treatment with DEN for 10 weeks led to a considerable increase in inflammatory cell (staining positive for F4/80 antigen27) infiltration into liver in both strains of mice (Fig. 3). However, when TJ-48 was administered concurrently with DEN, the number of F4/80-positive cells diminished dramatically. Expression of proinflammatory cytokines was evaluated by quantitative real-time PCR. In C57BL/6N mice, treatment with DEN for 10 weeks resulted in a significant increase in expression of Tnfα and IL-1β (Fig. 4), the effects that were abrogated by TJ-48. In C3H/HeN mice, expression of IL-1β, but not Tnfα, was increased by DEN and TJ-48 was also protective.
Figure 3.
TJ-48 blunts infiltration of inflammatory cells in the mouse model of DEN-induced liver carcinogenesis. Liver sections were obtained from C57BL/6N (left panel) and C3H/HeN (right panel) mice administered normal chow and tap water (Control), normal chow and DEN (25 mg/l)-containing drinking water (DEN), or TJ-48 (1.6% w/w)-supplemented chow and DEN-containing drinking water (DEN + TJ-48) for 10 weeks. Immunohistochemical analysis for F4/80-positive cells (intense brown staining of single cells in the sinusoids) was performed as detailed in Material and methods section. Original magnification, ×100. Representative photomicrographs.
Figure 4.
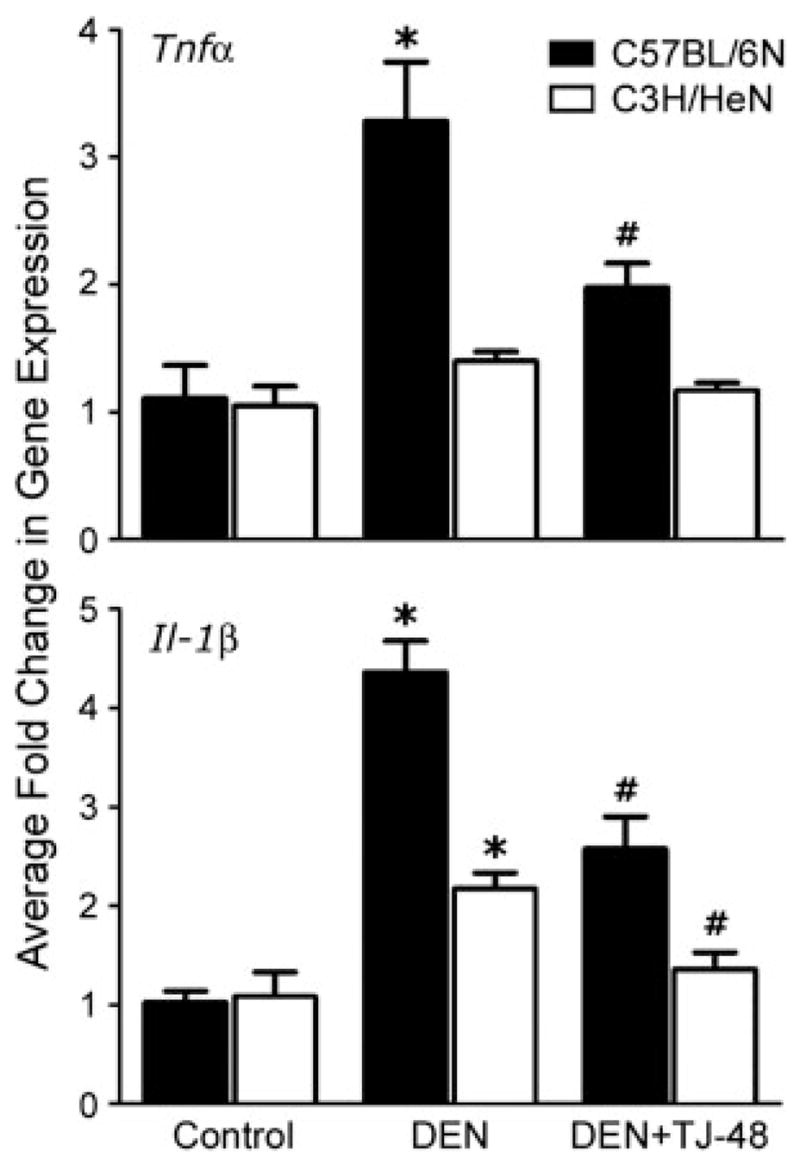
TJ-48 diminishes expression of proinflammatory cytokines in the mouse model of DEN-induced liver carcinogenesis. Liver samples were obtained from C57BL/6N (black bars) and C3H/HeN (open bars) mice administered normal chow and tap water (Control), normal chow and DEN (25 mg/l)-containing drinking water (DEN), or TJ-48 (1.6% w/w)-supplemented chow and DEN-containing drinking water (DEN + TJ-48) for 10 weeks. Expression of Tnfα (top) and IL-1β (bottom) mRNA was evaluated using quantitative RT-PCR as described in Materials and Methods. Fold change in gene expression (mean±S.E.M., n = 5 in each group) was derived from 2ΔΔCt values and normalized to expression of the housekeeping gene Gus-b. Asterisks denote statistically significant (p <0.05, one-way ANOVA with Newman-Keul’s post-hoc test) difference as compared to control (*), or DEN (#) groups within corresponding mouse strain.
To further characterize the effects of DEN and TJ-48 on liver inflammatory cells, C57BL/6N mice were administered DEN, or DEN + TJ-48 for 5 weeks and Kupffer cells were isolated and cultured. Protein levels for Tnfα, IL-1β and IL-6 were evaluated in the conditioned media from cultured cells. Kupffer cells isolated from mice treated with DEN were producing more proinflammatory cytokines than cells from naive mice, or mice treated with DEN + TJ-48 (Figs. 5a–5c).
Figure 5.
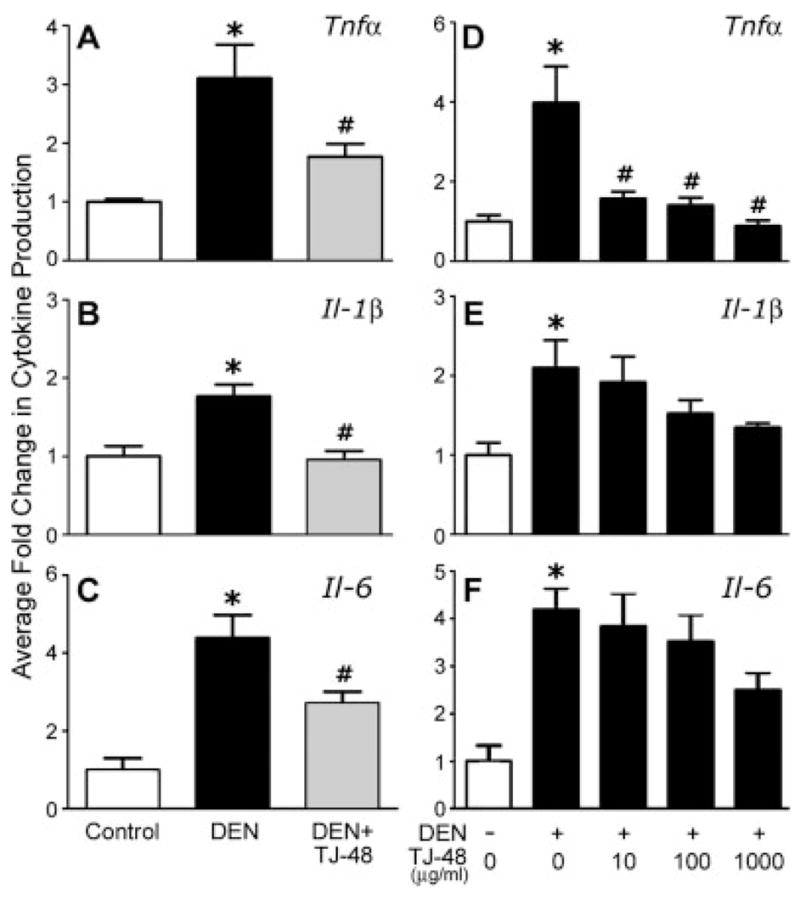
TJ-48 blocks release of pro-inflammatory cytokines from DEN-activated Kupffer cells both in vivo and in vitro. For in vivo experiments (a–c), Kupffer cells were isolated from C57BL/6N mice administered normal chow and tap water (Control, open bars), normal chow and DEN (25 mg/l)-containing drinking water (DEN, black bars), or TJ-48 (1.6% w/w)-supplemented chow and DEN-containing drinking water (DEN + TJ-48, grey bars) for 5 weeks. Cells were cultured and protein levels of Tnfα (a), Il-1β (b) and IL-6 (c) were measured in the media as detailed in Material and methods section. Absolute control values of each measurement were as follows Tnfα: 35.0 ± 3.0 pg/ml, IL-1β: 105.3 ± 27.5 pg/ml, and IL-6: 82.2 ± 49.1 pg/ml. For in vitro experiments (d–f), Kupffer cells were isolated from C57BL/6N mice administered normal chow and either tap water (DEN-, open bars), or DEN-containing drinking water (DEN +, closed bars) for 5 weeks. Cells were cultured in presence of TJ-48 (0–1,000 μg/mL) and protein levels of Tnfα (d), IL-1β (e) and IL-6 (f) were measured in the media as detailed in Material and methods section. Data are shown as mean ± S.E.M. (n = 5 in each group). Absolute control values in this experiment were as follows Tnfα: 22.5 ± 6.2 pg/ml, IL-1β: 80.2 ± 21.5 pg/ml, and IL-6: 73.0 ± 45.0 pg/ml. Asterisks denote statistically significant (p <0.05, one-way ANOVA with Newman-Keul’s post-hoc test) difference as compared to control (*), or DEN (#) groups.
Finally, immunohistochemical staining of liver sections for 8-OH-dG showed a significant increase in oxidative DNA damage after 10 and 22 weeks of DEN treatment in C57BL/6N mice, and after 10 weeks in C3H/HeN mice (Table IV). TJ-48 abrogated this effect of DEN significantly in the same groups. Although the overall trend in this endpoint was similar in C3H/HeN mice treated for 22 weeks, the data were not significantly different between groups.
To further evaluate whether TJ-48 has an effect on Kupffer cell-derived oxidants, C57BL/6N mice were fed a TJ-48-supplemented diet for 5 weeks; Kupffer cells isolated and cultured in presence of DEN. Addition of DEN to Kupffer cells isolated from naive mice led to a significant increase in oxidant production as measured by the oxidation of DCF-DA (Fig. 6a). However, DEN had no effect on oxidant production by the Kupffer cells isolated from TJ-48-treated mice.
Figure 6.
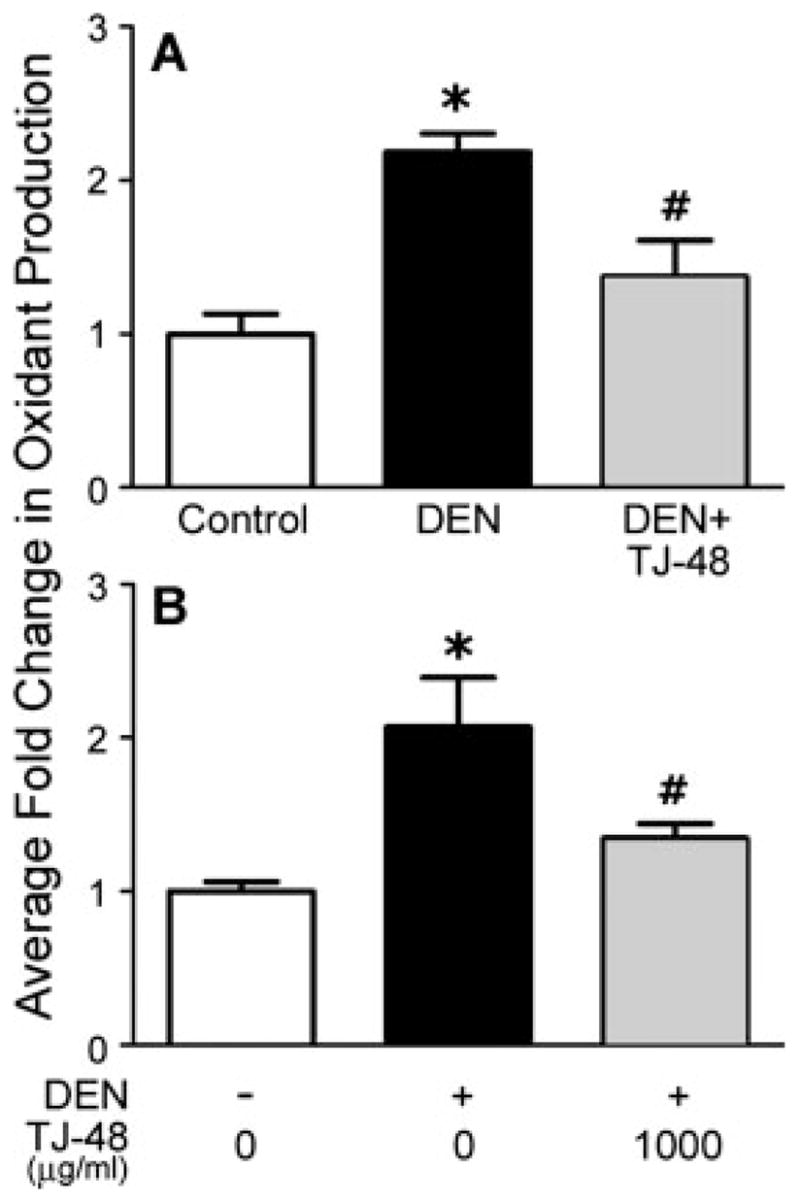
TJ-48 blocks oxidant production by DEN-activated Kupffer cells both in vivo and in vitro. For in vivo experiments (a), C57BL/6N mice were fed normal chow (control) or TJ-48 (1.6% w/w)-supplemented diets for 5 weeks. Kupffer cells were isolated and cultured in normal media (control), or media containing DEN (2.5 mg/ml) and production of oxidants was assessed as detailed in Material and methods section. For in vitro experiments (b), Kupffer cells were isolated from naive C57BL/6N mice and cultured with TJ-48 (0 or 1,000 μg/ml) and in absence or presence of DEN (2.5 mg/ml) and production of oxidants was assessed using DCF-fluorescence as detailed in Material and methods section. Data are shown as mean ± S.E.M. (n = 3 in each group). Asterisks denote statistically significant (p <0.05, one-way ANOVA with Newman-Keul’s post-hoc test) difference as compared to control (*), or DEN (#) groups.
TJ-48 blocks DEN-induced activation of the Kupffer cells in vitro
To determine if TJ-48 acts directly on the Kupffer cells to prevent production of pro-inflammatory cytokines and oxidants, we isolated Kupffer cells from C57BL/6N mice treated with DEN in vivo for 5 weeks and cultured them in presence of various concentrations of TJ-48. Production of TNFα, IL-1β and IL-6 was evaluated by measuring protein levels for these cytokines in the media (Figs. 5d–5f). As expected (see Figs. 5a–5c), Kupffer cells isolated from DEN-treated mice produced significantly more cytokines than the cells isolated from naive mice. Interestingly, addition of TJ-48 to the cell culture media lowered the production of cytokines significantly in a dose-responsive manner. To evaluate the effects of TJ-48 on the DEN-induced oxidant production in vitro, Kupffer cells were isolated from naive mice, and cultured with or without TJ-48. Addition of DEN to cell cultures led to an increase in production of oxidants in cells cultured without TJ-48 (Fig. 6b), but no effect was observed in cells cultured with TJ-48.
Discussion
Tumor resection is the choice of treatment for HCC; however, 5-year tumor recurrence rate exceeds 70%28–30 and improved therapies are badly needed to address low intrahepatic recurrence-free survival. Reappearance of HCC is thought to occur due to de novo formation of tumors or intrahepatic metastasis.31 It is likely that the same predisposition factors as those that lead to the formation of a primary tumor are causal in recurrence of most of HCC cases.
Oxidant-induced DNA damage has been strongly implicated as one of the primary causes of human cancers that are a result of chronic inflammation.3 Chronic hepatitis virus C infection results in a vicious cycle of inflammation, necrosis, and liver regeneration which are associated with infiltration of immune cells that are major producers of reactive oxygen and nitrogen species.32 It has been also suggested that HCV core protein may contribute to hepatic oxidative stress through mitochondrial dysfunction.33,34 Indeed, strong correlation between high intrahepatic inflammation and oxidative stress, and abysmal disease-free survival was observed in patients who underwent HCC removal8 which strongly suggests that inflammatory cells in liver may be a key source for reactive intermediates that exacerbate liver injury and lead to cancer recurrence.
Combating the causes that are thought to promote carcinogenesis, such as oxidative stress, may prove to be a powerful therapeutic strategy that will improve quality of life for HCC patients by extending the disease-free survival. Although antioxidants may or may not prove to be useful as treatment of cancers,35,36 traditional oriental herb preparations have been shown to be beneficial in a variety of diseases through mechanisms that may include antiinflammatory and other effects.13 In the present study, we show that administration of TJ-48, one of the commonly used herbal mixtures, improves the rate of intrahepatic recurrence of HCC after curative surgery. The median recurrence-free survival of patients receiving TJ-48 was 49 months, as compared to only 24 for the control group. Multivariate analysis showed that TJ-48 was the only independent factor associated with favorable recurrence outcome in the HCC patients. To our knowledge, this is the first report which analyzed the effect of TJ-48 on HCC recurrence after curative resection or MCT.
However, the clinical observation does not, by itself, provide sufficient clues as to what is a potential mechanism of protective action of TJ-48. Thus, in order to clarify how TJ-48 may stall tumorigenesis, we used a mouse model of hepatocarcinogenesis caused by DEN which is regarded to be similar to human HCC both histologicaly and mechanistically.37 On the basis of the data provided by our investigations in the mouse, we argue that Kupffer cells, not hepatocytes, play important role in the mechanisms of protective action by TJ-48.
Kupffer cell-derived inflammatory response, which involves both production of mitogenic cytokines and oxidants, plays a seminal role in DEN-induced hepatocarcinogenesis,26,38,39 likely via activation of Kupffer cells by mediators released from necrotic hepatocytes.40 In this study, Kupffer cells did not produce pro-inflammatory cytokines when treated with DEN in vitro (data not shown) even though DEN caused activation of production of reactive oxygen species by these cells both in vivo and in vitro. Although Maeda et al.26 have shown recently that activation of NF-κB in Kupffer cells may be a result of DEN-produced hepatocyte death, our data suggest that DEN may also have a direct effect on Kupffer cells to increase production of oxidants that, in turn, may lead to activation of NF-κB.41
DEN-induced hepatocarcinogenesis was reduced by TJ-9 (Sho-saiko-to), another Japanese herbal medicine, presumably through its effect on blocking the production of oxidants and the oxidative DNA damage.42 However, our study suggests that the mechanism of protective action of TJ-48, a similar herbal mixture, involves inhibition of Kupffer cells activation in both humans and mice, which lowers the production of pro-inflammatory cytokines and oxidants in liver, and thereby slowing the process of hepatocarcinogenesis. Our mouse experiment data suggest that Kupffer cells are the target for TJ-48 because it reduced the number of 8-OHdG-positive hepatocytes and F4/80-positive non-parenchymal cells, as well as diminished the expression of pro-inflammatory cytokines in liver. In addition, DEN-induced production of pro-inflammatory cytokines and reactive oxygen species by isolated Kupffer cells was blunted significantly by TJ-48 both in vivo and in vitro. TJ-48 is composed of 10 herbs and its individual ingredients have been reported to have antioxidant properties18,43,44; hence, it is presumable that antioxidant effect is important for TJ-48 to reduce liver carcinogenesis.
Several studies have suggested that anti-tumor effect of TJ-48 may be attributable to the activation of the immune system which facilitates the clearance of tumor cells from the liver.13,16,19 Specifically, natural killer (NK)T cells are known to exert prominent anti-tumor activity in the liver45 and it has been shown that TJ-48 activates NKT cells.46 Conversely, our results show that Kupffer cells, an important part of the innate immune response in liver, are affected in a protective way and TJ-48 causes attenuation of the production of proinflammatory cytokines. Thus, it is plausible that the effect of TJ-48 may be 2-fold—(i) promoting the phagocytosis of transformed cells and cell debris by NKT cells, (ii) preventing production of excessive amounts of oxidants and proinflammatory cytokines by Kupffer cells, which may facilitate secondary tumorigenesis. It is likely that both the factors play a role in protective mechanisms of TJ-48 in patients with HCC who underwent curative tumor resection or MCT and we suggest that modulation of immune system with TJ-48 may be a reasonable strategy as anti-cancer therapy given its lack of side effects and ease of use as compared with chemotherapy or interferon therapy. However, additional human studies are necessary to further evaluate the effectiveness of TJ-48 therapy in postoperative treatment of patients with HCC, as well as its potential benefit in combination with other established treatments.
Conclusion
This study presents new important information on the anti-cancer effect of TJ-48 in humans and provides mechanistic evidence that the protective effects are, at least in part, due to reduction in oxidant and cytokine production by Kupffer cells. In view of the fact that oxidative stress in the liver infected with HCV is associated with shortened intrahepatic recurrence-free survival of patients with HCC after primary tumor removal, combating oxidative stress and intrahepatic inflammation with TJ-48, or other remedies that are well-tolerated and easy to use, may prove to be beneficial in management of postoperative patients and improve their quality of life.
Acknowledgments
National Institutes of Health; Grant numbers: U19-ES11391, R01-AA16285, R01-ES12686.
References
- 1.Inoue H, Seitz HK. Viruses and alcohol in the pathogenesis of primary hepatic carcinoma. Eur J Cancer Prev. 2001;10:107–10. doi: 10.1097/00008469-200102000-00016. [DOI] [PubMed] [Google Scholar]
- 2.Kountouras J, Lygidakis NJ. New epidemiological data on liver oncogenesis. Hepatogastroenterology. 2000;47:855–61. [PubMed] [Google Scholar]
- 3.Hussain SP, Hofseth LJ, Harris CC. Radical causes of cancer. Nat Rev Cancer. 2003;3:276–85. doi: 10.1038/nrc1046. [DOI] [PubMed] [Google Scholar]
- 4.Koike K, Miyoshi H. Oxidative stress and hepatitis C viral infection. Hepatol Res. 2006;34:65–73. doi: 10.1016/j.hepres.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 5.De Maria N, Colantoni A, Fagiuoli S, Liu GJ, Rogers BK, Farinati F, Van Thiel DH, Floyd RA. Association between reactive oxygen species and disease activity in chronic hepatitis C. Free Radic Biol Med. 1996;21:291–5. doi: 10.1016/0891-5849(96)00044-5. [DOI] [PubMed] [Google Scholar]
- 6.Matsumoto K, Yoshimoto J, Sugo H, Kojima K, Futagawa S, Matsumoto T. Relationship between the histological degrees of hepatitis and the postoperative recurrence of hepatocellular carcinoma in patients with hepatitis C. Hepatol Res. 2002;23:196–201. doi: 10.1016/s1386-6346(01)00180-2. [DOI] [PubMed] [Google Scholar]
- 7.Jungst C, Cheng B, Gehrke R, Schmitz V, Nischalke HD, Ramakers J, Schramel P, Schirmacher P, Sauerbruch T, Caselmann WH. Oxidative damage is increased in human liver tissue adjacent to hepatocellular carcinoma. Hepatology. 2004;39:1663–72. doi: 10.1002/hep.20241. [DOI] [PubMed] [Google Scholar]
- 8.Maki A, Kono H, Gupta M, Asakawa M, Suzuki T, Matsuda M, Fujii H, Rusyn I. Predictive power of biomarkers of oxidative stress and inflammation in patients with hepatitis C virus-associated hepatocellular carcinoma. Ann Surg Oncol. 2007;14:1182–90. doi: 10.1245/s10434-006-9049-1. [DOI] [PubMed] [Google Scholar]
- 9.Forton D, Karayiannis P. Established and emerging therapies for the treatment of viral hepatitis. Dig Dis. 2006;24:160–73. doi: 10.1159/000090319. [DOI] [PubMed] [Google Scholar]
- 10.Levy C, Seeff LD, Lindor KD. Use of herbal supplements for chronic liver disease. Clin Gastroenterol Hepatol. 2004;2:947–56. doi: 10.1016/s1542-3565(04)00455-0. [DOI] [PubMed] [Google Scholar]
- 11.Cohen MR. Herbal and complementary and alternative medicine therapies for liver disease. A focus on Chinese traditional medicine in hepatitis C virus. Clin Liver Dis. 2001;5:461–78. doi: 10.1016/s1089-3261(05)70174-4. [DOI] [PubMed] [Google Scholar]
- 12.Oka H, Yamamoto S, Kuroki T, Harihara S, Marumo T, Kim SR, Monna T, Kobayashi K, Tango T. Prospective study of chemoprevention of hepatocellular carcinoma with Sho-saiko-to (TJ-9) Cancer. 1995;76:743–9. doi: 10.1002/1097-0142(19950901)76:5<743::aid-cncr2820760506>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 13.Saiki I. A Kampo medicine “Juzen-taiho-to”–prevention of malignant progression and metastasis of tumor cells and the mechanism of action. Biol Pharm Bull. 2000;23:677–88. doi: 10.1248/bpb.23.677. [DOI] [PubMed] [Google Scholar]
- 14.Saiki I, Yamaura T, Ohnishi Y, Hayakawa Y, Komatsu Y, Nunome S. HPLC analysis of juzen-taiho-to and its variant formulations and their antimetastatic efficacies. Chem Pharm Bull (Tokyo) 1999;47:1170–4. doi: 10.1248/cpb.47.1170. [DOI] [PubMed] [Google Scholar]
- 15.Kamiyama H, Takano S, Ishikawa E, Tsuboi K, Matsumura A. Anti-angiogenic and immunomodulatory effect of the herbal medicine “Juzen-taiho-to” on malignant glioma. Biol Pharm Bull. 2005;28:2111–16. doi: 10.1248/bpb.28.2111. [DOI] [PubMed] [Google Scholar]
- 16.Tatsuta M, Iishi H, Baba M, Nakaizumi A, Uehara H. Inhibition by shi-quan-da-bu-tang (TJ-48) of experimental hepatocarcinogenesis induced by N-nitrosomorpholine in Sprague-Dawley rats. Eur J Cancer. 1994;30A:74–8. doi: 10.1016/s0959-8049(05)80022-x. [DOI] [PubMed] [Google Scholar]
- 17.Dhuley JN. Anti-oxidant effects of cinnamon (Cinnamomum verum) bark and greater cardamom (Amomum subulatum) seeds in rats fed high fat diet. Indian J Exp Biol. 1999;37:238–42. [PubMed] [Google Scholar]
- 18.Keum YS, Park KK, Lee JM, Chun KS, Park JH, Lee SK, Kwon H, Surh YJ. Antioxidant and anti-tumor promoting activities of the methanol extract of heat-processed ginseng. Cancer Lett. 2000;150:41–8. doi: 10.1016/s0304-3835(99)00369-9. [DOI] [PubMed] [Google Scholar]
- 19.Chino A, Sakurai H, Choo MK, Koizumi K, Shimada Y, Terasawa K, Saiki I. Juzentaihoto, a Kampo medicine, enhances IL-12 production by modulating Toll-like receptor 4 signaling pathways in murine peritoneal exudate macrophages. Int Immunopharmacol. 2005;5:871–82. doi: 10.1016/j.intimp.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 20.Kawamata H, Ochiai H, Mantani N, Terasawa K. Enhanced expression of inducible nitric oxide synthase by Juzen-taiho-to in LPS-activated RAW264.7 cells, a murine macrophage cell line. Am J Chin Med. 2000;28:217–26. doi: 10.1142/S0192415X0000026X. [DOI] [PubMed] [Google Scholar]
- 21.Friedman SL, Roll FJ. Isolation and culture of hepatic lipocytes, Kupffer cells, and sinusoidal endothelial cells by density gradient centrifugation with. Stractan Anal Biochem. 1987;161:207–18. doi: 10.1016/0003-2697(87)90673-7. [DOI] [PubMed] [Google Scholar]
- 22.Peters JM, Rusyn I, Rose ML, Gonzalez FJ, Thurman RG. Peroxi-some proliferator-activated receptor α is restricted to hepatic parenchymal cells, not Kupffer cells: implications for the mechanism of action of peroxisome proliferators in hepatocarcinogenesis. Carcinogenesis. 2000;21:823–6. doi: 10.1093/carcin/21.4.823. [DOI] [PubMed] [Google Scholar]
- 23.Trayner ID, Rayner AP, Freeman GE, Farzaneh F. Quantitative multi-well myeloid differentiation assay using dichlorodihydrofluorescein diacetate (H2DCF-DA) or dihydrorhodamine 123 (H2R123) J Immunol Methods. 1995;186:275–84. doi: 10.1016/0022-1759(95)00152-z. [DOI] [PubMed] [Google Scholar]
- 24.Clapp NK, Craig AW. Carcinogenic effects of diethylnitrosamine in RF mice. J Natl Cancer Inst. 1967;39:903–16. [PubMed] [Google Scholar]
- 25.Ruebner BH, Gershwin ME, French SW, Meierhenry E, Dunn P, Hsieh LS. Mouse hepatic neoplasia: differences among strains and carcinogens. In: Popp JA, editor. Mouse liver neoplasia: current perspectives. Washington, DC: Hemisphere Publishing; 1984. pp. 115–44. [Google Scholar]
- 26.Maeda S, Kamata H, Luo JL, Leffert H, Karin M. IKKbeta couples hepatocyte death to cytokine-driven compensatory proliferation that promotes chemical hepatocarcinogenesis. Cell. 2005;121:977–90. doi: 10.1016/j.cell.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 27.Austyn JM, Gordon S. F4/80, a monoclonal antibody directed specifically against the mouse macrophage. Eur J Immunol. 1981;11:805–15. doi: 10.1002/eji.1830111013. [DOI] [PubMed] [Google Scholar]
- 28.Okada S, Shimada K, Yamamoto J, Takayama T, Kosuge T, Yamasaki S, Sakamoto M, Hirohashi S. Predictive factors for postoperative recurrence of hepatocellular carcinoma. Gastroenterology. 1994;106:1618–24. doi: 10.1016/0016-5085(94)90419-7. [DOI] [PubMed] [Google Scholar]
- 29.Minagawa M, Makuuchi M, Takayama T, Kokudo N. Selection criteria for repeat hepatectomy in patients with recurrent hepatocellular carcinoma. Ann Surg. 2003;238:703–10. doi: 10.1097/01.sla.0000094549.11754.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Adachi E, Maeda T, Matsumata T, Shirabe K, Kinukawa N, Sugimachi K, Tsuneyoshi M. Risk factors for intrahepatic recurrence in human small hepatocellular carcinoma. Gastroenterology. 1995;108:768–75. doi: 10.1016/0016-5085(95)90450-6. [DOI] [PubMed] [Google Scholar]
- 31.Chen YJ, Yeh SH, Chen JT, Wu CC, Hsu MT, Tsai SF, Chen PJ, Lin CH. Chromosomal changes and clonality relationship between primary and recurrent hepatocellular carcinoma. Gastroenterology. 2000;119:431–40. doi: 10.1053/gast.2000.9373. [DOI] [PubMed] [Google Scholar]
- 32.Murakami T, Kim T, Nakamura H. Hepatitis, cirrhosis, and hepatoma. J Magn Reson Imaging. 1998;8:346–58. doi: 10.1002/jmri.1880080214. [DOI] [PubMed] [Google Scholar]
- 33.Okuda M, Li K, Beard MR, Showalter LA, Scholle F, Lemon SM, Weinman SA. Mitochondrial injury, oxidative stress, and antioxidant gene expression are induced by hepatitis C virus core protein. Gastroenterology. 2002;122:366–75. doi: 10.1053/gast.2002.30983. [DOI] [PubMed] [Google Scholar]
- 34.Korenaga M, Wang T, Li Y, Showalter LA, Chan T, Sun J, Weinman SA. Hepatitis C virus core protein inhibits mitochondrial electron transport and increases reactive oxygen species (ROS) production. J Biol Chem. 2005;280:37481–8. doi: 10.1074/jbc.M506412200. [DOI] [PubMed] [Google Scholar]
- 35.Miller ER, III, Pastor-Barriuso R, Dalal D, Riemersma RA, Appel LJ, Guallar E. Meta-analysis: high-dosage vitamin E supplementation may increase all-cause mortality. Ann Intern Med. 2005;142:37–46. doi: 10.7326/0003-4819-142-1-200501040-00110. [DOI] [PubMed] [Google Scholar]
- 36.Kavanaugh CJ, Trumbo PR, Ellwood KC. The U.S. Food and Drug Administration’s evidence-based review for qualified health claims: tomatoes, lycopene, and cancer. J Natl Cancer Inst. 2007;99:1074–85. doi: 10.1093/jnci/djm037. [DOI] [PubMed] [Google Scholar]
- 37.Lee JS, Chu IS, Mikaelyan A, Calvisi DF, Heo J, Reddy JK, Thorgeirsson SS. Application of comparative functional genomics to identify best-fit mouse models to study human cancer. Nat Genet. 2004;36:1306–11. doi: 10.1038/ng1481. [DOI] [PubMed] [Google Scholar]
- 38.Teufelhofer O, Parzefall W, Kainzbauer E, Ferk F, Freiler C, Knasmuller S, Elbling L, Thurman R, Schulte-Hermann R. Superoxide generation from Kupffer cells contributes to hepatocarcinogenesis: studies on NADPH oxidase knockout mice. Carcinogenesis. 2005;26:319–29. doi: 10.1093/carcin/bgh320. [DOI] [PubMed] [Google Scholar]
- 39.Naugler WE, Sakurai T, Kim S, Maeda S, Kim K, Elsharkawy AM, Karin M. Gender disparity in liver cancer due to sex differences in MyD88-dependent IL-6 production. Science. 2007;317:121–4. doi: 10.1126/science.1140485. [DOI] [PubMed] [Google Scholar]
- 40.Scaffidi P, Misteli T, Bianchi ME. Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature. 2002;418:191–5. doi: 10.1038/nature00858. [DOI] [PubMed] [Google Scholar]
- 41.Rose ML, Rusyn I, Bojes HK, Belyea J, Cattley RC, Thurman RG. Role of Kupffer cells and oxidants in signaling peroxisome proliferator-induced hepatocyte proliferation. Mutat Res. 2000;448:179–92. doi: 10.1016/s0027-5107(99)00235-3. [DOI] [PubMed] [Google Scholar]
- 42.Shiota G, Maeta Y, Mukoyama T, Yanagidani A, Udagawa A, Oyama K, Yashima K, Kishimoto Y, Nakai Y, Miura T, Ito H, Murawaki Y, et al. Effects of Sho-Saiko-to on hepatocarcinogenesis and 8-hydroxy-2′-deoxyguanosine formation. Hepatology. 2002;35:1125–33. doi: 10.1053/jhep.2002.33066. [DOI] [PubMed] [Google Scholar]
- 43.Imamichi T, Hayashi K, Nakamura T, Kaneko K, Koyama J. A Chinese traditional medicine, juzentaihoto, inhibits the O2-generation by macrophages. J Pharmacobiodyn. 1989;12:693–9. doi: 10.1248/bpb1978.12.693. [DOI] [PubMed] [Google Scholar]
- 44.Lee YS, Han OK, Park CW, Yang CH, Jeon TW, Yoo WK, Kim SH, Kim HJ. Pro-inflammatory cytokine gene expression and nitric oxide regulation of aqueous extracted Astragali radix in RAW 264.7 macrophage cells. J Ethnopharmacol. 2005;100:289–94. doi: 10.1016/j.jep.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 45.Margalit M, Shibolet O, Klein A, Elinav E, Alper R, Thalenfeld B, Engelhardt D, Rabbani E, Ilan Y. Suppression of hepatocellular carcinoma by transplantation of ex-vivo immune-modulated NKT lymphocytes. Int J Cancer. 2005;115:443–9. doi: 10.1002/ijc.20889. [DOI] [PubMed] [Google Scholar]
- 46.Matsumoto T, Sakurai MH, Kiyohara H, Yamada H. Orally administered decoction of Kampo (Japanese herbal) medicine, “Juzen-Taiho-To” modulates cytokine secretion and induces NKT cells in mouse liver. Immunopharmacology. 2000;46:149–61. doi: 10.1016/s0162-3109(99)00166-6. [DOI] [PubMed] [Google Scholar]