Abstract
Escherichia coli K1 isolates synthesize a polysialic acid (polySia) capsule, are components of the adult gastrointestinal microbiota and may cause lethal bacteraemia and meningitis if acquired maternally by newborn infants. We used a neonatal rat pup K1 infection model to establish that prompt administration of a selective capsule depolymerase reverses the bacteraemic state and prevents death of almost all pups. In untreated animals, bacteria colonize the gastrointestinal tract and gain entry to the blood compartment, where they express the non-O-acetylated form of polySia. The bacteria invade the major organs of the host; histological and histochemical analysis of brain sections revealed that at least some bacteria enter the central nervous system through the blood–cerebrospinal fluid barrier at the choroid plexus prior to colonization of the meninges. Once in this location, they cease expression of polySia. The unexpected abrogation of polySia, a factor associated with the pathogenesis of meningitis and essential for transit through the blood, suggests that the neuropathogen dispenses with its protective capsule once it has colonized protected niches. Thus, systemic infections due to encapsulated pathogens may be resolved by capsule depolymerization only if the enzyme modifies the bacteria whilst they are in the blood compartment.
INTRODUCTION
Mortality and morbidity associated with neonatal bacterial meningitis (NBM) and neonatal sepsis remain significant despite advances in chemotherapy and supportive care (Polin & Harris, 2001). In the developed world, mortality has declined to around 10 %, although morbidity has not changed substantially over the last 30 years. Neurological sequelae in infants with meningitis due to Escherichia coli, one of the two predominant bacteria responsible for NBM, are, at around 60 %, higher than for those surviving group B streptococcal meningitis and other forms of the disease (Harvey et al., 1999). NBM usually arises following haematogenous spread and it has been suggested that bacteria enter the central nervous system (CNS) following translocation across the blood–brain barrier at the cerebral microvascular endothelium of the arachnoid membrane (Nassif et al., 2002; Kim, 2003), although the choroid plexus epithelium represents an alternative but little-studied portal for transit from blood to cerebrospinal fluid (CSF) (Parkkinen et al., 1988).
Of those bacteria capable of causing blood-borne infection, relatively few are able to overcome the host's protective barriers and invade the CNS. It is notable that the overwhelming majority of neuroinvasive pathogens elaborate a polysaccharide capsule, and non-encapsulated bacteria that cause bacteraemia, such as Streptococcus viridans, only infrequently invade the brain or CSF (Gaudreau et al., 1981). With E. coli, it has been consistently found that 80–85 % of isolates cultured from cases of NBM express the K1 capsule (Robbins et al., 1974; Korhonen et al., 1985), a homopolymer of α-2,8-linked polysialic acid (polySia) that mimics the molecular structure of the polySia modulator of neuronal plasticity in the human host.
E. coli NBM is invariably preceded by colonization of the infant with bacteria from the maternal gastrointestinal flora and by a threshold level of bacteraemia (Nassif et al., 2002; Kim, 2003), but the processes governing access to the CNS and later-stage pathogenic mechanisms are incompletely understood. Experimental models of meningitis frequently make use of adult rabbits or rats infected by direct intracisternal inoculation, bypassing the natural sequence of events and creating an artificial pathogenesis (Tunkel & Scheld, 1993). Recent insights into the interaction of E. coli K1 and cultured brain microvascular endothelial cells suggest a role for polySia in the modulation of intracellular trafficking and prevention of endosome–lysosome fusion (Kim, 2003). The focus on vascular endothelium rather than the epithelium of the choroid plexus derives from studies of the distribution of E. coli K1 in the brain of infant and adult rats following subcutaneous administration: bacteria were recovered from CSF and found in the subarachnoid space, predominantly around perivascular areas, but not in the choroid plexus (Kim et al., 1992).
The polySia capsule protects E. coli K1 from humoral (Cross et al., 1986; Mushtaq et al., 2004) and cellular (Bortolussi et al., 1979; Mushtaq et al., 2005) components of the host's immune system and is a key determinant of the capacity of E. coli K1 to cause infection in experimental animals (Kim et al., 1992). We hypothesized that absence of the capsule during the early stages of systemic E. coli K1 infection enables the host's defences to eliminate the pathogen (Taylor et al., 2002); this approach could form the basis of a novel therapy for systemic infections due to encapsulated bacteria. To evaluate this concept, we adapted an animal model of E. coli K1 infection that mimics key features of human neonatal disease (Glode et al., 1977; Pluschke et al., 1983). In this model, susceptibility to infection is strongly age-dependent, bacteraemia is preceded by colonization of the host and invading bacteria appear to follow the ‘natural’ tropism. Newborn rats were fed E. coli K1 and the bacteria rapidly colonized the gastrointestinal tract; the animals subsequently developed bacteraemia that led to death within a few days. Administration during the early phases of infection of small doses of phage-derived recombinant endosialidase E (endoE), an enzyme that rapidly and selectively degrades the polySia capsule (Tomlinson & Taylor, 1985; Leggate et al., 2002), prevented bacteraemia and death in the large majority of infected animals (Mushtaq et al., 2004, 2005). These observations imply that expression of polySia at the bacterial surface is required during the early, critical phases of the infection and endoE-mediated removal of the polysaccharide disrupts the processes that lead to lethal outcome. The present study was undertaken to determine bacterial capsule expression in the experimental E. coli K1 infection model and to examine its role in the modulation of host–bacteria interactions. The results indicate that E. coli K1 expresses the non-O-acetylated form of polySia in the blood circulation but ceases to express the capsule after penetration of the surface of the brain.
METHODS
Rats, bacteria and capsule depolymerase
Litters (9–14 individuals) of 2-day-old Wistar rat pups were either purchased from Harlan UK or bred in-house, and were retained with the natural mothers in a single cage. All procedures involving these animals conformed to national and European legislation and were approved in full by the institutional Ethics Committee and the UK Home Office. E. coli A192 (O18 : K1) was isolated from a patient with septicaemia and its properties are described by Achtman et al. (1983); the strain was obtained from the Deutsche Sammlung von Mikroorganismen und Zellkulturen. The virulence of A192 was enhanced by passage in neonatal rats and a colony isolated from blood following the second recovery was designated A192PP (Mushtaq et al., 2004). A192PPK1− is a stable capsule-free mutant derived from A192PP by selection for resistance to the K1-specific phage K1E. E. coli EV36 is a K12/K1 hybrid that produces a non-O-acetylated K1 capsule (Vimr & Troy, 1985; Steenbergen et al., 2006); EV36+pSX785 contains a high-copy-number ampicillin-resistance plasmid carrying the K1 O-acetyltransferase gene neuO and produces a K1 capsule in which ∼15 % of the sialyl residues are O-acetylated (Deszo et al., 2005; Steenbergen et al., 2006). These two strains were provided by Eric Vimr, University of Illinois at Urbana-Champaign, IL, USA. His6-tagged endoE was produced in E. coli BL21(DE3) and purified using Ni-affinity chromatography as described previously (Mushtaq et al., 2004). Recovered protein was pure as judged by SDS-PAGE, with no proteins detectable other than the 76 kDa recombinant endoE fusion product. Kinetic properties and stability of the recombinant enzyme are reported elsewhere (Leggate et al., 2002; Mushtaq et al., 2004). For intraperitoneal (i.p.) administration, 20 μg doses of endoE for individual pups were formulated in 100 μl PBS μl−1.
Histochemical reagents
Rabbit polyclonal antibody against E. coli O18 LPS surface antigen was the gift of Tom Cheasty (Health Protection Agency, Colindale, UK) and was used at a dilution of 1 : 2500. Binding was detected with a polyclonal goat anti-rabbit IgG labelled with Alexa Fluor 546 (Invitrogen) and used at a dilution of 1 : 1 000. Murine monoclonal IgG2b antibody Ab8064, which recognizes the O-acetylated but not the non-O-acetylated form of K1, was obtained from Abcam and used at a dilution of 1 : 1000; binding was detected with a polyclonal goat anti-mouse IgG labelled with Cy5 (1 : 100; Abcam). PolySia is poorly immunogenic and thus a probe consisting of a catalytically inactive endosialidase that recognizes α-2,8 linkages in polysialyl chains was used to detect K1 polymer. The endosialidase–GFP fusion protein (PK1A-GFP) used in this study has been extensively characterized (Jokilammi et al., 2004) and it efficiently and selectively detects polySia in bacteria and tissues. The efficiency of binding of active endosialidases to their substrate is unaffected by O-acetylation of the polymer (Tomlinson & Taylor, 1985).
Infection of neonatal rats
Two-day-old rat pups were fed 20 μl of a mid-exponential-phase Mueller–Hinton broth culture (37 °C) of E. coli A192PP (OD600 0.55; 2–6×106 c.f.u. administered) using a micropipette. All litter members were infected in identical fashion and at the same time. Intestinal colonization was assessed at 24 h intervals by MacConkey agar culture of perianal swabs; K1 expression by isolated colonies was determined using K1-specific phages as described previously (Mushtaq et al., 2004). Bacteraemia was detected by MacConkey agar culture of blood from superficial veins in the footpad and K1 expression by colonies confirmed with K1E phage (obtained from Tom Cheasty). Animals were killed by decapitation and blood for visualization of bacteria in the systemic circulation was removed; samples (∼100 μl) from all pups in a litter were each diluted in 300 μl heparin (2 units ml−1) in PBS. Blood smears were made on glass slides and dried in air. Pooled blood samples were made up to 4 ml−1 in PBS and layered on to 3 ml Ficoll-Paque PLUS (GE Healthcare Bio-Sciences). After centrifugation (800 g, 45 min), the bacteria were found at the buffer/Ficoll interface; 30 μl aliquots were spotted on to polylysine-coated slides, air-dried and fixed for 15 min in ice-cold acetone prior to staining.
Post-mortem, the brain, heart, lungs, liver, spleen and kidneys were removed asceptically from each member of the litter, transferred to 1 ml cold PBS and the organ gently washed to remove blood. The washing process was repeated twice. Spread-plate counting on MacConkey agar was used to determine the bacterial content of each organ. Tissues were homogenized in 1 ml PBS using an Ultra-Turrax T8 homogenizer (IKA Werke). Homogenate (500 μl for brain, heart and kidney and 50 μl for lung, liver and spleen) was plated without dilution. If more colonies were evident following overnight incubation at 37 °C than could be reliably counted, a further 50 μl of homogenate was serially diluted and plated.
Preparation of tissue sections
Each organ was weighed and cut into two pieces along the longitudinal axis of symmetry. One portion was homogenized in 1 ml PBS as described above and E. coli K1 enumerated by the spread-plate method. The other was fixed in 10 % (v/v) neutral buffered formalin for 24–48 h and processed (2 h each) in ascending grades of ethanol (70–90 %, v/v), three baths of absolute alcohol, three of xylene and three of molten wax. The final process was conducted under vacuum. Tissue was then embedded in molten wax contained within metal moulds; after the wax had set the resultant blocks were cooled on ice, and 7 μm sequential sections were cut on a Shandon RM 2165 rotary microtome (Thermo Fisher Scientific) and dried overnight at room temperature.
Staining procedures
Modified (Drury & Wallington, 1980) Gram–Twort stain was used to visualize bacteria in tissue sections. For histochemical and immunochemical detection of O18 surface antigen and K1 polysaccharide, sections were immersed in Histo-Clear clearing agent HS-200 (National Diagnostics) for 10 min with one change of reagent prior to rehydration in decreasing concentrations of ethanol (100, 90, 70 %, v/v, in PBS). For indirect immunostaining, non-specific binding was blocked (20 min) with serum from the host of the secondary antibody followed by 1 h in diluted primary antibody, three 5 min washes in PBS, 1 h in diluted secondary conjugated antibody and three 5 min washes in PBS. For detection of K1 polymer with PK1A-GFP, the reagent was applied for 1 h prior to three PBS washes. Stained sections were mounted in Shandon Immunomount (Thermo Fisher Scientific).
Microscopy
Wide-field fluorescent microscopy was undertaken using a Zeiss Axioskop 2 plus microscope with Axiovision software (Carl Zeiss). Confocal microscopy was performed using a Zeiss LSM 510 Meta laser scanning microscope and Zeiss AIM software. Green and red fluorescence signals were quantified using Volocity4 software (Improvision).
RESULTS
Experimental E. coli K1 systemic infection involves invasion of major tissues
E. coli A192PP efficiently colonized the intestinal tract of neonatal rats; within 24–48 h of feeding of the bacterial suspension to postnatal day 2 (P2) pups, K1+ colonies could be recovered from the perianal area of all animals. Colonization of the gut, which persisted throughout the course of the infection, was followed by translocation of bacteria from the gut into the bloodstream to produce persistent bacteraemia in 80–100 % of rat pups, followed by death of bacteraemic animals within 1 week of gut colonization. Administration of a single daily endoE dose of 20 μg at P3, 1 day after infection by the oral route, reduced mortality from E. coli K1 infection by 77–90 %. A similar, but not greater, degree of protection against lethal bacteraemia was afforded by five daily 20 μg doses administered over P3–P7 (Mushtaq et al., 2004). Dosing with endoE did not significantly reduce intestinal colonization by A192PP. The capacity of A192PP to invade the bloodstream was dependent on the presence of polySia, as demonstrated by experiments in which the O18 : K1-negative strain A192PPK− was used to infect pups at P2 by the oral route and the animals were monitored for gut colonization and bacteraemia over the next 8 days. A192PPK1− rapidly and efficiently colonized the pups in the litter (9/10) but was unable to invade and survive in the blood (0/10); all animals survived the period of the experiment and all appeared healthy.
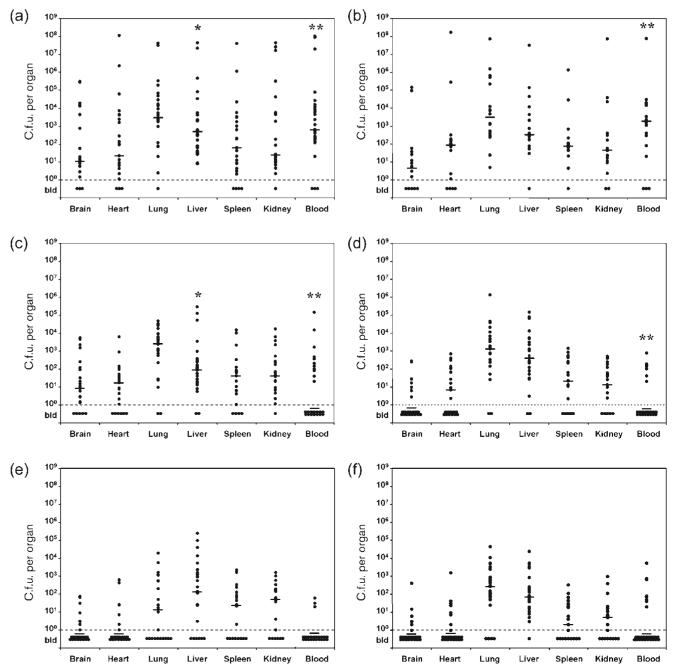
The association of the bacteria with the major organs (brain, heart, lung, liver, spleen and kidney) was determined during the early stages of infection in animals infected at P2 by the oral route (Fig. 1a, b). For each time point, data were obtained using three litters infected on different occasions and the data points for individual pups pooled as indicated. Less than 10 % of animals fed A192PP failed to develop systemic infection as judged by the recovery of viable bacteria from the blood at P4 and P5. Bacteria could be cultured from all organs of infected animals, although there were wide variations in the number of bacteria recovered from each tissue within litters. Counts for individual organs ranged from less than 10 bacteria per tissue to values of 108–109. Due to the lethal nature of the infection, the number of survivors was insufficient to continue the experiments beyond P5.
Fig. 1.
Progression of systemic E. coli K1 infection in neonatal rat pups. (a, b) Viable bacteria were cultured from blood and homogenized tissues 2 days (a) and 3 days (b) after feeding of A192PP to P2 rats. (c–e) Groups of A192PP-fed P2 rats were also dosed via the i.p. route with 20 μg endoE 1 day after feeding of bacteria (at P3) and blood and tissue colony counting performed at P4 (c), P5 (d) and P6 (e). Other animals were given daily i.p. doses of 20 μg endoE and bacteria enumerated at P6 (f). Data points represent bacterial counts per organ for individual pups; three litters were infected on separate occasions and the data grouped as indicated. The range of bacterial counts obtained for each of the three datasets within experiments was comparable. The median for each dataset is indicated. Counts were not performed on organs from pups that died during the course of the infection (number of dead pups/total pups: a, 3/30; b, 12/30; c, 3/30; d, 7/30; e, 5/29; f, 7/34). The differences in blood counts between untreated and endoE-treated pups at P4 and P5 were highly significant (**, P<0.001; Mann–Whitney two-tailed U-test); organ counts were not significantly different at these time points with the exception of liver at P4 (*, P<0.05). bld, below limit of detection.
Survival following administration of endoE is associated with clearance of K1 bacteria from the blood and a progressive reduction in the number of bacteria cultivated from tissue homogenates (Fig. 1c–e). At P4, 1 day after endoE administration to infected pups, more than half of the animals were abacteraemic (Fig. 1c) and the trend towards recovery continued through P5 (Fig. 1d) and P6 (Fig. 1e). Daily dosing of endoE did not enhance survival or recovery compared to single doses administered at P3. Levels of bacteria in blood and tissues of pups given three daily doses at P3–P5 are shown at P6 in Fig. 1(f) and should be compared to P6 data from P6 animals that received a single endoE dose (20 μg) at P3 (Fig. 1e).
E. coli K1 invades the choroid plexus and meninges, but not brain parenchyma
Spontaneous bacterial meningitis involves haematogenous spread into the CNS, leading to inflammation of the meninges (Fowler et al., 2004). We investigated the distribution of bacteria in brain sections using the Gram–Twort stain, as a prelude to determination of K1 capsule expression in infected tissue. As there was wide variation in bacterial numbers associated with the tissues of infected pups, the brain was removed and dissected along the longitudinal (sagittal) line of symmetry; one segment was used to determine bacterial content and the other for histological evaluation. Bacteria could not be readily located in sections prepared from brain tissue harbouring low numbers of K1; histological evaluation was therefore undertaken using tissue from brains containing bacterial populations greater than 5×105. No macroscopic differences between the brains of uninfected, lightly infected or heavily infected pups of the same age at sacrifice were evident. There was no difference in the weight of brains removed from animals given one endoE dose compared to those receiving daily doses over the period P3–P5. There were only minor differences in the weights of other organs between untreated and endoE-treated pups.
In mid-line sagittal sections, Gram-negative bacteria appeared to be restricted to two areas of the neonatal rat brain: choroid plexus and meninges (Fig. 2). Extensive microscopic examination of 7 μm sagittal sections failed to reveal any bacteria in the brain parenchyma. Bacteria were found scattered amongst the ependymal cells of the choroid plexus (Fig. 2a) and on or in the outermost structural feature of the meninges, the dura mater, including the meningeal folds of the cerebellum (Fig. 2b–f).
Fig. 2.
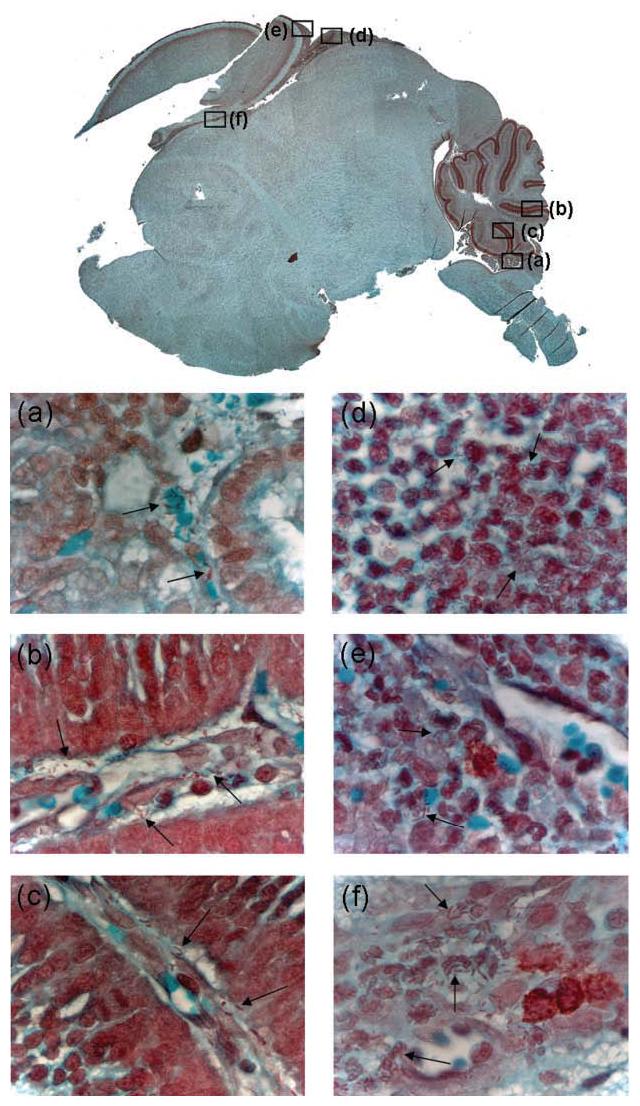
Bacteria are found in the choroid plexus and meninges of rat pups infected via the oral route with E. coli K1. Gram–Twort-stained medial sagittal section of brain from a rat pup fed K1 at P2 and culled at P5. The brain from which this section was taken contained 1.6×107 c.f.u. The main image was formed as a composite of fields (taken at ×5, reproduced at ×3) viewed by light microscopy. Higher magnification (taken at ×100, reproduced at ×60) images of regions of the choroid plexus (a), cerebral folds (b, c) and meninges (d–f) are shown; the locations of these fields within the sagittal section of the composite image are represented by the black squares and bacteria are indicated with arrows. Absence of bacteria within stained blood vessels indicated that contaminating blood was removed by the washing procedure described in Methods.
E. coli K1 in the bloodstream express polySia capsular polysaccharide
Many O18 : K1 strains are lysogenized by prophage CUS-3, which carries the neuO gene encoding an O-acetyltransferase responsible for non-stoichiometric O-acetylation of sialyl residues at positions C-7 and C-9 (Deszo et al., 2005; Vimr & Steenbergen, 2006). To characterize the expression of the K1 capsule in blood and tissues of infected neonatal rat pups, bacteria were probed with reagents specific for capsule (PK1A-GFP) and polySia O-acetyl groups (Ab8064). The bacterial surface was located using a polyclonal O18 antibody.
In a mid-exponential-phase culture of A192PP bacteria, the great majority of cells (>95 %) expressed K1 capsule, detected with PK1A-GFP. When the bacteria were treated with endoE to remove K1 capsule, no staining with PK1A-GFP was observed (data not shown). The confocal images shown in Fig. 3 illustrate a high degree of heterogeneity in capsule expression. In line with earlier studies on O-acetyl form variation in O18 : K1 strains (Ørskov et al., 1979), about 5 % of cells expressed O-acetyl-polySia in broth culture (Fig. 3). The large majority of E. coli EV36+pSX785 cells, which served as a control, expressed O-acetyl-polySia.
Fig. 3.
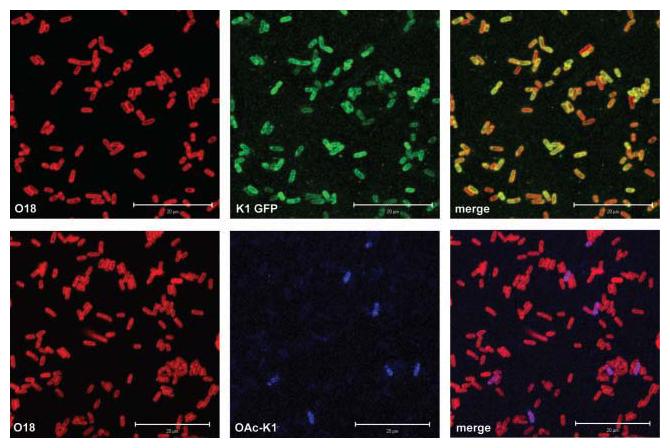
Expression of polySia capsule by E. coli K1 in vitro. First row: visualization of the K1 capsule expressed by mid-exponential-phase broth cultures of strain A192PP using PK1A-GFP. The O18 surface antigen was detected with rabbit polyclonal O18 antibody and Alexa Fluor 546 (red)-labelled second antibody. Second row: detection of O-acetylated polySia produced by broth-grown A192PP using monoclonal antibody Ab8064 and Cy5-labelled second antibody (blue). Images were obtained with the confocal microscope. Scale bars, 20 μm.
Blood samples obtained post-mortem from P4 infected untreated pups, containing large numbers of bacteria (>10 000 c.f.u. ml−1), were examined for bacterial K1 capsule. Staining of smears revealed that the majority (>95 %) of K1 cells in blood expressed K1 in the non-O-acetylated form (Fig. 4). Extensive examination by confocal microscopy of areas within a number of different blood smears stained with the O-acetyl-polySia antibody Ab8064 both alone and in combination with the other reagents failed to provide any evidence of O-acetylation of polySia. Examination at high magnification suggested that polySia was located predominantly at the poles of the bacterial rods present in blood; different patterns of capsule distribution were seen when bacteria were recovered from blood using Ficoll-Paque gradients (data not shown). Less than 5 % of bacteria failed to express capsule. Attempts were made to visualize bacteria in blood samples from endoE-treated P4–P6 pups. Despite an extensive search of a large number of samples, only small numbers of predominantly encapsulated bacteria could be visualized by fluorescence microscopy. These cells appeared to have evaded endoE-mediated capsule removal, at least up to the point of sampling.
Fig. 4.
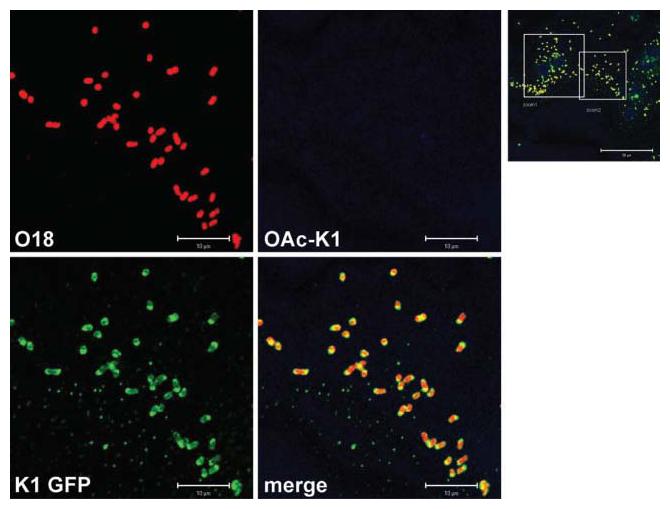
Expression of polySia capsule by E. coli K1 in the bloodstream of infected neonatal rat pups: visualization of the A192PP K1 capsule in blood obtained post mortem at P4 from infected untreated rat pups. Smears were made on glass slides, air-dried, fixed and stained with O18 polyclonal and O-acetyl-polySia-specific monoclonal antibodies. PolySia was visualized with the PK1A-GFP reagent. The main images (scale bars 10 μm) represent the area covered by the larger square in the lower-magnification image (scale bar 50 μm).
E. coli A192PP polySia is reduced following migration into the choroid plexus
PolySia promotes plasticity in cell–cell interactions, is abundantly expressed in the vertebrate embryonic brain and plays a critical role in the orderly development of this organ (Weinhold et al., 2005). Levels of polySia progressively decrease after birth and expression of relatively low amounts of the polymer persists in the adult brain in order to preserve a potential for morphological and physiological plasticity (Mühlenhoff et al., 1998). PK1A-GFP therefore detects polySia associated with brain tissue, in addition to its capacity to visualize the K1 capsule (Jokilammi et al., 2004). Bacteria located in the choroid plexus, visualized initially with the Gram–Twort stain (Fig. 2a), were localized with anti-O18 antibody (Fig. 5). Although some polySia is found in association with bacterial cells, capsule expression is reduced (Fig. 5): the ratio of green/red fluorescence of bacteria in the choroid plexus (0.39±0.03; n=13) contrasts with that for bacteria prepared for feeding to pups (1.56±0.06; n=67). No evidence was found for expression of O-acetyl-polySia. PolySia is expressed at moderate levels by the host tissue.
Fig. 5.
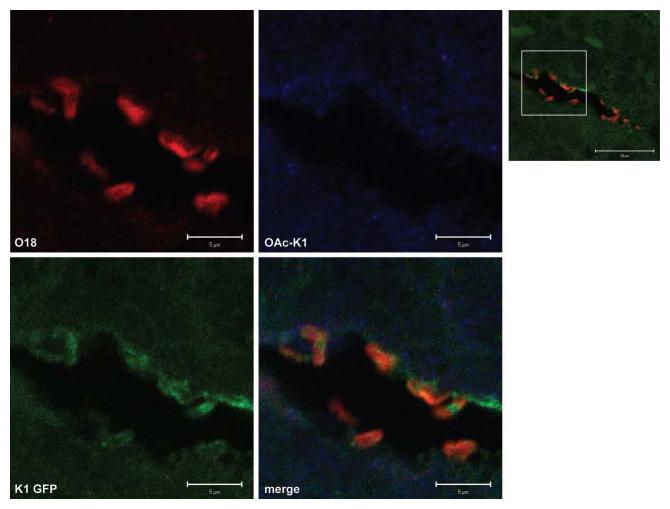
Reduced expression of polySia by A192PP cells localized within the choroid plexus. Confocal images of bacteria within the choroid plexus of an infected untreated rat pup, sacrificed at P5. Sections were stained with anti-O18 polyclonal antibody, anti-O-acetyl-polySia-specific monoclonal antibody and PK1A-GFP reagent for polySia as described in Methods. Optical slices of 1 μm were visualized. Ratio of green/red fluorescence associated with bacteria: 0.39±0.03 (mean±sem; n=13). The higher-magnification images (scale bars 5 μm) were located within the zone indicated in the lower-magnification image (scale bar 20 μm).
The structure and physiology of the brain (Saunders et al., 2000; Moody, 2006) indicates that colonization of the choroid plexus by neuropathogens such as E. coli K1 should enable them to gain access to the CSF and the ventricles. We therefore searched for bacteria in the ventricular system in off-mid-line sagittal sections of brain from infected P5 pups; mid-line sagittal sections, as used for Gram–Twort staining, expose little of the ventricular system (Fig. 2). A192PP cells expressing non-O-acetyl-polySia were found in association with the ventricular wall; a representative image is shown in Fig. 6. Green/red fluorescence ratios for bacteria located on the ventricular wall were similar to those found for bacteria in the choroid plexus (0.44±0.07; n=20). The ventricular surface expressed polySia.
Fig. 6.
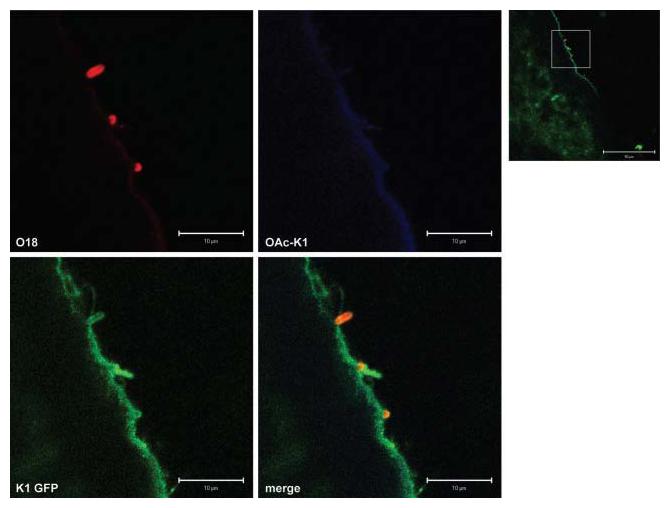
E. coli K1 cells located on the fourth ventricular ependymal wall express polySia. Confocal images of bacteria adherent to the ependymal cells lining a ventricle of an infected untreated rat pup, sacrificed at P5. Optical slices of 1 μm were visualized. Ratio of green/red fluorescence associated with bacteria: 0.44±0.07 (mean±sem; n=20). The higher-magnification images (scale bars 10 μm) were located within the zone indicated in the lower magnification image (scale bar 50 μm). The areas selected for examination corresponded to stereotaxic coordinates (−12,+8) of Fig. 162 in Paxinos & Wilson (2007).
E. coli K1 colonizing the meninges do not express polySia
Bacteria expressing O18 antigen could be located by fluorescence and confocal microscopy in the meninges of P5 infected untreated pups (Fig. 7). Adjoining cerebral tissue contained readily detectable polySia; meningeal tissue did not react with PK1A-GFP. Large numbers of bacteria were located in sagittal sections of P5 infected rat brain and no evidence for expression of capsule by A192PP bacteria was found (green/red fluorescence ratio 0.04±0.002; n=247). No bacteria located in the meninges bound O-acetyl-polySia antibody Ab8064 (Fig. 7), indicating that failure of PK1A-GFP to react with O18+ cells was not due to inhibition of binding by extensive O-acetylation of capsule.
Fig. 7.
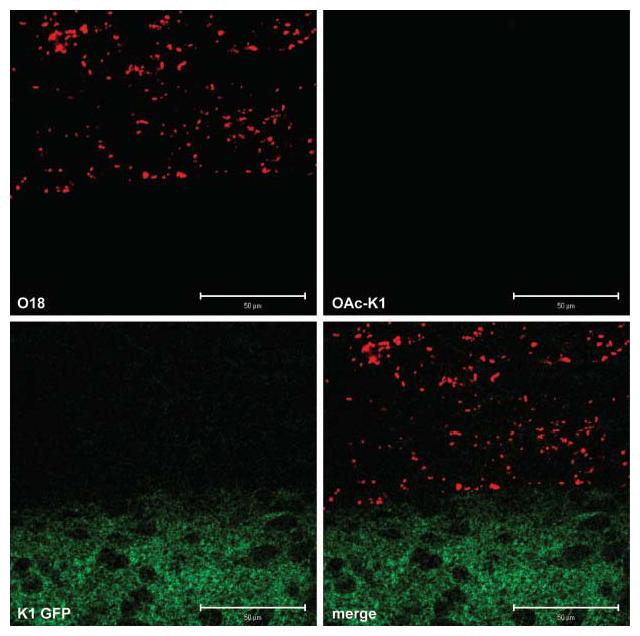
E. coli K1 colonizing the meninges do not express polySia. Bacterial cells at the meninges–cerebrum interface: confocal images of brain sections from an infected untreated pup sacrificed at P5. Optical slice of 1 μm; scale bars 50 μm. Ratio of green/red fluorescence associated with bacteria: 0.04±0.002 (mean±sem; n=247). The area selected for examination corresponded to stereotaxic coordinates (−4,+0) of Fig. 162 in Paxinos & Wilson (2007).
K1 bacteria cultured from brain tissue express O-acetyl-polySia
To ensure that K1 cells invading brain tissue had not lost the capacity to synthesize polySia, bacteria recovered by culture from the brain and other organs were examined for susceptibility to the K1-specific phage K1E. All colonies obtained from all organs were susceptible to K1E, indicating that selection for K1− forms had not occurred during transit from gut to brain. Mid-exponential-phase broth cultures were prepared from homogenates of pooled brain tissue from infected untreated P5 pups and examined for polySia. Staining patterns indicated that the distribution of polySia and O-acetyl-polySia was indistinguishable from that displayed by cells prepared for oral administration to P2 pups (data not shown).
DISCUSSION
Neuropathogenic K1 strains belong to a limited number of well-preserved clones associated with the O1, O7 or O18 O-groups, with O7 : K1 and O18 : K1 strains displaying a high degree of virulence for the neonate (Achtman et al., 1983; Pluschke et al., 1983). Human neonatal K1 infection is preceded by colonization of the intestine and this process is likely to be facilitated by the lack of a complex microbial community characteristic of the adult (Palmer et al., 2007). We exploited neonatal susceptibility to K1 infection to establish an in vivo model with a high rate of intestinal colonization (100 %) and bloodstream infection (80–100 %), in order to reduce redundancy of animal use and provide a platform for the evaluation of endoE administration. The virulence of O18 : K1 strain A192 was enhanced by animal passage and the enhanced strain A192PP introduced orally during the short period (P1–3) when non-K1 aerobic or facultative anaerobic intestinal bacteria were absent or present in low numbers.
Neonatal immune responses to polySia-bearing bacteria such as E. coli K1 are likely to be restricted due to the high degree of structural similarity of the capsule to the polySia moiety of NCAM, the mammalian neural cell adhesion molecule (Mühlenhoff et al., 1998; Stein et al., 2006). PolySia impedes activation of the alternative complement pathway, abrogating antibody-independent bactericidal action and opsonophagocytosis (Taylor, 1993). Immunogenicity, and possibly virulence, of K1 are increased by O-acetylation (Colino & Outschoorn, 1999; King et al., 2007). We therefore estimated the degree of O-acetylation of A192PP cells during the infection to determine if the advantages bestowed by phase variation outweighed those gained by reduced immune visibility.
Around 5 % of mid-exponential-phase A192PP cells expressed the O-acetylated form of polySia at any one time. After feeding of the bacteria to P2 pups, the intestinal tract was efficiently colonized and, soon after, invasion of the bloodstream occurred. The large majority of bacteria visualized in blood expressed polySia; the capsule most likely provided protection against complement attack and opsonization. We consistently found a small proportion of blood-derived bacteria that appeared to express no capsule. No evidence was found for O-acetylation in blood-derived K1+ bacteria, suggesting either downregulation of the rate of neuO transcription in response to exposure to immune mediators or, less likely, selection of bacteria that had lost the capacity for phase variation.
It is generally accepted that E. coli K1 bacteria invade the CNS due to their capacity to traverse the microvascular endothelium of the blood–brain barrier (Nassif et al., 2002; Kim, 2003) and this has given rise to extensive investigation of the processes involved in bacterial trafficking across cultured human brain endothelial cells (Xie et al., 2004; Kim et al., 2005). However, evidence for this route of entry is modest and derives from experiments with newborn and adult rats infected subcutaneously (Kim et al., 1992), an invasive model of haematogenous spread that neither replicates the age dependency of the spontaneous infection nor utilizes the natural route of infection. The model used here mimics key features of spontaneous infection in neonates and we detected large numbers of bacteria in the choroid plexus. This observation is compatible with the view that the choroid plexus is a portal of entry for bacteria into the CSF: the blood–brain endothelium has a very high resistance to transport, the choroid plexus much less so (Moody, 2006). Distribution of E. coli K1 in the human infection (Siegel & McCracken, 1981) and in our rat model is restricted to the meninges. Transit from blood to CSF via the choroid plexus would account for the observed distribution of A192PP cells. Bacteria emerging from the choroid plexus, the site of production of CSF, would be directed through the subarachnoid space of the ventricular system and over the brain surface; adhesion to the surface would precede meningeal penetration and produce the distribution pattern noted here.
A192PP bacteria in the choroid plexus expressed lower amounts of polySia compared to control cells; in this location they are likely to be protected from attack by humoral immune effectors and this may allow a degree of downregulation of capsule expression. No evidence for O-acetylation of polySia in choroid plexus was found. The presence of E. coli K1 in CSF is a characteristic of the rat neonatal model (Glode et al., 1977) and K1+ bacteria attached to the ventricular wall expressed levels of non-O-acetylated polySia similar to bacteria in the choroid plexus. In the meninges, A192PP bacteria were detected with anti-O18 antibodies but they did not react with PK1A-GFP reagent. We considered the unlikely possibility that, although the rate of hydrolysis of polySia by α-2,8-specific endoneuraminidases (from which PK1A-GFP was derived) is unaffected by the presence of the O-acetyl group (Tomlinson & Taylor, 1985), failure of the PK1A-GFP reagent to bind to bacteria in the meninges was due to high levels of O-acetylation. This was not the case, as O-acetyl-specific antibody failed to bind to O18+ bacteria in tissue sections. We suggest that once E. coli K1 penetrates the meninges, likely to be a protected site in the early stages of the infection, the bacteria no longer express polySia. The synthetic, regulatory and export components for K1 capsule expression are encoded in three functionally distinct regions of the 20 kb kps pathogenicity island (Cieslewicz & Vimr, 1997) and it is our intention to explore the transcriptional basis of repression of polySia expression in the meninges.
Tracking the progress of A192PP bacteria from the blood to the meninges has revealed differential expression of polySia: extensive expression of the non-O-acetylated form of the capsule in compartments (blood, cerebral spinal) rich in immune defence mediators contrasts with reduced expression or complete shutdown of expression in sites that may afford protection from immune attack. These differences were not due to selection for K1− forms during progression from gut to brain, as cultured bacterial colonies, from isolates taken from tissue beds, including the brain, expressed capsule. Bacteria cultured from brain homogenates retained the capacity to O-acetylate polySia to an extent comparable to parent cultures. Expression of the non-O-acetylated polymer, particularly in blood, limits the capacity of the immature host to mount an immune response and reinforces the view that this type of form variation is not critical to the virulence of E. coli K1 in neonatal infection (Colino & Outschoorn, 1999). Experimental endoE therapy is most effective during the early stages of infection; a single dose of the enzyme given 24 h after A192PP feeding was more effective in preventing bacteraemia and death than delayed administration (Mushtaq et al., 2004). Thus, it is likely that removal of the capsule from bacteria in the blood compartment, before arrival at the choroid plexus or other portals of entry into the CNS, leads to complement deposition, opsonization and C5b-9-mediated killing (Taylor, 1993). Timely administration of endoE led to bacterial clearance from the bloodstream and reductions in the bacterial burden of the organs of surviving rat pups, suggesting that the pool of A192PP in the blood acted as a reservoir for seeding of tissues. Lack of polySia expression by bacteria in the meninges would make them refractory to modulation by endoE even if, as seems unlikely (Saunders et al., 2000), the enzyme penetrated meningeal tissue.
In conclusion, E. coli O18 : K1 A192PP causes an age-dependent systemic infection in neonatal rat pups when given orally. The bacteria colonize the intestinal tract and rapidly relocate to the blood compartment, where they express non-O-acetylated polySia capsule. Prompt administration of the selective capsule depolymerase endoE resolves the infection and prevents death in almost all pups. In untreated animals, the bacteria gain entry to the CNS, in part via the choroid plexus, and colonize and penetrate the meninges. Once established in this tissue, they appear not to express polySia. Differential expression of polySia and O-acetyl-polySia is phenotypic in nature. Therapeutic modulation of capsule expression could form the basis of a new approach to the treatment of systemic infections due to encapsulated pathogens.
ACKNOWLEDGEMENTS
This work was funded by a research grant (G0400268) from the MRC. We thank Eric Vimr (University of Illinois) for stimulating discussion, Ann Kingsbury (University College London) for advice on the anatomy of the rat brain and Roger Jee (School of Pharmacy London) for statistical insight.
Abbreviations
- CSF
cerebrospinal fluid
- CNS
central nervous system
- endoE
endosialidase E
- i.p.
intraperitoneal
- NBM
neonatal bacterial meningitis
- PK1A-GFP
endosialidase–GFP fusion protein
- polySia
polysialic acid
REFERENCES
- Achtman M, Mercer A, Kusećek B, Pohl A, Heuzenroeder M, Aaronson W, Sutton A, Silver RP. Six widespread bacterial clones among Escherichia coli K1 isolates. Infect Immun. 1983;39:315–335. doi: 10.1128/iai.39.1.315-335.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bortolussi R, Ferrieri P, Björkstén B, Quie PG. Capsular K1 polysaccharide of Escherichia coli: relationship to virulence in newborn rats and resistance to phagocytosis. Infect Immun. 1979;25:293–298. doi: 10.1128/iai.25.1.293-298.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cieslewicz M, Vimr E. Reduced polysialic acid capsule expression in Escherichia coli K1 mutants with chromosomal defects in kpsF. Mol Microbiol. 1997;26:237–249. doi: 10.1046/j.1365-2958.1997.5651942.x. [DOI] [PubMed] [Google Scholar]
- Colino J, Outschoorn I. The form variation of the capsular polysaccharide K1 is not a critical virulence factor of Escherichia coli in a neonatal mouse model of infection. Microb Pathog. 1999;27:187–196. doi: 10.1006/mpat.1999.0291. [DOI] [PubMed] [Google Scholar]
- Cross AS, Kim KS, Wright DC, Sadoff JC, Gemski P. Role of lipopolysaccharide and capsule in the serum resistance of bacteremic Escherichia coli. J Infect Dis. 1986;154:497–503. doi: 10.1093/infdis/154.3.497. [DOI] [PubMed] [Google Scholar]
- Deszo EL, Steenbergen SM, Freedberg DI, Vimr ER. Escherichia coli K1 polysialic acid O-acetyltranferase gene, neuO, and the mechanism of capsule form variation involving a mobile contingency locus. Proc Natl Acad Sci U S A. 2005;102:5564–5569. doi: 10.1073/pnas.0407428102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drury RAB, Wallington EA. Carleton's Histological Technique. 5th edn. Oxford University Press; New York: 1980. [Google Scholar]
- Fowler MI, Weller RO, Heckels JE, Christodoulides M. Different meningitis-causing bacteria induce distinct inflammatory responses on interaction with cells of the human meninges. Cell Microbiol. 2004;6:555–567. doi: 10.1111/j.1462-5822.2004.00382.x. [DOI] [PubMed] [Google Scholar]
- Gaudreau C, Delage G, Rousseau D, Cantor ED. Bacteremia caused by viridans streptococci in 71 children. Can Med Assoc J. 1981;125:1246–1249. [PMC free article] [PubMed] [Google Scholar]
- Glode MP, Sutton A, Moxon ER, Robbins JB. Pathogenesis of neonatal Escherichia coli meningitis: induction of bacteremia and meningitis in infant rats fed E. coli K1. Infect Immun. 1977;16:75–80. doi: 10.1128/iai.16.1.75-80.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey D, Holt DE, Bedford H. Bacterial meningitis in the newborn: a prospective study of mortality and morbidity. Semin Perinatol. 1999;23:218–225. doi: 10.1016/s0146-0005(99)80066-4. [DOI] [PubMed] [Google Scholar]
- Jokilammi A, Ollikka P, Korja M, Jakobsson E, Loimaranta V, Haataja H, Finne J. Construction of antibody mimics from a noncatalytic enzyme – detection of polysialic acid. J Immunol Methods. 2004;295:149–160. doi: 10.1016/j.jim.2004.10.006. [DOI] [PubMed] [Google Scholar]
- Kim KS. Pathogenesis of bacterial meningitis: from bacteraemia to neuronal injury. Nat Rev Neurosci. 2003;4:376–385. doi: 10.1038/nrn1103. [DOI] [PubMed] [Google Scholar]
- Kim KS, Itabashi H, Gemski P, Sadoff J, Warren RL, Cross AS. The K1 capsule is the critical determinant in the development of Escherichia coli meningitis in the rat. J Clin Invest. 1992;90:897–905. doi: 10.1172/JCI115965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim BY, Kang J, Kim KS. Invasion processes of pathogenic Escherichia coli. Int J Med Microbiol. 2005;295:463–470. doi: 10.1016/j.ijmm.2005.07.004. [DOI] [PubMed] [Google Scholar]
- King MR, Steenbergen SM, Vimr ER. Going for baroque at the Escherichia coli K1 cell surface. Trends Microbiol. 2007;15:196–202. doi: 10.1016/j.tim.2007.03.006. [DOI] [PubMed] [Google Scholar]
- Korhonen TK, Valtonen MV, Parkkinen J, Väïsänen-Rhen V, Finne J, Ørskov F, Ørskov I, Svenson SB, Mäkelä PH. Serotypes, hemolysin production, and receptor recognition of Escherichia coli strains associated with neonatal sepsis and meningitis. Infect Immun. 1985;48:486–491. doi: 10.1128/iai.48.2.486-491.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leggate DR, Bryant JM, Redpath MB, Head D, Taylor PW, Luzio JP. Expression, mutagenesis and kinetic analysis of recombinant K1E endosialidase to define the site of proteolytic processing and requirements for catalysis. Mol Microbiol. 2002;44:749–760. doi: 10.1046/j.1365-2958.2002.02908.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moody DM. The blood-brain barrier and blood-cerebral spinal fluid barrier. Semin Cardiothorac Vasc Anesth. 2006;10:128–131. doi: 10.1177/1089253206288992. [DOI] [PubMed] [Google Scholar]
- Mühlenhoff M, Eckhardt M, Gerardy-Schahn R. Polysialic acid: three-dimensional structure, biosynthesis and function. Curr Opin Struct Biol. 1998;8:558–564. doi: 10.1016/s0959-440x(98)80144-9. [DOI] [PubMed] [Google Scholar]
- Mushtaq N, Redpath MB, Luzio JP, Taylor PW. Prevention and cure of systemic Escherichia coli K1 infection by modification of the bacterial phenotype. Antimicrob Agents Chemother. 2004;48:1503–1508. doi: 10.1128/AAC.48.5.1503-1508.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mushtaq N, Redpath MB, Luzio JP, Taylor PW. Treatment of experimental Escherichia coli infection with recombinant bacteriophage-derived capsule depolymerase. J Antimicrob Chemother. 2005;56:160–165. doi: 10.1093/jac/dki177. [DOI] [PubMed] [Google Scholar]
- Nassif X, Bourdoulous S, Eugène E, Couraud P-O. How do extracellular pathogens cross the blood-brain barrier? Trends Microbiol. 2002;10:227–232. doi: 10.1016/s0966-842x(02)02349-1. [DOI] [PubMed] [Google Scholar]
- Ørskov F, Ørskov I, Sutton A, Schneerson R, Lin W, Egan W, Hoff GE, Robbins JB. Form variation in Escherichia coli K1: determined by O-acetylation of the capsular polysaccharide. J Exp Med. 1979;149:669–685. doi: 10.1084/jem.149.3.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer C, Bik EM, DiGiulio DB, Relman DA, Brown PO. Development of the human infant intestinal microbiota. PLoS Biol. 2007;5:e177. doi: 10.1371/journal.pbio.0050177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkkinen J, Korhonen TK, Pere A, Hacker J, Soinila S. Binding sites in the rat brain for Escherichia coli S fimbriae associated with neonatal meningitis. J Clin Invest. 1988;81:860–865. doi: 10.1172/JCI113395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Wilson C. The Rat Brain in Stereotaxic Coordinates. 6th edn. Academic Press; London: 2007. [Google Scholar]
- Pluschke G, Mercer A, Kusećek B, Pohl A, Achtman M. Induction of bacteremia in newborn rats by Escherichia coli K1 is correlated with only certain O (lipopolysaccharide) antigen types. Infect Immun. 1983;39:599–608. doi: 10.1128/iai.39.2.599-608.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polin RA, Harris MC. Neonatal bacterial meningitis. Semin Neonatol. 2001;61:157–172. doi: 10.1053/siny.2001.0045. [DOI] [PubMed] [Google Scholar]
- Robbins JB, McCracken GH, Gotschlich EC, Ørskov F, Ørskov I, Hanson LA. Escherichia coli K1 capsular polysaccharide associated with neonatal meningitis. N Engl J Med. 1974;290:1216–1220. doi: 10.1056/NEJM197405302902202. [DOI] [PubMed] [Google Scholar]
- Saunders NR, Knott GW, Dziegelewska KM. Barriers in the immature brain. Cell Mol Neurobiol. 2000;20:29–40. doi: 10.1023/A:1006991809927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel JD, McCracken GH. Sepsis neonatorum. N Engl J Med. 1981;304:642–647. doi: 10.1056/NEJM198103123041105. [DOI] [PubMed] [Google Scholar]
- Steenbergen SM, Lee Y-C, Vann WF, Vionnet J, Wright LF, Vimr ER. Separate pathways for O-acetylation of polymeric and monomeric sialic acids and identification of sialyl O-acetyl esterase in Escherichia coli K1. J Bacteriol. 2006;188:6195–6206. doi: 10.1128/JB.00466-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein DM, Robbins J, Miller MA, Lin F-YC, Schneerson R. Are antibodies to the capsular polysaccharide of Neisseria meningitidis group B and Escherichia coli K1 associated with immunopathology? Vaccine. 2006;24:221–228. doi: 10.1016/j.vaccine.2005.07.084. [DOI] [PubMed] [Google Scholar]
- Taylor PW. Non-immunoglobulin activators of the complement system. In: Sim RB, editor. Activators and Inhibitors of Complement. Kluwer Academic Publishers; Dordrecht, The Netherlands: 1993. pp. 37–68. [Google Scholar]
- Taylor PW, Stapleton PD, Luzio JP. New ways to treat bacterial infections. Drug Discov Today. 2002;7:1086–1091. doi: 10.1016/s1359-6446(02)02498-4. [DOI] [PubMed] [Google Scholar]
- Tomlinson S, Taylor PW. Neuraminidase associated with coliphage E that specifically depolymerizes the Escherichia coli K1 capsular polysaccharide. J Virol. 1985;55:374–378. doi: 10.1128/jvi.55.2.374-378.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tunkel AR, Scheld WM. Pathogenesis and patho-physiology of bacterial meningitis. Clin Microbiol Rev. 1993;6:118–136. doi: 10.1128/cmr.6.2.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vimr ER, Steenbergen SM. Mobile contingency locus controlling Escherichia coli polysialic acid capsule acetylation. Mol Microbiol. 2006;60:828–837. doi: 10.1111/j.1365-2958.2006.05158.x. [DOI] [PubMed] [Google Scholar]
- Vimr ER, Troy FA. Identification of an inducible catabolic system for sialic acids (nan) in Escherichia coli. J Bacteriol. 1985;164:845–853. doi: 10.1128/jb.164.2.845-853.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinhold B, Seidenfaden R, Röckle I, Mühlenhoff M, Schertzinger F, Conzelmann S, Marth JD, Gerardy-Schahn R, Hildebrandt H. Genetic ablation of polysialic acid causes severe neurodevelopmental defects rescued by deletion of the neural cell adhesion molecule. J Biol Chem. 2005;280:42971–42977. doi: 10.1074/jbc.M511097200. [DOI] [PubMed] [Google Scholar]
- Xie Y, Kim KJ, Kim KS. Current concepts on Escherichia coli K1 translocation of the blood-brain barrier. FEMS Immunol Med Microbiol. 2004;42:271–279. doi: 10.1016/j.femsim.2004.09.001. [DOI] [PubMed] [Google Scholar]