Abstract
In its simplest aspect, this review is an attempt to describe the major ligand classes of the aryl hydrocarbon receptor (AHR). A grander objective is to provide models that may help define the physiological activator or “endogenous ligand” of the AHR. We begin by presenting evidence that supports a developmental function for the AHR. This is followed by proposing mechanisms by which an endogenous ligand and consequent AHR activation might be important during normal physiology and development. With this background, we then present a survey of the known xenobiotic, endogenous, dietary and “un-conventional” activators of the AHR. When possible, this includes information about their induction potency, receptor binding affinity and potential for exposure. Because of the essential function of the AHR in embryonic development, we discuss the candidacy of each of these compounds as physiologically important activators.
1. INTRODUCTION
It is now clear that the biological response to many environmental pollutants is a direct consequence of their interactions with the aryl hydrocarbon receptor. Experiments demonstrating a role for the aryl hydrocarbon receptor (AHR) in the metabolism of benzo[a]pyrene, as well as in the acute toxicity of halogenated-dioxins are important examples of how certain classes of hazardous chemicals elicit their toxicity and how mammalian organisms adapt to such exposures. In recent years, interest in AHR biology has grown beyond a toxicological perspective as research has uncovered a physiological role for this receptor in normal development. Consequently, the study of AHR pharmacology has garnered additional attention as investigators begin to look at known receptor ligands for insights into the structure of the putative endogenous ligand and for help in unlocking the therapeutic potential that may come from modulating this system.
In this review, we will first provide a brief description of the underlying AHR signal transduction pathway and summarize the evidence that this receptor is both a player in chemical toxicity and an important component of normal development. Given the numerous reviews on the role of the AHR in chemical toxicity (1–3), we will highlight developmental aspects of AHR signaling. Specifically, we will be presenting the case for the existence of an endogenous activator. In the second part of this review, we will provide a discussion of known agonists of the AHR, with an emphasis on those compounds that may be relevant to the putative endogenous ligand and the future pharmacopoeia of the AHR.
2. BACKGROUND
The Ah receptor and adaptive metabolism
The field of AHR research has its origins in vertebrate toxicology. In the 1960s and 1970s, polycyclic aromatic hydrocarbons (PAHs) were common model compounds used in attempts to understand carcinogen bioactivation and detoxification. In these early studies, the metabolism of PAHs was found to be much more efficient upon secondary exposures (4–7). Examination of the underlying mechanism revealed that the primary exposure led to the induction of a battery of cytochrome P450-dependent monooxygenases and conjugating enzymes such as UDP-glucuronosyl transferase and glutathione-S-transferase (8, 9). Observations that the upregulated enzymes led to increased metabolism of the inducing compounds provided early evidence that this response might serve as a protective mechanism against xenobiotics (10).
Experiments to describe this metabolic adaptation resulted in the observation that certain mouse strains differed in their inductive response after PAH exposure (11, 12). Analysis of crosses between “responsive” and “nonresponsive” strains demonstrated that a single autosomal dominant locus played a central role. This locus was designated Ah, for aryl hydrocarbon responsiveness (13). Evidence that the Ah locus encoded a receptor was drawn from toxicology studies of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD). Because of its induction potency, this highly toxic environmental contaminant proved to be a useful probe of the Ah system. Through analysis with radiolabeled TCDD, a receptor species was identified from hepatic cytosol of C57BL/6J mice (14). The proof that this site was a “receptor” was two-fold. First, this site bound chlorinated-dioxin congeners with an affinity that was proportional to the individual compound’s potency as an inducer of monooxygenases. Second, the TCDD-binding affinity for this site segregated with the responsive and nonresponsive genotype (15, 16). Taken in sum, these experiments provided pharmacological and genetic proof that this binding site was a bona fide receptor and that it was encoded by the Ah-locus (13). We now refer to this PAH- and dioxin-binding protein as the Ah-receptor.
3. THE AHR SIGNAL TRANSDUCTION PATHWAY
The basics of signaling
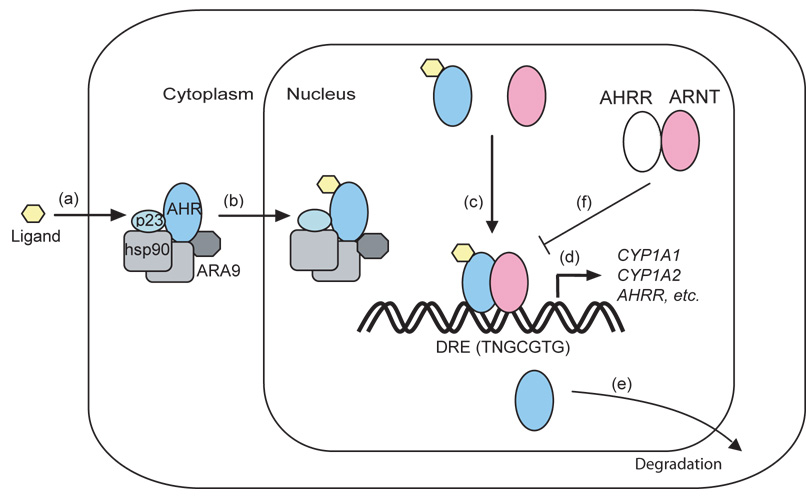
Investigations into the role of the AHR in adaptive metabolism resulted in a detailed understanding of the signal transduction pathway that links toxicant-receptor binding to the induction of xenobiotic metabolizing enzymes. Biochemical studies demonstrate that in the absence of agonist, the AHR exists as an inactive complex with two molecules of the chaperone Hsp90, as well as molecules of the co-chaperones, ARA9 (also known as AIP1 or XAP2) and p23 (17–22). Molecular cloning studies reveal that the AHR contains a basic-helix-loop-helix (bHLH) domain similar to that present in many DNA-binding proteins (23–25). It also contains a PER-ARNT-SIM (PAS) homology domain similar to that found in other regulators of cellular and organismal responses to the environment (25–28). Most notable among the bHLH-PAS superfamily are the hypoxia inducible factors that regulate responses to low oxygen tension and the Clock-Mop3 proteins that serve at the core of the mammalian circadian clock (26).
In current models of signaling, the binding of an agonist at the PAS domain of the AHR leads to a conformational change in the receptor (Fig. 1). This change alters associations with chaperones and exposes a nuclear localization signal on the AHR (18, 29, 30). As a result, the receptor complex migrates to the nucleus, where the AHR heterodimerizes with another bHLH-PAS protein known as the aryl hydrocarbon receptor nuclear translocator (ARNT) (31). The interaction of the AHR with ARNT increases their capacity to bind specific enhancer sequences adjacent to target promoters termed “dioxin responsive elements” (DREs) (32–35). An assembly of coactivators and general transcription factors, including p300, SRC-1, p/CIP and transcription factor IIB, then interacts with gene promoters and potentiates the expression of target loci (36). The most well studied of these responsive genes include the Cyp1a1, Cyp1a2 and Cyp1b1 loci that encode the xenobiotic metabolizing monooxygenases central to the adaptive metabolic response (10).
Figure 1.
The AHR is also subject to negative regulation. Following ligand-induced activation and nuclear export (29, 37), the receptor is apparently degraded via a 26S proteosome pathway (38–41). The activity of the AHR-ARNT complex is also attenuated by a second mechanism, the upregulation of a transcriptional repressor known as the aryl hydrocarbon receptor repressor (AHRR) (42). The AHRR is another bHLH-PAS protein with high sequence similarity to the AHR. The AHRR represses AHR transcriptional activity by binding ARNT and by the repressive activity derived from the interaction of the ARNT and AHRR complex with DREs (42). This attenuation of AHR activity by means of a negative feedback loop and receptor degradation may serve to protect the organism from the consequences of transcriptional hyperstimulation by potent agonists and to provide precise temporal control of this pathway.
4. A PHYSIOLOGICAL/ DEVELOPMENTAL ROLE FOR THE AHR
Three independent lines of evidence are in keeping with the idea that the AHR plays an important role in normal physiology. The evolutionary conservation of the AHR, the aberrant phenotypes of Ahr-mutant mice and the expression of DRE-responsive genes during development are all consistent with a physiological function of this receptor system. By extension, these observations also support the existence of an endogenous ligand or activator. In the next section, we will review the evidence that strengthens the idea that the AHR has dual roles in normal biology: That is, one role as a mediator of an adaptive response to xenobiotics and a second role as a mediator of normal embryonic development and adult physiology.
4.1. The AHR has been highly conserved throughout evolution
If the physiological role of the AHR were limited to regulating a metabolic response to foreign chemicals, it would follow that this xenobiotic stress would be relatively conserved in most environmental niches. If the xenobiotic or environmental stress were not similar, it would follow that receptor structure and function would vary significantly in organisms from differing ecosystems. A phylogenetic analysis of the AHR reveals that the primary amino acid sequence of the ligand binding PAS domain protein is highly conserved across vertebrate species from marine, terrestrial and avian environments (43). In this regard, the amino acid sequence of the chicken PAS domain shares 81–86% similarity with that of the amphibian and mammalian AHR, and 64–69% similarity with that of fish species (43). In addition to structural similarities in the PAS domain, the vertebrate AHR orthologs appear to dimerize with ARNT and drive transcription from DREs (44). Moreover, in fish, rodents and birds, the upregulated target genes appear to be orthologs of the Cyp1 family of monooxygenases (45, 46). We take these observations as an indication that the ligand binding specificity of the AHR does not differ significantly across species found in terrestrial, marine and avian environments, signifying that the evolutionary stressor influencing ligand specificity of the AHR is an endogenous compound, not an exogenous one.
Additional support for the existence of an endogenous activator can also be garnered from the observation that AHR orthologs found in invertebrate species possess physiological functions, yet do not appear to bind xenobiotics. In Drosophila melanogaster, the spineless and tango loci encode bHLH-PAS proteins that bear homology to the mammalian AHR and ARNT, respectively (47, 48). Mutations in either spineless or tango disrupt the lengthening of distal segments in the antennal and leg, demonstrating their essential roles in the organism’s development (47, 48). In addition, spineless expression is also crucial for generating the retinal photoreceptor pattern required for fly color vision (49). Proteins with structural similarity to AHR and ARNT have also been identified in Caenorhabditis elegans, where deficiency of the AHR ortholog (AHR-1) leads to impaired neuronal differentiation (50, 51). Despite sharing protein sequence homology, invertebrate AHRs do not appear to bind agonists recognized by the vertebrate receptor (e.g., β-naphthoflavone, TCDD or the radioligand, 2-azido-3-[125I]iodo-7,8-dibromodibenzo-p-dioxin) (47, 50, 52). The lack of known xenobiotic agonists of invertebrate AHRs provides support for the idea that the mammalian AHR has a biological role other than being an evolutionary adaptor to environmental pollution (50, 53, 54).
4.2. Defects in AHR null mice
Perhaps the most compelling argument for the existence of an endogenous activator of the AHR comes from studies of recombinant mouse models where the Ahr and Arnt genes have been mutated. Independent labs studying the physiology and development of Ahr null mice have described developmental aberrations and pathological endpoints that arise when AHR signaling is compromised by receptor deletion (55, 56). A number of allele specific phenotypes has also been reported. Given these reported differences, we refer to the recombinant animals as either Δ1/Δ1 or Δ2/Δ2 mice, indicating the exon targeted for excision (57). For example, a 40-50% neonatal lethality rate, inflammation of the bile ducts and an 80% depletion of splenic lymphocytes is reported for 3-week old Δ1/Δ1 mice (55). As the Δ1/Δ1 mice age, they also exhibit cardiohypertrophy, skin lesions, portal vascular hypertrophy and pyloric hyperplasia of the gastrointestinal tract (58). In contrast, the Δ2/Δ2 mice commonly exhibit normal perinatal survival and normal lymphocyte numbers. While an explanation for the different phenotypes would likely require extensive experimental effort, contributing factors might include the animals’ different genetic background, targeting strategies or even the housing environment and pathogen status of the mice (57).
In our laboratory, the most penetrant and easily observed phenotypes in both Δ1/Δ1 and Δ2/Δ2 mice are vascular in nature. In this regard, Ahr null mice have a 100% frequency of patent ductus venosus (DV). The DV is a fetal porto-caval shunt of the developing liver that normally closes immediately after birth. In Ahr null mice, this shunt fails to close, and the mutants display aberrant hepatovascular blood flow and altered disposition of small molecules requiring hepatic clearance (59, 60). Other vascular aberrations noted in the Ahr null mouse model include a persistence of the hyaloid artery and an altered limbal vasculature within the developing eye. Like the DV, the hyaloid artery represents a second fetal vascular structure that normally resolves postnatally, yet persists in the eyes of adult Ahr null mice (59). These highlighted phenotypes are evidence supporting a function of the AHR in normal development. Based upon these early studies, we have concluded that the role of the AHR is in regulating normal vascular or hematopoietic development.
Studies using AHR hypomorphic animals have also provide evidence that receptor activation is a required step in normal mammalian development. When gene targeting was used to generate a mouse with a marked decrease in the level of the AHR (i.e., hypomorphic), these mice displayed a high frequency of patent DV, similar to that seen in the corresponding null animals. Exposure of these hypomorphs to TCDD between embryonic days E12.5-E18.5 leads to the complete closure of the DV and the maturation of a normal-sized liver by adulthood (61). The compensation of a hypomorphic AHR signaling pathway by a potent agonist during gestation supports the proposal that an endogenous agonist activates the AHR to regulate vascular development during normal ontogeny.
4.3. Activation of the AHR during development
If the AHR plays a role in normal ontogeny, one would predict that evidence of receptor activation during development would be detectable. In keeping with this prediction, there are multiple reports to suggest that upregulation of AHR-target genes occur during normal embryonic development. In this regard, evidence for Cyp1a1 expression was observed in mice that carry the Cyp1a1 promoter driving a lacZ transgene (62). The analysis of β-galactosidase activity in tissue sections indicated expression of Cyp1a1 in the hindbrain, midbrain, heart, kidney and tail during embryonic days E8 through E14. Transcriptional activity was also detected in the liver at day E13, skin and muscle at days E13 and E14 (62). Using PCR-based assays, pools of cDNA from whole mouse embryos revealed the expression of Cyp1a1 transcript at day E7 and Cyp1b1 transcripts at beginning at embryonic day E11 (63). Analyses of human tissues also showed strong expression of the CYP1A1 transcript in the fetal adrenal, lung and liver (64). Taken in sum, these reports of Cyp1b1 and Cyp1a1 expression during embryogenesis provide evidence that the AHR may be signaling in response to some tightly regulated developmental cue.
5. THE SEARCH FOR ENDOGENOUS ACTIVATORS OF THE AHR
Appreciation that the AHR is a developmentally important molecule has renewed our interest in the search for the bona fide “endogenous ligand.” An analysis of ligand studies carried out over the last twenty years yields a long list of AHR-activators, including environmental contaminants, therapeutics, naturally occurring chemicals and small molecules isolated from mammalian tissues (65, 66). To help sort out this literature, we will use the next section to provide models that we propose can help us define the characteristics of a true endogenous activator of the AHR. We will then go on to review what is known about the current list of candidate activators and comment on the data that each candidate compound is an endogenous ligand of the AHR.
Models of endogenous signaling
One model to explain AHR signaling is based on the idea that the AHR has arisen to mediate the adaptive metabolism of harmful chemicals generated endogenously. Such harmful endogenous compounds could be the byproducts of enzymatic reactions or normal physiological chemistry. If this model were correct, it would follow that the developmental pathologies observed in the Ahr-deficient animals result from the unchecked levels of endogenously generated toxins that adversely affect ontogeny. In such a defensive system, the AHR could be a receptor that recognizes a spectrum of structurally diverse compounds, or could be specifically targeted to upregulate the metabolism of a single highly toxic chemical structure. Under such a system, the AHR would participate in normal development by promoting clearance of biological molecules that harbor toxic potential.
A second model is that the AHR is part of an essential signaling pathway that is initiated by binding of its endogenous ligand, via a mechanism analogous to that seen in signal transduction of the nuclear receptor family of transcription factors (67, 68). In contrast to the protective mechanism described above, the endogenous AHR agonist under this system would induce, rather than impede developmental processes. Under such a model, the capacity to respond and regulate xenobiotics, such as PAHs, could have arisen independently and be of secondary importance developmentally.
A third model represents a combination of the two already mentioned. In this model, the foremost physiological role of the AHR is to mediate normal development by transducing the chemical signal of the endogenous agonist to transcriptional events. Similar to the androgen receptors that mediate sexual differentiation, the physiological function of the AHR would likely be highly regulated, such that an endogenous agonist would require clearance at critical time points during the embryonic development or normal physiology. Consistent with the activation of the AHR under precise temporal and spatial parameters, this model would favor the existence of a specific endogenous agonist that via ligand binding, signals to influence cellular events and upregulates its own metabolism through CYP1 gene products so that receptor action is not prolonged.
In developing models to explain AHR biology, it is important to consider the possible existence of an endogenous activator that is not a classical ligand (i.e., unlike PAHs or TCDD). This concept is important because presently, we cannot rule out the possibility of endogenous AHR signaling occurring in the absence of a small molecule ligand. Under such a system of ligand-independent signaling, a protein or other macromolecule that catalytically modifies the receptor or influences its shape or localization could activate the receptor signals. For example, an upstream event that leads to the phosphorylation of the receptor on key residues could transform the receptor and enhance its affinity for ARNT and its DRE-regulated targets (69).
6. KNOWN LIGANDS OF THE AHR
A search for endogenous ligands of the AHR can benefit from a prediction of what ligands of the AHR typically “look like”. To this end, we can rely in part on known structure-activity relationships (SAR) for classes of agonists. By relating the binding affinities of known agonists with their structural properties, the physicochemical parameters for a ligand of a given class can be used to predict the likelihood that a similar structure can bind the AHR. Most compounds known to bind and activate the AHR are hydrophobic molecules that commonly fall under two structural classes, PAHs or halogenated aromatic hydrocarbons (HAHs) (70). While many AHR agonists are planar compounds, the SAR analysis of polychlorinated biphenyls revealed that absolute planarity is not a requirement for receptor binding; however, coplanarity does influence a ligand’s steric fit of the receptor (71). This idea is consistent with findings from a comparative molecular field analysis of ~100 analogues of halogenated –dibenzo-pdioxin, -dibenzofurans, -naphthalenes, -biphenyls and the non-halogenated derivatives of indolo[3,2-b]carbazole. Using this data set, it was estimated that an AHR ligand would be between 12.0 Å -14.0 Å in length, less than 12 Å in width and no more than 5.0 Å deep (72). For halogenated aromatic ligands, increased receptor affinity and activation are controlled in part by the polarizability of the substituent groups (71, 73, 74). A number of recent studies that applied regression analyses to similar sets of test compounds identified electronegativity, hydrophobic and hydrogen-bonding as properties that could also contribute to receptor interaction (75, 76).
7. XENOBIOTIC LIGANDS
7.1. Halogenated-dioxins and related compounds
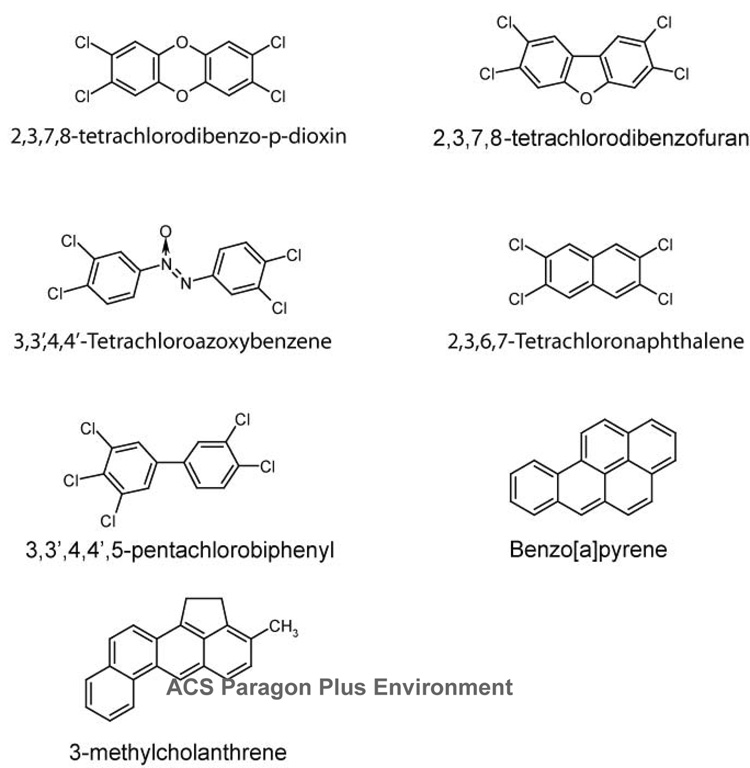
The halogenated-dibenzo-p-dioxins, -dibenzofurans, -azo(xy)benzenes and -naphthalenes comprise a family of important, structurally related AHR agonists (Fig. 2). In general, these chemicals enter the environment through contamination of commercial products, as the result of industrial accident or as products of waste incineration. When these compounds are extensively halogenated, they are metabolically and environmentally stable. When these compounds are halogenated at lateral positions of the coplanar rings, they are often found to be potent agonists of the AHR. Although these compounds can vary significantly in their binding affinity for the receptor, certain compounds, such as TCDD are among the most potent AHR agonists known (Fig. 2). In C57BL/6J mice, TCDD induces hepatic monooxygenase activity with an ED50 of 1 × 10−9 mol/kg. This potency is about 1000-fold greater than that of PAHs such as 3-methylcholanthrene or benzanthracene (11, 77).
Figure 2.
Chlorinated members of this class of agonists elicit a characteristic pattern of toxicity that is mediated via their binding to the AHR. These toxic responses include epithelial hyperplasia, tumor promotion, teratogenesis, thymic involution and death. In the mouse and rat, the oral LD50 of TCDD occurs at doses of 114 µg/kg and 22–45 µg/kg, respectively (78–81). A rich body of pharmacological and genetic evidence supports a role for the AHR in the toxicity of halogenated-dibenzo-p-dioxins, -dibenzofurans and related compounds (77, 82–86). This evidence includes the observation that sensitivity to TCDD toxicity segregates with the Ahrb and Ahrd loci (83, 87–89). In addition, the toxic potencies of halogenated dibenzo-p-dioxin and dibenzofuran congeners correspond to their relative binding affinities to the AHR (90, 91). Furthermore, Ahr null mice are not susceptible to the toxic activities of TCDD (86).
7.2. Polychlorinated biphenyls
There are 209 isomers and congeners of polychlorinated biphenyls (PCBs) (92), many of which are biologically inactive (93). Because of their chemical stability, PCBs are constituents of numerous commercial products, including insulators, flame retardants and adhesives (94). Consequently, the environmental pervasiveness of this chemical family and their trace contamination of wildlife and humans have generated concerns about their health risks. A pattern of toxicity reminiscent of TCDD poisoning has also been observed in animals and humans who were accidentally exposed to high doses of select PCBs (95).
Comparative SAR studies have determined that the presence of halogens on lateral positions of the benzene rings contribute to the potency of PCBs for activating the AHR (70), where maximal activity is achieved when halogens exist at both para (4 and 4’) and two or more meta positions (3, 3’, 6, and 6’) (92). The degree of substitution at the biphenyl bridge also influences the potency for AHR induction by the biphenyls. Because the phenyl rings can rotate about the linking bond, the presence of halogens at the ortho positions (e.g., 2 and 2’) is believed to minimize coplanarity and thus reduce their binding to the AHR (70). The affinity of PCBs for binding the AHR, in turn, correlates with their relative potency for activating the AHR (96).
Among the most potent PCB congeners, 3,3’,4,4’,5’-PentaCB (Fig. 1) is about 100 times less potent than TCDD for inducing AHR-regulated enzyme activity in the Wistar rat. In this species, the observed EC50 value of 3,3’,4,4’,5’-PentaCB is 0.50 µmol/kg and that of TCDD is 0.004 µmol/kg (97). In rat hepatoma H4IIE cells, where ligand metabolism might be less rapid than in the whole organism (97), the potency for CYP1A1 induction by 3,3’,4,4’,5’-PentaCB (EC50 = 2.4 × 10−10 M) is only ~4-fold lower than that of TCDD (EC50 = 7.2 × 10−11 M) (98). Using this cell system, 3,3’,4,4’,5,5’-HexaCB (EC50 = 6.0 × 10−8 M) ; 3,3’,4,4’-TetraCB (3.5 × 10−8 M) and 2,3,3’,4,4’-PentaCB (EC50 = 8.8 × 10−8 M) were shown to be 100 to 1000-fold less active. Displaying EC50 values that were 4–5 orders of magnitude greater than that of TCDD, the mono-ortho analogs are comparable to the polycyclic aromatic hydrocarbon, 3-methylcholanthrene, in their ability to induce CYP1A1 activity in this experimental system (98).
7.3. Polycyclic aromatic hydrocarbons
Polycyclic aromatic hydrocarbons represent a large class of AHR agonists that commonly contain four or more conjugated benzene rings (99). As products of combustion processes that can be found in chimney soot, charbroiled foods and smoke exhaust, PAHs have been studied extensively for their induction of AHR-mediated enzymes (8). Typified by the compounds benzo[a]pyrene and 3-methylcholanthrene (Fig. 2), PAHs induce AHR signaling with a potency that is lower than that of TCDD by 3-4 orders of magnitude (100). As substrates of the monooxygenases and conjugating enzymes they induce, these agonists normally stimulate their own metabolism. The enzyme-mediated conjugation of O-, N- and S-containing moieties to the aromatic structures enhances the polarity and elimination of PAHs and their derivatives. However, the same enzymatic activities can also biotransform select PAHs to carcinogens. A well studied example is the reactive diol epoxide intermediates of benzo[a]pyrene that can covalently bind macromolecules to form potentially genotoxic DNA and protein adducts (8). Therefore, while PAHs elicit the metabolic response of the AHR, select compounds can be metabolically activated to toxicants.
8. ENDOGENOUS LIGANDS
In the previous section, we reviewed AHR signaling induced by compounds that come primarily from anthropogenic sources. That is, PAHs, halogenated-dioxins, -biphenyls and -dibenzofurans are not commonly synthesized by living organisms. Although PAHs may have been generated through forest fires and other natural combustion processes, significant exposure to the compounds described above is probably a relatively new event from the standpoint of evolutionary pressure on AHR structure-function. That is, it is difficult to think of these pollutants as having provided the evolutionary pressure to explain the emergence and selection of the AHR signaling in vertebrate systems. Based on this idea, we argue that our search for insights into the natural ligand of the AHR must be extended to additional structural classes. Of particular interest are those that are known to be endogenously synthesized in higher organisms. To this end, we provide a brief review of those candidate endogenous AHR ligands that have been isolated from mammalian tissues.
8.1. Indigoids
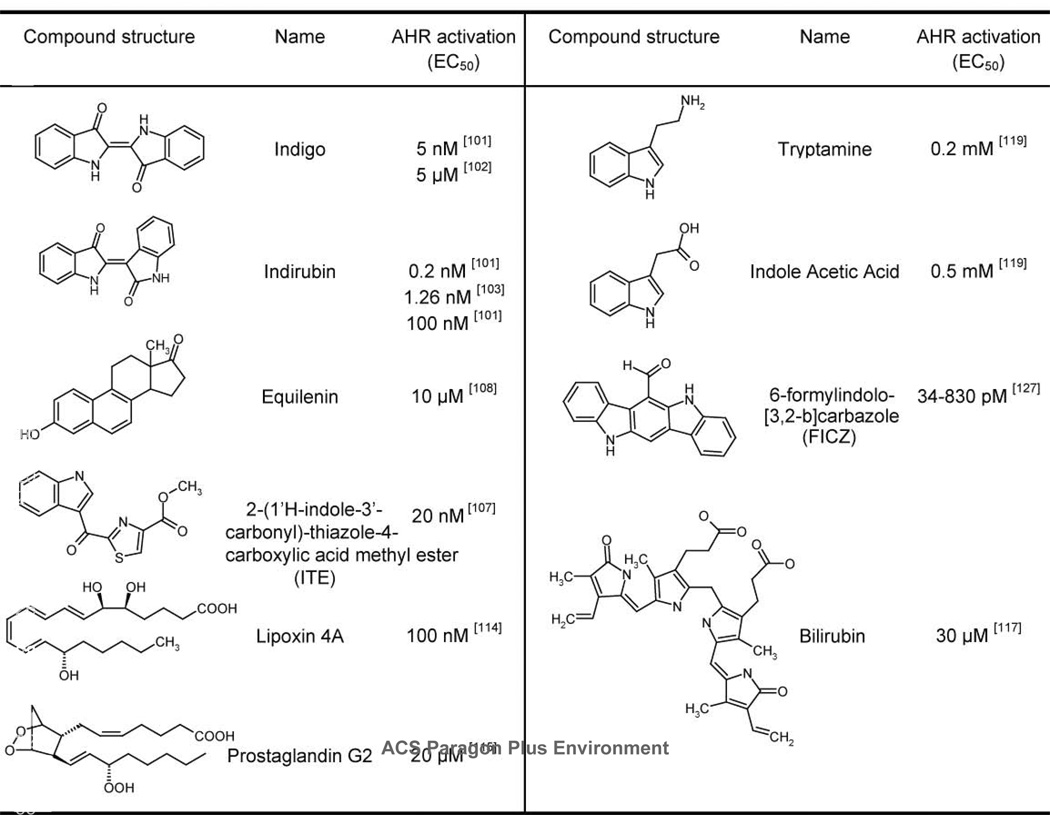
Indigo and indirubin have been suggested to be endogenous AHR agonists (Fig. 3) (101). Indirubin, the more potent AHR agonist of the two, has an EC50 for AHR activation of 0.2 nM when measured in a yeast reporter system, where the EC50 value derived from TCDD treatment was 9 nM (101). However, the dose response curves generated by treating mammalian cells estimate the potency of indirubin to be within 100-fold lower than that of TCDD (102). Due to the CYP1A-mediated metabolism of this compound in mammalian systems (102, 103), the length of ligand exposure and cell type could contribute to an observed range of potency for receptor activation. The EC50 values of indirubin-induced DRE-driven reporter is 100 nM when human hepatoma cells were treated for 24 hours (102), and 1.26 nM for induction of CYP1A-mediated ethoxyresorufin O-deethylase (EROD) activity when MCF-7 cells were treated for 4 hours (103). Indigo and indirubin also appear to be specific AHR agonists, as they compete for receptor occupancy with TCDD and upregulate CYP1A1 monooxygenase activity, but do not appear to stimulate other xenobiotic sensor systems in rodent models (102, 104).
Figure 3.
Historically recognized as plant compounds traditionally used for textile coloring, indigo and indirubin have been isolated from human urine and bovine serum (101). While synthesis of these indigoids in higher organisms has not been shown, the enzymatic conversion of indole to the indigo precursor, 3-hydroxyindole has been demonstrated (105). Arguing against their role as an endogenous ligand for the AHR is the observation these indigoids are present in fairly low levels in vertebrate tissues. The average levels of indigo and indirubin in human urine are in the picomolar range, well below the nanomolar concentrations required to significantly upregulate classic DRE-driven responses in mammalian cell systems (101, 102). Nevertheless, their candidacy as physiologically relevant ligands cannot be disregarded, since their local concentrations may be higher at certain sites, which could allow these compounds to reach physiologically active levels in vivo. A better understanding of the spatial and temporal distribution of these compounds, as well as additional searches for more active isomers is necessary before we can make firm conclusions regarding the influence of these structures on endogenous AHR signaling.
8.2. 2-(1’H-indole-3’-carbonyl)-thiazole-4-carboxylic acid methyl ester (ITE)
An agonist of the AHR was isolated from porcine lung tissue and identified as 2-(1’H-indole-3’-carbonyl)-thiazole-4-carboxylic acid methyl ester or ITE (Fig. 3). Three experiments support the conclusion that ITE is an AHR agonist. First, the purified compound was shown to compete with [3H]TCDD for binding to the human, murine, killifish and zebrafish AHR (106). Second, saturation binding isotherms indicated a high affinity interaction between ITE and the AHR from Hepa cell cytosol (i.e., KD of 6.5 nM) (107). Finally, ITE exposure transformed the AHR to a DRE-binding conformation, augmented CYP1A1 protein expression and induced DRE-dependent luciferase activity in a concentration and time-dependent manner (106, 107). Based on dose-response curves generated in murine hepatoma cells (107), ITE has an estimated EC50 value of ~20 nM, or a potency that is approximately 100-fold lower than that of TCDD. The administration of ITE to transgenic DRE-lacZ pregnant dams showed activation of the AHR in fetal tissues, indicating an in vivo bioactivity. Furthermore, the compound does not appear to be cytotoxic, as reflected by the absence of cleft palate in exposed embryos, a characteristic defect induced by the gestational exposure to TCDD.
The potency that ITE displayed for activating the AHR, coupled with its isolation from tissue has led to the suggestion that ITE is a physiologically relevant endogenous ligand of the AHR. Although this idea is intriguing, it requires further validation. One issue that should be clarified is whether ITE actually exists in mammalian tissues. In this regard, the method of isolating ITE from lung tissue employed high temperature and acidic conditions. This raises the concern that ITE may be a byproduct of the isolation process. A demonstration of the route of biological synthesis or direct measurement of ITE in biological samples, without such reactive isolation conditions, would provide an important contribution to the body of evidence suggesting a physiological role for ITE.
8.3. Equilenin [3-hydroxy-1,3,5(10),6,8-estrapentaen-17-one]
Equilenin is an equine estrogen and a constituent of the widely prescribed hormone replacement drug, Premarin™ (Fig. 3). Produced by pregnant mares and excreted in equine urine, equilenin has been shown to be an AHR agonist. A potentially weak ligand, equilenin is capable of binding the murine AHR with an estimated affinity of about 1/30,000 that of benzo[a]pyrene (108). In human HepG2 cells, 30 µM of this compound produced a 15-fold increase in CYP1A1 mRNA and approximately 5-fold induction of DRE-mediated reporter activity. Based on the dose responses produced under these systems, the EC50 of equilenin for AHR activation is estimated to be ~10 µM.
Interestingly, equilenin was also reported to produce similar levels of enzyme induction in the C57BL/6J and DBA/2J mice, strains that harbor the Ahrb and Ahrd alleles, respectively. This observation is particularly interesting because the Ahrd allele encodes a murine form of the AHR that binds exogenous ligands, such as TCDD, with a ten-fold lower affinity than receptors encoded by the Ahrb allele (16, 109). The ability of equilenin to induce similar levels of EROD activity in the DBA and C57BL6 mice is an interesting aspect of this compound that is rarely noted about other AHR agonists. However, the weak binding of this compound to the AHR leaves room for speculation about the mechanism by which equilenin activates the receptor. Does the compound interact directly with AHR to induce the apparent similar response by the two forms of the receptor?
8.4. Arachidonic acid metabolites
Arachidonic acid and its related structures have long been considered potential endogenous ligands of the AHR. A number of associations between these pathways provide hints of such a relationship. For example, many cytochrome P450-dependent monooxygenases have metabolic activity toward arachidonic acid and its metabolites (110, 111). In addition, the exposure of cultured hepatocytes to TCDD induces the expression of COX2, providing a potential link between AHR activation and prostaglandin synthesis (112). Less direct arguments include the observation that changes in cell shape, as induced by shear stress, have been shown to activate AHR signaling (see below), a phenomenon that is also associated with prostaglandin release (113).
The above arguments led to an AHR agonist screen of endogenous lipophilic substances, with a focus on arachidonic metabolites. From this screen, lipoxin 4A, a metabolite of arachidonic acid, was shown to be an AHR agonist (114). The activation of the AHR by lipoxin 4A has been demonstrated by measuring multiple endpoints, including competitive binding with TCDD for receptor occupancy, induction of a DRE-driven reporter assay, and stimulation of CYP1A1 and CYP1A2 mediated enzyme activity (114). Not only does lipoxin 4A induce the CYP1A monooxygenases, but it also appears to be a substrate of these enzymes (114). This observation is consistent of a model where this compound or related structures are regulating their own metabolism, in a manner similar to xenobiotics such as the PAHs. Interestingly, lipoxin 4A differs structurally from classical AHR agonists, as it contains no rings and bears a negative charge at physiological pH. With an EC50 of 100 nM for induction of a DRE-driven reporter in mouse hepatoma cells, it has been suggested that activation of the AHR by the nanomolar level of physiological lipoxin 4A could occur (114). The biological role of this compound would benefit from future studies showing that lipoxin 4A-induced Ah-receptor activation regulates the developmental features that have been attributed to AHR signaling.
In light of a potential relationship between the AHR and arachidonic acid metabolism, a screen employing both a cell-based reporter assay and a gel retardation assay was conducted to investigate the ability of prostaglandins to activate the AHR (115). Among the 25 compounds tested, six prostaglandins (Prostaglandin B2, D2, F3α, G2, H1 and H2) induced receptor activity. Dose-response assays conducted in a murine hepatoma cell line indicated that the selected prostaglandins are rather weak inducers, with activity detected at concentrations of 10 µM or higher. The most active molecule among these, prostaglandin G2 (Fig. 3), is active at 1µM and can induce DRE-dependent transcription with an estimated EC50 value of 20 µM. By comparison, lipoxin A4 has an EC50 in the nM range. Interestingly, the reporter response induced by 100 µM of prostaglandin G2 exceeded that generated from 1 nM TCDD. Competitive binding analysis of prostaglandin G2 indicates that it may be a weak ligand of the AHR, represented by a 27% displacement of [3H]TCDD binding when prostaglandin G2 was co-incubated at a 20,000-fold excess (115). Based on the low biological potency of these prostaglandins and lipoxins as AHR agonists, it seems unlikely that these particular structures are biologically relevant. However, related structures having higher affinities and potencies that are in range with their biological levels may be important candidates with physiological roles in AHR biology.
8.5. Heme metabolites
Metabolites of heme are also intriguing candidates as endogenous AHR ligands. Studies of congenitally jaundiced rats link an elevated level of bilirubin with increased CYP1A1 activity (116). The biosynthesis of bilirubin begins with the rate-limiting conversion of heme to biliverdin, which in turn, is metabolized to bilirubin. Given that CYP1A1 upregulation is a hallmark of AHR activation, these heme derivatives were tested for their regulation of Cyp1a1 gene and induction of the AHR (117, 118). The treatment of mouse hepatoma cells with micro molar concentrations of biliverdin, bilirubin, or hemin (the Fe3+ oxidation product of heme) led to the time and dose-dependent expression of Cyp1a1 mRNA, EROD induction and DRE-driven reporter activity (117). The absences of these activities in cell lines that are deficient in AHR or ARNT expression indicate that these compounds stimulate classical AHR signal transduction.
It has been suggested that bilirubin is the most significant AHR agonist derived from heme metabolism (117). In this regard, in vitro gel shift assays performed with cytosols from mouse hepatoma cells and guinea pig hepatic tissue showed AHR transformation by bilirubin, but not biliverdin and hemin. These results, coupled with the rank order potency of bilirubin > biliverdin > hemin for CYP1A1 induction, suggest that hemin and biliverdin are precursors, while bilirubin is the ultimate activator of the AHR (117). Importantly, the relative significance of bilirubin is up for some debate, as an independent study has indicated that both bilirubin and biliverdin are active inducers in vivo and in vitro (118). These latter studies also provided evidence that both bilirubin and biliverdin can compete with [3H]TCDD for receptor occupancy, indicating that they both may be true ligands of the AHR.
There are arguments for and against bilirubin and related heme metabolites as endogenous ligands of the AHR. Arguing against is the observation that heme mainly exists bound to serum albumin. Therefore, while the normal level of total heme in human plasma is 5-20 µM, a relatively small fraction is accessible to other proteins or to intracellular signaling machinery. However, under pathological conditions where the bilirubin-conjugating enzyme is impaired, the level of free bilirubin may be high enough to activate the AHR. In this regard, patients of the Crigler-Najjar syndrome have plasma levels of bilirubin that reach 400–800 µM, a range that is ~10 times the estimated EC50 of 30 µM for AHR activation in mouse hepatoma cells (117).
8.6. Tryptophan metabolites
The aromaticity of tryptophan has led to the speculation that this amino acid or its metabolites may be endogenous agonists of the AHR. Independent groups have reported AHR induction by tryptamine (TA) and indole acetic acid (IAA). Tryptamine and IAA can stimulate AHR-DRE binding in vitro with an EC50 in the 0.2 –0.5 mM range (119). By comparison, the EC50 of TCDD is about one million times lower (i.e., approximately 0.1 nM). Tryptamine and IAA both appear to weakly bind the AHR, as they compete with [3H]TCDD for AHR occupancy when used at a 200,000-fold excess (119). At the tested concentration of 2 mM, both compounds activated DRE-regulated luciferase reporter in a murine hepatoma cell line. Although IAA was inactive, TA can inhibit enzymatic activity of rat hepatic microsomes, indicating metabolism by CYP1A1 and CYP1A2. In a recombinant yeast system, AA and TA were also demonstrated to activate the AHR (120).
The reported physiological level of tryptophan ranges from 70 to 150 µM (119), while plasma level of IAA is approximately 2–3 nM. Given their weak potency as inducers of CYP1A1 activity in cell culture, it is unlikely that IAA and TA can affect AHR signaling under physiological conditions. However, under certain pathological states, tryptophan metabolites may become relevant to AHR signaling. In this regard, it has been reported that in the presence of inhibitors of monoamine oxygenase, the tissue level of TA was as high as 700 µM, suggesting that certain physiological conditions can promote AHR activation by tryptophan derivatives.
8.7. Ultraviolet photoproducts of tryptophan
Ultraviolet (UV) irradiation of tryptophan generates compounds that display high affinity for the AHR and potency as inducers of CYP1 monooxygenases (121, 122). Chromatographic and structural analyses of the two most active tryptophan photoproducts led to the identification of 6-formylindolo[3,2-b]carbazole (FICZ) (Fig. 3) and 6,12-diformylindolo[3,2-b]carbazole (dFICZ) (123). In competitive binding assays, FICZ and dFICZ displaced TCDD with KD values of 0.07 nM and 0.44 nM respectively, suggesting an affinity for the AHR that is in the range of that observed for TCDD (e.g., KD of 0.48 nM in these experiments) (121). Due to its metabolism by the upregulated CYP1A1 and CYP1B1 enzymes, the AHR activity induced by FICZ is transient, peaking at 3 hours (124–126). Dose-response curves produced from exposing MH1C1 rat hepatoma cells to FICZ yield an EC50 of 34 pM, compared to 830 pM when cells were incubated for 24 hours (127).
A compelling body of data provides indirect evidence that the synthesis of FICZ could occur in vivo. Among these, the exposure of human skin to ultraviolet-B was shown to induce CYP1A1 and CYP1B1 mRNAs and proteins in the epidermis and dermis (128). In addition, UV-irradiation of rats and hairless mice increased hepatic CYP1A-dependent monooxygenase activity (129–131). A recent report also showed that FICZ could be formed from the prolonged exposure of tryptophan to sunlight (132). Based on some of these and other findings, AHR has been suggested to play a role in mediating the cellular response to oxidative stress caused by UV light, and that FICZ would be the endogenous agonist that induces the AHR-mediated biological response (133). Although the photooxidation of tryptophan can produce FICZ, it remains to be shown that UV exposure leads to the formation of this compound in vivo and that cellular levels of tryptophan can support this biosynthesis.
9. DIETARY COMPOUNDS
If we are looking for physiologically relevant ligand(s) that might relate to the phenotypes seen in Ahr null mice, the diet is an obvious potential source. If they are physiologically relevant, we could think of these dietary ligands as toxicants that, when inefficiently cleared, lead to patent ductus venosus. Alternatively, they could also be likened to an unknown vitamin that is required for normal vascular development. If Ahr null phenotypes such as patent DV are the result of an inefficiently metabolized toxicant, it seems most likely that chemistry or fermentative processes within the gastro-intestinal tract should generate such a toxicant. This conclusion is based upon the observation that animals raised on purified diets have never been reported to harbor the phenotypes of Ahr null mice. If the molecule is more like a vitamin, the same argument holds; it must be present in purified diets or it must be generated from simple constituents in vivo. Below is a short review of those dietary compounds that have been found to activate the AHR and the arguments before and against their role as the physiologically relevant activator of this receptor.
9.1. Indole-3-carbinol derivatives
Found in vegetables such as broccoli and Brussels sprouts, indole-3-carbinol (I3C) has been studied for its potential anticancer properties (Fig. 4). Many studies have reported the therapeutic potential of the IC3 derivatives in cancer treatments based on their ability to modify the metabolism of chemical carcinogens, inhibit the growth tumors or induce apoptosis of cancer cells (134–137). However, it is currently unclear how much of their activity may be attributed to their induction of the AHR (138).
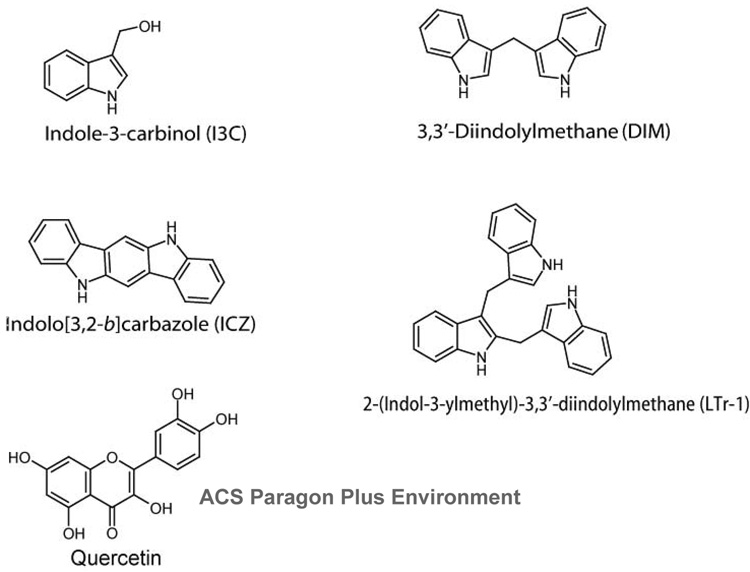
Figure 4.
Indole-3-carbinol is a secondary metabolite of glucobrassicin, a tryptophan-derived glucosinolate found in Cruciferous plants. While dietary I3C has been shown to upregulate classical AHR regulated monooxygenases (139), its structure does not conform to many of the models generated for AHR ligands. The observation that an oral--but not intraperitoneal--administration of I3C increased hepatic monooxygenase activity led to the conclusion that I3C is converted to higher order AHR agonists in the acidic environment of the stomach (140). Chemical analysis of the I3C acid condensation products revealed that acidic conditions generated numerous di- and trimeric condensation derivatives (141). Among the I3C derivates displaying the greatest AHR potency are indolo[3,2-b]carbazole (ICZ), 3,3’-diindolylmethane (DIM) and 2-(indol-3-ylmethyl)-3,3’-diindolylmethane (LTr-1) (Fig. 4) (141). The discovery of these derivatives from the stomach and intestinal contents of rats dosed orally with I3C confirmed the synthesis of these compounds in vivo (141).
Competitive binding analysis of I3C metabolites with [125I]2-iodo-7,8-dibromodibenzo-p-dioxin indicates that the AHR binds ICZ with a surprisingly high affinity. The KD value for ICZ is 1.9 × 10−10 and that of TCDD is 7.1 × 10−12 M (141). When Hepa1c1c7 cells were incubated with ICZ for four hours, ICZ induced Cyp1a1 mRNA expression with a potency that reflects its binding affinity, approximately two orders of magnitude lower than that of TCDD (142). However, when the treatment time was lengthened to 48 hours, ICZ is ~4 orders of magnitude less potent than TCDD at inducing EROD activity, reflected by the EC50 of 36 pM for TCDD and 260 nM for ICZ (141). Unlike TCDD, ICZ appears to be metabolically unstable and readily converted to inactive metabolites. In this regard, an ICZ sample that was preincubated in rat hepatic microsomes produced a time-dependent decrease in the HPLC-detected peak. In contrast, no loss of the compound occurred when the control sample was incubated in buffer or heat-inactivated microsomes (142). Therefore, while ICZ appears to be a potent agonist that is generated in vivo, it is readily metabolized to compounds without AHR activity. It was estimated that as much as 1-5 nmol ICZ could be generated from the consumption of a 100g portion of Brussels sprouts.
Another acid-condensation product of I3C, LTr-1, has also been reported to bind the AHR and induce receptor activity (143). The LTr-1 can also stimulate DRE-binding and monooxygenase activity, but more weakly than comparable doses of ICZ. DIM, another major isolate of the acid condensation products of I3C, also binds to the receptor and activates the AHR, but with a lower potency than ICZ. (144–146).
9.2. Natural Flavonoids
Ubiquitously found in fruits and vegetables, naturally occurring flavonoids encompass the most abundant class of plant polyphenols (147). Because plant foods are rich in flavonoids, it is estimated that their dietary intake can be as high as 1g per day (148). Numerous reports have suggested links of flavonoid consumption with health benefits (or risks) owing to their antioxidant, antiproliferative, estrogenic or antiestrogenic properties (147). Interest in their effect on AHR signaling thus stems from the investigation of phytocompounds as dietary modulators of chemical carcinogenesis. Of the six major subgroups of flavonoids, some flavones, flavonols and isoflavones have been identified as activators of the AHR.
In a comparative analysis involving mammalian cell reporter systems, the flavonoids chrysin, galangin, baicalein, genistein, daidzein and apigenin were reported to activate the AHR (149). Despite their demonstration of AHR activity, it remains to be shown whether these natural flavonoids can bind the receptor or whether they are metabolized to agonists. Another natural flavonoid, quercetin (Fig. 4), also activated the AHR, as evidenced by multiple measures, including its induction of EROD activity and the de novo induction of CYP1A1 mRNA transcription in a time and dose-dependent fashion in MCF-7 cells (150). By means of semi-quantitative RT-PCR analysis, an approximately 22-fold increase of CYP1A1 expression was detected when cells were treated with a 10 µM dose of quercetin. Furthermore, quercetin was demonstrated to compete with TCDD for receptor binding. While these flavonoids are relatively weak inducers, their prevalence in plant foods warrants consideration of their effect on AHR signaling and xenobiotic metabolism in general. Furthermore, the activation of the AHR by naturally occurring flavonoids can be interpreted to mean that the AHR may have a role in detoxifying and/or metabolizing compounds from dietary sources.
10. NONLIGAND ACTIVATORS
There are compelling reasons to consider the possibility that the endogenous activator of the AHR does not bind the receptor as xenobiotic ligands do. As noted above, mouse strains harboring Ahrb or Ahrd alleles differ in their binding affinities for the same xenobiotic ligands by an order of magnitude. Yet, mice harboring the low affinity binding allele (Ahrd) do not display developmental defects reminiscent of the null allele (i.e., patent ductus venosus). By comparison, mouse models of the testicular feminization mutation, an amino acid substitution on the androgen receptor that reduces testosterone binding affinity by nearly ten-fold, exhibit external female sexual characteristics while being genetically male (151–153). This suggests that either the binding pocket of the Ahrd and Ahrb alleles recognize the endogenous ligand with the same affinity or developmental activation of the receptor occurs through a distinct mechanism, one that is independent of the ligand binding pocket (i.e., via post-translational modification or protein-protein interaction).
While the AHR is commonly known as a ligand-induced transcription factor, numerous reports have also documented the activation of the AHR in the absence of addition of small molecule ligands. In this regard, it has been observed that culturing techniques can alter AHR regulated transcriptional activity in cells. Among the earliest examples of this relationship is the observation that suspension of human keratinocytes and murine hepatoma cells with methylcellulose enhances CYP1A1 transcription in an AHR- and ARNT-dependent manner (154–156). More recently, it has been observed that the disruption of cell-cell contact can activate DRE-driven reporter activity in fibroblast cell culture and that sparsely seeded cells are more likely than confluent cells to display nuclear localization of the AHR and drive the transcription of DRE-driven reporter genes (157, 158). The relationship between the AHR and cell shape is also supported by the demonstration that the absence of AHR expression in a mutant mouse hepatoma cell line leads to spindle-shaped cell morphology (159).
The observation that the second messenger cAMP modulates AHR signaling also indicates a mechanism of receptor activation that is ligand independent. In this regard, the exposure of murine hepatoma cells with a membrane permeable cAMP derivative or the cAMP inducer, forskolin, was shown to induce nuclear localization and transformation of the AHR to bind DNA (160).
A recent effort to explore the role of the AHR in vascular development has led to the finding that low-density lipoprotein (LDL) can activate the AHR (161). This conclusion was derived from the initial observation that exposure of sera to hydrodynamic shear stress can stimulate CYP1A1 mRNA expression and DRE-dependent transcription of reporter genes in human and rodent hepatoma cells (161–164). Following a series of fractionation steps, sheared LDL was identified as the AHR inducing agent. The modification of LDL by sodium hypochlorite, an oxidizing reagent, also induced AHR activity. Interestingly, the sheared sera of AHR null, but not heterozygous, mice induced DRE-mediated transcriptional activity. The observations from this study thus indicate LDL may be a physiologically relevant inducer of the AHR and that this transcription factor may play a role in mediating the metabolism of LDL (161).
Collectively, these reports provide ample evidence of xenobiotic-free AHR activation. Furthermore, these results suggest a role of the AHR in responding to cell-cell contact or cell shape. Although no xenobiotic ligand was added to the cell cultures in the highlighted studies, one cannot exclude the possibility that the loss of cell-cell contact or the suspension and shearing of cells can mobilize an endogenous agonist.
11. PROAGONISTS, WEAK AGONISTS, OR NON-SPECIFIC LIGANDS
A number of AHR-inducing compounds that differ from the classical description of an agonist have also been described. Below, we will review examples of small molecules that may activate the receptor through a mechanism that lies outside their binding to the same pocket that recognizes TCDD and other xenobiotics. For more extensive discussions on these non-classic AHR agonists, the reader is referred to earlier literature reviews (65, 66). Because of their structural deviation from classic agonists or their lack of receptor binding, multiple proposals have been raised regarding their mechanism of receptor induction. While their mode of action remains to be clarified, analysis of these compounds may provide insights into the endogenous signaling of the AHR.
11.1. Benzimidazoles
Unlike most xenobiotic ligands of the AHR, benzimidazoles do not exhibit extended planarity and do not bind the AHR with high affinity. Recognition of this new group of AHR activators began with the early studies of omeprazole (Fig. 5), a proton pump inhibitor whose pharmaceutical formulations are widely used in the treatment of gastric ulcers or dyspepsia (165). Other benzimidazoles that have been demonstrated to activate the AHR include lansoprazole, and the compounds albendazole, mebendazole, thiabendazole, fenbendazole and oxfendazole that are used as antihelminthics.
Figure 5.
Because of their broad clinical and veterinary applications, extensive research efforts have been made to examine their secondary effects, including their action on xenobiotic metabolizing enzymes and the influence on the metabolic half-life of drugs taken concurrently. Evidence that benzimidazoles activate the AHR is provided by numerous studies that demonstrate their potency as inducers of DRE-driven reporter systems, CYP1A1 and CYP1A2 gene expression or related monooxygenase activity (166–171).
Two interesting aspects of the benzimidazoles appear to distinguish them from other known AHR agonists. First, the benzimidazole-stimulated activity appears to species-specific. A comparative study showed that omeprazole, thiabendazole and lansoprazole were unable to activate the AHR in the mouse cell line, Hepa1c1c7, while they induce receptor activity in the human HepG2 cells (172, 173). Second, in contrast to the classic AHR agonists, competitive binding analyses of oxfendazole, omeprazole, thiabendazole or Lansoprazole revealed that these benzimidazoles were incapable of displacing [3H]TCDD from the AHR in vitro (166, 171, 174–176). As an exception to this trend, a recent study found that by preincubating thiabendazole with guinea pig cytosol, competitive displacement of [3H]TCDD can occur (177). In general, the benzimidazoles possess weak to moderate AHR activation potency, as significant receptor response was achieved by the treatment of cultured cells with micro molar range concentrations of the compounds.
These features of the benzimidazoles’ activity form the basis of two suggested hypotheses to explain the mechanisms by which they activate the AHR. One proposal is that the omeprazole-induced AHR signaling is ligand-dependent, but stimulated by a metabolic derivative of omeprazole that can bind and activate the receptor (178). A second proposal is that this class of compound induces AHR activity via a ligand-independent mechanism. Support of this view comes from the demonstration by independent labs that the protein kinase inhibitors daidzein, herbimycin A and a series of tyrphostins decreased CYP1A1 expression induced by omeprazole, but not TCDD or B[a]P (69, 176, 179). In addition, the small molecule antagonist, PD98059, which binds the AHR, did not effect receptor induction by omeprazole (180). It was therefore suggested that omeprazole activates the AHR indirectly, possibly by inducing a signaling cascade that involves the phosphorylation of the AHR (69, 179).
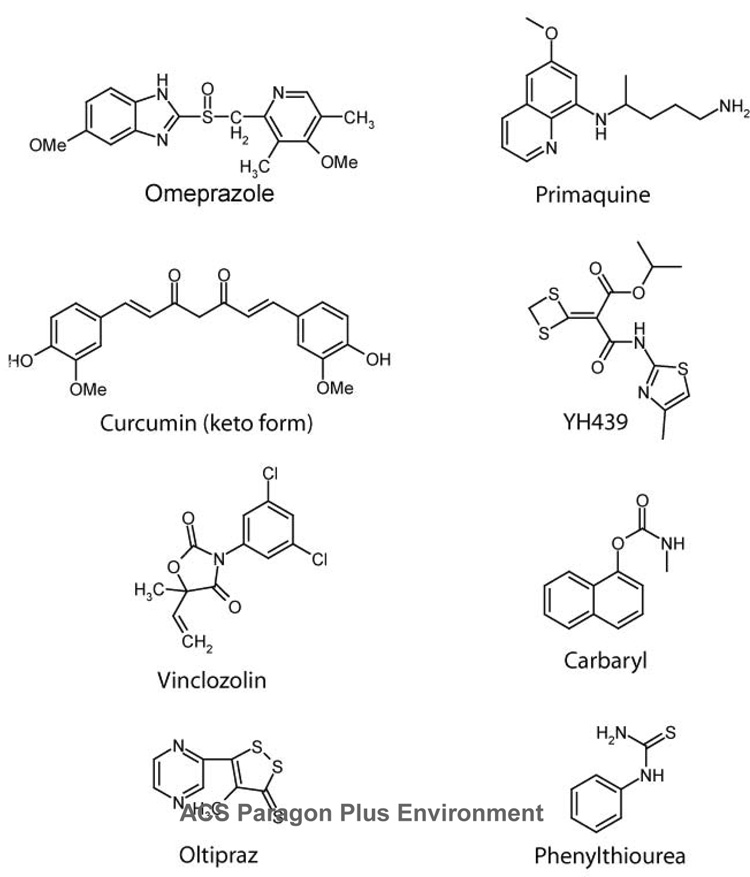
11.2. Unusual structures
A number of compounds that bear little structural resemblance to classic agonists have also been reported to induce the AHR. As shown in Fig. 4, compounds such as primaquine, vinclozolin, YH439, phenylthiourea, curcumin and Oltipraz lack the conjugated aromatic rings of the PAHs (181–186). In general, these compounds are weak inducers of the AHR that require doses in the mid to high micromolar range to elicit receptor activity.
Regarding their specific induction of the AHR, little is known or conflicting results are reported about the ability of these non-aromatic compounds to bind the receptor. For example, opposing conclusions have been drawn about the commonly prescribed anti-malarial medication, primaquine. While one study indicated that this molecule did not compete with [3H]TCDD for receptor binding (181), by reducing the concentration of radioligand, another group has observed competitive binding of the AHR by primaquine (187). Altered conditions of competitive binding assay might also explain the different findings on receptor binding by Carbaryl, the widely used carbamate insecticide. Contrasting the suggestion that this compound induces CYP1A1 gene transcription without direct receptor interaction (188–190), a 40,000-fold excess of Carbaryl (191), or the preincubation of hepatic cytosol with Carbaryl prior to the addition of [3H]TCDD (177) did lead to competitive displacement of the radioligand.
Taken in sum, the inconsistent competitive binding data and relatively weak potency for AHR activation by the benzimidazoles and the compounds reviewed in this section imply that they might be proagonists, weak ligands or non-specific AHR agonists. Support of this latter conclusion comes from the observation that vinclozolin, in addition to inducing CYP1A1, also elevated increased the expression of CYP3A and CYP4A (185), target enzymes of the pregnane X receptor and the peroxisome proliferator-activated receptor, respectively.
12. CONCLUSION
Advances of recent years have affirmed a role for the aryl hydrocarbon receptor as an important regulator of normal development. Future understanding of the receptor’s physiological function will ultimately be linked to identification of the AHR’s endogenous activator. While the identity of the physiologically relevant endogenous agonist remains elusive, the search has yielded numerous candidates. Although these candidates are often of biological interest, none has yet been proven to be an agonist with relevance to the regulatory mechanism underlying the receptor’s endogenous function. Future efforts to elucidate this compound should address these following points: 1) activation of the AHR at biologically relevant concentrations, 2) its similar potency for activation of the polymorphic murine receptors and 3) the ability of the compound to stimulate the developmental closure of the ductus venosus.
Abbreviations
- AHR
aryl hydrocarbon receptor
- PAHs
polycyclic aromatic hydrocarbons
- TCDD
2,3,7,8-tetrachlorodibenzo-p-dioxin
- bHLH
basic-helix-loop-helix
- PAS
PER-ARNT-SIM
- ARNT
aryl hydrocarbon receptor nuclear translocator
- DREs
dioxin responsive elements
- AHRR
aryl hydrocarbon receptor repressor
- DV
ductus venosus
- SAR
structure-activity relationship
- HAHs
halogenated aromatic hydrocarbons
- PCBs
polychlorinated biphenyls
- EROD
ethoxyresorufin O-deethylase
- ITE
2-(1’H-indole-3’-carbonyl)-thiazole-4-carboxylic acid methyl ester
- TA
tryptamine
- IAA
indole acetic acid
- FICZ
6-formylindolo[3,2-b]carbazole
- dFICZ
6,12-diformylindolo[3,2-b]carbazole
- I3C
indole-3-carbinol
- ICZ
indolo[3,2-b]carbazole
- DIM
3,3’-diindolylmethane
- LTr-1
2-(indol-3-ylmethyl)-3,3’-diindolylmethane
- LDL
low-density lipoprotein
REFERENCES
- 1.Mimura J, Fujii-Kuriyama Y. Functional role of AhR in the expression of toxic effects by TCDD. Biochim Biophys Acta. 2003;1619:263–268. doi: 10.1016/s0304-4165(02)00485-3. [DOI] [PubMed] [Google Scholar]
- 2.Wilson CL, Safe S. Mechanisms of ligand-induced aryl hydrocarbon receptor-mediated biochemical and toxic responses [see comments] Toxicologic Pathology. 1998;26:657–671. doi: 10.1177/019262339802600510. [DOI] [PubMed] [Google Scholar]
- 3.Nebert DW, Puga A, Vasiliou V. Role of the Ah receptor and the dioxin-inducible [Ah] gene battery in toxicity, cancer, and signal transduction. Ann N Y Acad Sci. 1993;685:624–640. doi: 10.1111/j.1749-6632.1993.tb35928.x. [DOI] [PubMed] [Google Scholar]
- 4.Schlede E, Kuntzman R, Haber S, Conney AH. Effect of enzyme induction on the metabolism and tissue distribution of benzo(alpha)pyrene. Cancer Res. 1970;30:2893–2897. [PubMed] [Google Scholar]
- 5.Levin W, Conney AH. Stimulatory effect of polycyclic hydrocarbons and aromatic azo derivatives on the metabolism of 7,12-dimethylbenz(alpha)anthracene. Cancer Res. 1967;27:1931–1938. [PubMed] [Google Scholar]
- 6.Conney AH, Davison C, Gastel R, Burns JJ. Adaptive increases in drug-metabolizing enzymes induced by phenobarbital and other drugs. J Pharmacol Exp Ther. 1960;130:1–8. [PubMed] [Google Scholar]
- 7.Conney AH, Miller EC, Miller JA. Substrate-induced synthesis and other properties of benzpyrene hydroxylase in rat liver. J Biol Chem. 1957;228:753–766. [PubMed] [Google Scholar]
- 8.Conney AH. Induction of microsomal enzymes by foreign chemicals and carcinogenesis by polycyclic aromatic hydrocarbons: G. H. A. Clowes Memorial Lecture. Cancer Res. 1982;42:4875–4917. [PubMed] [Google Scholar]
- 9.Shimada T. Xenobiotic-metabolizing enzymes involved in activation and detoxification of carcinogenic polycyclic aromatic hydrocarbons. Drug Metab Pharmacokinet. 2006;21:257–276. doi: 10.2133/dmpk.21.257. [DOI] [PubMed] [Google Scholar]
- 10.Schrenk D. Impact of dioxin-type induction of drug-metabolizing enzymes on the metabolism of endo-and xenobiotics. Biochem Pharmacol. 1998;55:1155–1162. doi: 10.1016/s0006-2952(97)00591-1. [DOI] [PubMed] [Google Scholar]
- 11.Niwa A, Kumaki K, Nebert DW, Poland AP. Genetic expression of aryl hydrocarbon hydroxylase activity in the mouse. Distinction between the "responsive" homozygote and heterozygote at the Ah locus. Arch Biochem Biophys. 1975;166:559–564. doi: 10.1016/0003-9861(75)90420-8. [DOI] [PubMed] [Google Scholar]
- 12.Poland AP, Glover E, Robinson JR, Nebert DW. Genetic expression of aryl hydrocarbon hydroxylase activity. Induction of monooxygenase activities and cytochrome P1-450 formation by 2,3,7,8-tetrachlorodibenzo-p-dioxin in mice genetically "nonresponsive" to other aromatic hydrocarbons. Journal of Biological Chemistry. 1974;249:5599–5606. [PubMed] [Google Scholar]
- 13.Poland A, Glover E, Taylor BA. The murine Ah locus: a new allele and mapping to chromosome 12. Molecular Pharmacology. 1987;32:471–478. [PubMed] [Google Scholar]
- 14.Poland A, Glover E, Kende AS. Stereospecific, high affinity binding of 2,3,7,8-tetrachlorodibenzo-p-dioxin by hepatic cytosol. Evidence that the binding species is receptor for induction of aryl hydrocarbon hydroxylase. Journal of Biological Chemistry. 1976;251:4936–4946. [PubMed] [Google Scholar]
- 15.Poland A, Glover E, Kende AS. Stereospecific, high affinity binding of 2,3,7,8-tetrachlorodibenzo-p-dioxin by hepatic cytosol. J. Biol. Chem. 1976;251:4936–4946. [PubMed] [Google Scholar]
- 16.Poland A, Palen D, Glover E. Analysis of the four alleles of the murine aryl hydrocarbon receptor. Mol. Pharmacol. 1994;46:915–921. [PubMed] [Google Scholar]
- 17.Perdew GH. Association of the Ah receptor with the 90-kDa heat shock protein. J. Biol. Chem. 1988;263:13802–13805. [PubMed] [Google Scholar]
- 18.Wilhelmsson A, Cuthill S, Denis M, Wikstrom AC, Gustafsson JA, Poellinger L. The specific DNA binding activity of the dioxin receptor is modulated by the 90 kd heat shock protein. EMBO J. 1990;9:69–76. doi: 10.1002/j.1460-2075.1990.tb08081.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Carver LA, Bradfield CA. Ligand-dependent interaction of the aryl hydrocarbon receptor with a novel immunophilin homolog in vivo. Journal of Biological Chemistry. 1997;272:11452–11456. doi: 10.1074/jbc.272.17.11452. [DOI] [PubMed] [Google Scholar]
- 20.Meyer BK, Pray-Grant MG, Vanden Heuvel JP, Perdew GH. Hepatitis B virus X-associated protein 2 is a subunit of the unliganded aryl hydrocarbon receptor core complex and exhibits transcriptional enhancer activity. Molecular & Cellular Biology. 1998;18:978–988. doi: 10.1128/mcb.18.2.978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ma Q, Whitlock JP., Jr A novel cytoplasmic protein that interacts with the Ah receptor, contains tetratricopeptide repeat motifs, and augments the transcriptional response to 2,3,7,8-tetrachlorodibenzo-p-dioxin. J. Biol. Chem. 1997;272:8878–8884. [PubMed] [Google Scholar]
- 22.Kazlauskas A, Poellinger L, Pongratz I. Evidence that the co-chaperone p23 regulates ligand responsiveness of the dioxin (Aryl hydrocarbon) receptor. Journal of Biological Chemistry. 1999;274:13519–13524. doi: 10.1074/jbc.274.19.13519. [DOI] [PubMed] [Google Scholar]
- 23.Fukunaga BN, Probst MR, Reisz-Porszasz S, Hankinson O. Identification of functional domains of the aryl hydrocarbon receptor. Journal of Biological Chemistry. 1995;270:29270–29278. doi: 10.1074/jbc.270.49.29270. [DOI] [PubMed] [Google Scholar]
- 24.Ema M, Sogawa K, Watanabe N, Chujoh Y, Matsushita N, Gotoh O, Funae Y, Fujii-Kuriyama Y. cDNA cloning and structure of mouse putative Ah receptor. Biochem. Biophys. Res. Comm. 1992;184:246–253. doi: 10.1016/0006-291x(92)91185-s. [DOI] [PubMed] [Google Scholar]
- 25.Burbach KM, Poland A, Bradfield CA. Cloning of the Ah receptor cDNA reveals a distinctive ligand-activated transcription factor. Proc. Natl. Acad. Sci. USA. 1992;89:8185–8189. doi: 10.1073/pnas.89.17.8185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gu Y-Z, Hogenesch J, Bradfield C. Annu. Rev. Pharmacol. Toxicol. Academic Press; 2000. The PAS superfamily: Sensors of environmental and developmental signals; pp. 519–561. [DOI] [PubMed] [Google Scholar]
- 27.Crews ST, Fan CM. Remembrance of things PAS: regulation of development by bHLH-PAS proteins. Current Opinion in Genetics & Development. 1999;9:580–587. doi: 10.1016/s0959-437x(99)00003-9. [DOI] [PubMed] [Google Scholar]
- 28.Dolwick KM, Swanson HI, Bradfield CA. In vitro analysis of Ah receptor domains involved in ligand-activated DNA recognition. Proc. Natl. Acad. Sci. USA. 1993;90:8566–8570. doi: 10.1073/pnas.90.18.8566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ikuta T, Eguchi H, Tachibana T, Yoneda Y, Kawajiri K. Nuclear localization and export signals of the human aryl hydrocarbon receptor. J. Biol. Chem. 1998;273:2895–2904. doi: 10.1074/jbc.273.5.2895. [DOI] [PubMed] [Google Scholar]
- 30.Petrulis JR, Kusnadi A, Ramadoss P, Hollingshead B, Perdew GH. The hsp90 Co-chaperone XAP2 alters importin beta recognition of the bipartite nuclear localization signal of the Ah receptor and represses transcriptional activity. J Biol Chem. 2003;278:2677–2685. doi: 10.1074/jbc.M209331200. [DOI] [PubMed] [Google Scholar]
- 31.Reyes H, Reisz-Porszasz S, Hankinson O. Identification of the Ah receptor nuclear translocator protein (Arnt) as a component of the DNA binding form of the Ah receptor. Science. 1992;256:1193–1195. doi: 10.1126/science.256.5060.1193. [DOI] [PubMed] [Google Scholar]
- 32.McLane KE, Whitlock JP., Jr DNA sequence requirements for Ah receptor/Arnt recognition determined by in vitro transcription. Receptor. 1994;4:209–222. [PubMed] [Google Scholar]
- 33.Tukey RH, Hannah RR, Negishi M, Nebert DW, Eisen HJ. The Ah locus: Correlation of intranuclear appearance of inducer-receptor complex with induction of cytochrome P1-450 mRNA. Cell. 1982;31:275–284. doi: 10.1016/0092-8674(82)90427-5. [DOI] [PubMed] [Google Scholar]
- 34.Shen ES, Whitlock JP., Jr Protein-DNA interactions at a dioxin-responsive enhancer. Mutational analysis of the DNA-binding site for the liganded Ah receptor. J Biol Chem. 1992;267:6815–6819. [PubMed] [Google Scholar]
- 35.Lusska A, Shen E, Whitlock JP., Jr Protein-DNA interactions at a dioxin-responsive enhancer. Analysis of six bona fide DNA-binding sites for the liganded Ah receptor. J. Biol. Chem. 1993;268:6575–6580. [PubMed] [Google Scholar]
- 36.Hankinson O. Role of coactivators in transcriptional activation by the aryl hydrocarbon receptor. Arch Biochem Biophys. 2005;433:379–386. doi: 10.1016/j.abb.2004.09.031. [DOI] [PubMed] [Google Scholar]
- 37.Ikuta T, Tachibana T, Watanabe J, Yoshida M, Yoneda Y, Kawajiri K. Nucleocytoplasmic shuttling of the aryl hydrocarbon receptor. J Biochem (Tokyo) 2000;127:503–509. doi: 10.1093/oxfordjournals.jbchem.a022633. [DOI] [PubMed] [Google Scholar]
- 38.Pollenz RS. The aryl-hydrocarbon receptor, but not the aryl-hydrocarbon receptor nuclear translocator protein, is rapidly depleted in hepatic and nonhepatic culture cells exposed to 2,3,7,8-tetrachlorodibenzo-p-dioxin. Mol. Pharmacol. 1996;49 [PubMed] [Google Scholar]
- 39.Roberts BJ, Whitelaw ML. Degradation of the basic helix-loop-helix/Per-ARNT-Sim homology domain dioxin receptor via the ubiquitin/proteasome pathway. Journal of Biological Chemistry. 1999;274:36351–36356. doi: 10.1074/jbc.274.51.36351. [DOI] [PubMed] [Google Scholar]
- 40.Davarinos NA, Pollenz RS. Aryl hydrocarbon receptor imported into the nucleus following ligand binding is rapidly degraded via the cytosplasmic proteasome following nuclear export. Journal of Biological Chemistry. 1999;274:28708–28715. doi: 10.1074/jbc.274.40.28708. [DOI] [PubMed] [Google Scholar]
- 41.Ma Q, Baldwin KT. 2,3,7,8-tetrachlorodibenzo-p-dioxin-induced degradation of aryl hydrocarbon receptor (AhR) by the ubiquitin-proteasome pathway. Role of the transcription activaton and DNA binding of AhR. J Biol Chem. 2000;275:8432–8438. doi: 10.1074/jbc.275.12.8432. [DOI] [PubMed] [Google Scholar]
- 42.Mimura J, Ema M, Sogawa K, Fujii-Kuriyama Y. Identification of a novel mechanism of regulation of Ah (dioxin) receptor function. Genes & Development. 1999;13:20–25. doi: 10.1101/gad.13.1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Walker MK, Heid SE, Smith SM, Swanson HI. Molecular characterization and developmental expression of the aryl hydrocarbon receptor from the chick embryo. Comp Biochem Physiol C Toxicol Pharmacol. 2000;126:305–319. doi: 10.1016/s0742-8413(00)00119-5. [DOI] [PubMed] [Google Scholar]
- 44.Hahn M. Aryl hydrocarbon receptors: diversity and evolution(1) Chem Biol Interact. 2002;141:131. doi: 10.1016/s0009-2797(02)00070-4. [DOI] [PubMed] [Google Scholar]
- 45.Goldstone HM, Stegeman JJ. A revised evolutionary history of the CYP1A subfamily: gene duplication, gene conversion, and positive selection. J Mol Evol. 2006;62:708–717. doi: 10.1007/s00239-005-0134-z. [DOI] [PubMed] [Google Scholar]
- 46.Prasad JC, Goldstone JV, Camacho CJ, Vajda S, Stegeman JJ. Ensemble Modeling of Substrate Binding to Cytochromes P450: Analysis of Catalytic Differences between CYP1A Orthologs. Biochemistry. 2007;46:2640–2654. doi: 10.1021/bi062320m. [DOI] [PubMed] [Google Scholar]
- 47.Emmons RB, Duncan D, Estes PA, Kiefel P, Mosher JT, Sonnenfeld M, Ward MP, Duncan I, Crews ST. The spineless-aristapedia and tango bHLH-PAS proteins interact to control antennal and tarsal development in Drosophila. Development. 1999;126:3937–3945. doi: 10.1242/dev.126.17.3937. [DOI] [PubMed] [Google Scholar]
- 48.Duncan DM, Burgess EA, Duncan I. Control of distal antennal identity and tarsal development in Drosophila by spineless-aristapedia, a homolog of the mammalian dioxin receptor. Genes & Development. 1998;12:1290–1303. doi: 10.1101/gad.12.9.1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wernet MF, Mazzoni EO, Celik A, Duncan DM, Duncan I, Desplan C. Stochastic spineless expression creates the retinal mosaic for colour vision. Nature. 2006;440:174–180. doi: 10.1038/nature04615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Powell-Coffman JA, Bradfield CA, Wood WB. Caenorhabditis elegans orthologs of the aryl hydrocarbon receptor and its heterodimerization partner the aryl hydrocarbon receptor nuclear translocator. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:2844–2849. doi: 10.1073/pnas.95.6.2844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Qin H, Powell-Coffman JA. The Caenorhabditis elegans aryl hydrocarbon receptor, AHR-1, regulates neuronal development. Dev Biol. 2004;270:64–75. doi: 10.1016/j.ydbio.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 52.Butler RA, Kelley ML, Powell WH, Hahn ME, Van Beneden RJ. An aryl hydrocarbon receptor (AHR) homologue from the soft-shell clam, Mya arenaria: evidence that invertebrate AHR homologues lack 2,3,7,8-tetrachlorodibenzo-p-dioxin and beta-naphthoflavone binding. Gene. 2001;278:223–234. doi: 10.1016/s0378-1119(01)00724-7. [DOI] [PubMed] [Google Scholar]
- 53.Dey A, Nebert DW. Markedly increased constitutive CYP1A1 mRNA levels in the fertilized ovum of the mouse. Biochem Biophys Res Commun. 1998;251:657–661. doi: 10.1006/bbrc.1998.9519. [DOI] [PubMed] [Google Scholar]
- 54.Hahn ME, Karchner SI, Evans BR, Franks DG, Merson RR, Lapseritis JM. Unexpected diversity of aryl hydrocarbon receptors in non-mammalian vertebrates: insights from comparative genomics. J Exp Zoolog A Comp Exp Biol. 2006;305:693–706. doi: 10.1002/jez.a.323. [DOI] [PubMed] [Google Scholar]
- 55.Fernandez-Salguero P, Pineau T, Hilbert DM, McPhail T, Lee SS, Kimura S, Nebert DW, Rudikoff S, Ward JM, Gonzalez FJ. Immune system impairment and hepatic fibrosis in mice lacking the dioxin-binding Ah receptor [see comments] Science. 1995;268:722–726. doi: 10.1126/science.7732381. [DOI] [PubMed] [Google Scholar]
- 56.Schmidt JV, Su GH, Reddy JK, Simon MC, Bradfield CA. Characterization of a murine Ahr null allele: involvement of the Ah receptor in hepatic growth and development. Proc Natl Acad Sci U S A. 1996;93:6731–6736. doi: 10.1073/pnas.93.13.6731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lahvis GP, Bradfield CA. Ahr null alleles: distinctive or different? Biochem Pharmacol. 1998;56:781–787. doi: 10.1016/s0006-2952(98)00134-8. [DOI] [PubMed] [Google Scholar]
- 58.Fernandez-Salguero PM, Ward JM, Sundberg JP, Gonzalez FJ. Lesions of aryl-hydrocarbon receptor-deficient mice. Veterinary Pathology. 1997;34:605–614. doi: 10.1177/030098589703400609. [DOI] [PubMed] [Google Scholar]
- 59.Lahvis G, Lindell S, Thomas R, McCuskey R, Murphy C, Glover E, Bentz M, Southard J, Bradfield C. Portosystemic shunts and persistent fetal vascular structures in Ah-receptor deficient mice. Proc Natl Acad Sci U S A. 2000;97:10442–10447. doi: 10.1073/pnas.190256997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Thomae TL, Glover E, Bradfield CA. A maternal Ahr null genotype sensitizes embryos to chemical teratogenesis. J Biol Chem. 2004;279:30189–30194. doi: 10.1074/jbc.M403690200. [DOI] [PubMed] [Google Scholar]
- 61.Walisser JA, Bunger MK, Glover E, Bradfield CA. Gestational exposure of Ahr and Arnt hypomorphs to dioxin rescues vascular development. Proc Natl Acad Sci U S A. 2004;101:16677–16682. doi: 10.1073/pnas.0404379101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Campbell SJ, Henderson CJ, Anthony DC, Davidson D, Clark AJ, Wolf CR. The murine Cyp1a1 gene is expressed in a restricted spatial and temporal pattern during embryonic development. J Biol Chem. 2005;280:5828–5835. doi: 10.1074/jbc.M412899200. [DOI] [PubMed] [Google Scholar]
- 63.Choudhary D, Jansson I, Schenkman JB, Sarfarazi M, Stoilov I. Comparative expression profiling of 40 mouse cytochrome P450 genes in embryonic and adult tissues. Arch Biochem Biophys. 2003;414:91–100. doi: 10.1016/s0003-9861(03)00174-7. [DOI] [PubMed] [Google Scholar]
- 64.Omiecinski CJ, Redlich CA, Costa P. Induction and developmental expression of cytochrome P450IA1 messenger RNA in rat and human tissues: detection by the polymerase chain reaction. Cancer Res. 1990;50:4315–4321. [PubMed] [Google Scholar]
- 65.Denison MS, Nagy SR. Activation of the aryl hydrocarbon receptor by structurally diverse exogenous and endogenous chemicals. Annu Rev Pharmacol Toxicol. 2003;43:309–334. doi: 10.1146/annurev.pharmtox.43.100901.135828. [DOI] [PubMed] [Google Scholar]
- 66.Denison MS, Seidel SD, Rogers RA, Ziccardi M, Winter GM, Health-Pagliuso S. Natural and Synthetic Ligands for the Ah Receptor. In: Puga A, Wallace KB, editors. Molecular Biology of the Toxic Response. Philadelphia: Taylor & Francis; 1999. pp. 393–410. [Google Scholar]
- 67.Aranda A, Pascual A. Nuclear hormone receptors and gene expression. Physiol Rev. 2001;81:1269–1304. doi: 10.1152/physrev.2001.81.3.1269. [DOI] [PubMed] [Google Scholar]
- 68.Ordentlich P, Downes M, Evans RM. Corepressors and nuclear hormone receptor function. Curr Top Microbiol Immunol. 2001;254:101–116. doi: 10.1007/978-3-662-10595-5_5. [DOI] [PubMed] [Google Scholar]
- 69.Backlund M, Johansson I, Mkrtchian S, Ingelman-Sundberg M. Signal transduction-mediated activation of the aryl hydrocarbon receptor in rat hepatoma H4IIE cells. Journal of Biological Chemistry. 1997;272:31755–31763. doi: 10.1074/jbc.272.50.31755. [DOI] [PubMed] [Google Scholar]
- 70.Poland A, Glover E. Chlorinated biphenyl induction of aryl hydrocarbon hydroxylase activity: a study of the structure-activity relationship. Molecular Pharmacology. 1977;13:924–938. [PubMed] [Google Scholar]
- 71.McKinney JD, Singh P. Structure-activity relationships in halogenated biphenyls: unifying hypothesis for structural specificity. Chem Biol Interact. 1981;33:271–283. doi: 10.1016/0009-2797(81)90046-6. [DOI] [PubMed] [Google Scholar]
- 72.Waller CL, McKinney JD. Three-dimensional quantitative structure-activity relationships of dioxins and dioxin-like compounds: model validation and Ah receptor characterization. Chem Res Toxicol. 1995;8:847–858. doi: 10.1021/tx00048a005. [DOI] [PubMed] [Google Scholar]
- 73.Miller G, Sontum S, Crosby DG. Electron-acceptor properties of chlorinated dibenzo-p-dioxins. Bull Environ Contam Toxicol. 1977;18:611–616. doi: 10.1007/BF01684008. [DOI] [PubMed] [Google Scholar]
- 74.Long G, McKinney J, Pedersen L. Polychlorinated Dibenzofuran (PCDF) Binding to the Ah Receptor(s) and Associated Enzyme Induction. Theoretical Model Based on Molecular Parameters. Quant. Struct.-Act. Relat. 1987;6:1–7. [Google Scholar]
- 75.Arulmozhiraja S, Morita M. Structure-activity relationships for the toxicity of polychlorinated dibenzofurans: approach through density functional theory-based descriptors. Chem Res Toxicol. 2004;17:348–356. doi: 10.1021/tx0300380. [DOI] [PubMed] [Google Scholar]
- 76.Ashek A, Lee C, Park H, Cho SJ. 3D QSAR studies of dioxins and dioxin-like compounds using CoMFA and CoMSIA. Chemosphere. 2006;65:521–529. doi: 10.1016/j.chemosphere.2006.01.010. [DOI] [PubMed] [Google Scholar]
- 77.Poland A, Knutson JC. 2,3,7,8-tetrachlorodibenzo-p-dioxin and related halogenated aromatic hydrocarbons: examination of the mechanism of toxicity. Annual Review of Pharmacology & Toxicology. 1982;22:517–554. doi: 10.1146/annurev.pa.22.040182.002505. [DOI] [PubMed] [Google Scholar]
- 78.Higginbotham GR, Huang A, Firestone D, Verrett J, Ress J, Campbell AD. Chemical and toxicological evaluations of isolated and synthetic chloro derivatives of dibenzo-p-dioxin. Nature. 1968;220:702–703. doi: 10.1038/220702a0. [DOI] [PubMed] [Google Scholar]
- 79.Schwetz BA, Norris JM, Sparschu GL, Rowe UK, Gehring PJ, Emerson JL, Gerbig CG. Toxicology of chlorinated dibenzo-p-dioxins. Environ Health Perspect. 1973;5:87–99. doi: 10.1289/ehp.730587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Vos JG, Moore JA, Zinkl JG. Toxicity of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) in C57B1/6 mice. Toxicol Appl Pharmacol. 1974;29:229–241. doi: 10.1016/0041-008x(74)90060-x. [DOI] [PubMed] [Google Scholar]
- 81.McConnell EE, Moore JA, Haseman JK, Harris MW. The comparative toxicity of chlorinated dibenzo-p-dioxins in mice and guinea pigs. Toxicol Appl Pharmacol. 1978;44:335–356. doi: 10.1016/0041-008x(78)90195-3. [DOI] [PubMed] [Google Scholar]
- 82.Bradfield CA, Kende AS, Poland A. Kinetic and equilibrium studies of Ah receptor-ligand binding: use of [125I]2-iodo-7,8-dibromodibenzo-p-dioxin. Molecular Pharmacology. 1988;34:229–237. [PubMed] [Google Scholar]
- 83.Poland A, Glover E. 2,3,7,8,-Tetrachlorodibenzo-p-dioxin: segregation of toxocity with the Ah locus. Molecular Pharmacology. 1980;17:86–94. [PubMed] [Google Scholar]
- 84.Mimura J, Yamashita K, Nakamura K, Morita M, Takagi TN, Nakao K, Ema M, Sogawa K, Yasuda M, Katsuki M, Fujii-Kuriyama Y. Loss of teratogenic response to 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) in mice lacking the Ah (dioxin) receptor. Genes to Cells. 1997;2:645–654. doi: 10.1046/j.1365-2443.1997.1490345.x. [DOI] [PubMed] [Google Scholar]
- 85.Fernandez-Salguero PM, Hilbert DM, Rudikoff S, Ward JM, Gonzalez FJ. Aryl-hydrocarbon receptor-deficient mice are resistant to 2,3,7,8-tetrachlorodibenzo-p-dioxin-induced toxicity. Toxicology & Applied Pharmacology. 1996;140:173–179. doi: 10.1006/taap.1996.0210. [DOI] [PubMed] [Google Scholar]
- 86.Peters JM, Narotsky MG, Elizondo G, Fernandez-Salguero PM, Gonzalez FJ, Abbott BD. Amelioration of TCDD-induced teratogenesis in aryl hydrocarbon receptor (AhR)-null mice. Toxicol. Sci. 1999;47:86–92. doi: 10.1093/toxsci/47.1.86. [DOI] [PubMed] [Google Scholar]
- 87.Hassoun E, d'Argy R, Dencker L, Sundstrom G. Teratological studies on the TCDD congener 3,3',4,4'-tetrachloroazoxybenzene in sensitive and nonsensitive mouse strains: evidence for direct effect on embryonic tissues. Arch Toxicol. 1984;55:20–26. doi: 10.1007/BF00316581. [DOI] [PubMed] [Google Scholar]
- 88.Vecchi A, Sironi M, Canegrati MA, Recchia M, Garattini S. Immunosuppressive effects of 2,3,7,8-tetrachlorodibenzo-p-dioxin in strains of mice with different susceptibility to induction of aryl hydrocarbon hydroxylase. Toxicol Appl Pharmacol. 1983;68:434–441. doi: 10.1016/0041-008x(83)90288-0. [DOI] [PubMed] [Google Scholar]
- 89.Chapman DE, Schiller CM. Dose-related effects of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) in C57BL/6J and DBA/2J mice. Toxicol Appl Pharmacol. 1985;78:147–157. doi: 10.1016/0041-008x(85)90314-x. [DOI] [PubMed] [Google Scholar]
- 90.Kerkvliet NI, Baecher-Steppan L, Smith BB, Youngberg JA, Henderson MC, Buhler DR. Role of the Ah locus in suppression of cytotoxic T lymphocyte activity by halogenated aromatic hydrocarbons (PCBs and TCDD): structure-activity relationships and effects in C57Bl/6 mice congenic at the Ah locus. Fundam Appl Toxicol. 1990;14:532–541. doi: 10.1016/0272-0590(90)90257-k. [DOI] [PubMed] [Google Scholar]
- 91.Mason G, Sawyer T, Keys B, Bandiera S, Romkes M, Piskorska-Pliszczynska J, Zmudzka B, Safe S. Polychlorinated dibenzofurans (PCDFs): correlation between in vivo and in vitro structure-activity relationships. Toxicology. 1985;37:1–12. doi: 10.1016/0300-483x(85)90108-8. [DOI] [PubMed] [Google Scholar]
- 92.Safe S, Bandiera S, Sawyer T, Robertson L, Safe L, Parkinson A, Thomas PE, Ryan DE, Reik LM, Levin W, et al. PCBs: structure-function relationships and mechanism of action. Environ Health Perspect. 1985;60:47–56. doi: 10.1289/ehp.856047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.McFarland VA, Clarke JU. Environmental occurrence, abundance, and potential toxicity of polychlorinated biphenyl congeners: considerations for a congener-specific analysis. Environ Health Perspect. 1989;81:225–239. doi: 10.1289/ehp.8981225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Safe S. Toxicology, structure-function relationship, and human and environmental health impacts of polychlorinated biphenyls: progress and problems. Environ Health Perspect. 1993;100:259–268. doi: 10.1289/ehp.93100259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Safe S. Polychlorinated biphenyls (PCBs): mutagenicity and carcinogenicity. Mutat Res. 1989;220:31–47. doi: 10.1016/0165-1110(89)90007-9. [DOI] [PubMed] [Google Scholar]
- 96.Bandiera S, Safe S, Okey AB. Binding of polychlorinated biphenyls classified as either phenobarbitone-, 3-methylcholanthrene- or mixed-type inducers to cytosolic Ah receptor. Chem Biol Interact. 1982;39:259–277. doi: 10.1016/0009-2797(82)90045-x. [DOI] [PubMed] [Google Scholar]
- 97.Safe S. Polychlorinated biphenyls (PCBs), dibenzo-p-dioxins (PCDDs), and related compounds: environmental and mechanistic considerations which support the development of toxic equivalency factors (TEFs) Critical Reviews In Toxicology. 1990;21:51–88. doi: 10.3109/10408449009089873. [DOI] [PubMed] [Google Scholar]
- 98.Sawyer T, Safe S. PCB isomers and congeners: induction of aryl hydrocarbon hydroxylase and ethoxyresorufin O-deethylase enzyme activities in rat hepatoma cells. Toxicol Lett. 1982;13:87–93. doi: 10.1016/0378-4274(82)90142-4. [DOI] [PubMed] [Google Scholar]
- 99.Barron MG, Heintz R, Rice SD. Relative potency of PAHs and heterocycles as aryl hydrocarbon receptor agonists in fish. Mar Environ Res. 2004;58:95–100. doi: 10.1016/j.marenvres.2004.03.001. [DOI] [PubMed] [Google Scholar]
- 100.Poland A, Glover E. Comparison of 2,3,7,8-tetrachlorodibenzo-p-dioxin, a potent inducer of aryl hydrocarbon hydroxylase, with 3-methylcholanthrene. Molecular Pharmacology. 1974;10:349–359. [PubMed] [Google Scholar]
- 101.Adachi J, Mori Y, Matsui S, Takigami H, Fujino J, Kitagawa H, Miller CA, 3rd, Kato T, Saeki K, Matsuda T. Indirubin and indigo are potent aryl hydrocarbon receptor ligands present in human urine. J Biol Chem. 2001;276:31475–31478. doi: 10.1074/jbc.C100238200. [DOI] [PubMed] [Google Scholar]
- 102.Guengerich FP, Martin MV, McCormick A, Nguyen LP, Glover E, Bradfield CA. Aryl hydrocarbon receptor response to indigoids in vitro and in vivo. Arch Biochem Biophys. 2004;423:309–316. doi: 10.1016/j.abb.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 103.Spink BC, Hussain MM, Katz BH, Eisele L, Spink DC. Transient induction of cytochromes P450 1A1 and 1B1 in MCF-7 human breast cancer cells by indirubin. Biochem Pharmacol. 2003;66:2313–2321. doi: 10.1016/j.bcp.2003.08.019. [DOI] [PubMed] [Google Scholar]
- 104.Sugihara K, Kitamura S, Yamada T, Okayama T, Ohta S, Yamashita K, Yasuda M, Fujii-Kuriyama Y, Saeki K, Matsui S, Matsuda T. Aryl hydrocarbon receptor-mediated induction of microsomal drug-metabolizing enzyme activity by indirubin and indigo. Biochem Biophys Res Commun. 2004;318:571–578. doi: 10.1016/j.bbrc.2004.04.066. [DOI] [PubMed] [Google Scholar]
- 105.Gillam EM, Aguinaldo AM, Notley LM, Kim D, Mundkowski RG, Volkov AA, Arnold FH, Soucek P, DeVoss JJ, Guengerich FP. Formation of indigo by recombinant mammalian cytochrome P450. Biochem Biophys Res Commun. 1999;265:469–472. doi: 10.1006/bbrc.1999.1702. [DOI] [PubMed] [Google Scholar]
- 106.Song J, Clagett-Dame M, Peterson RE, Hahn ME, Westler WM, Sicinski RR, DeLuca HF. A ligand for the aryl hydrocarbon receptor isolated from lung. Proc Natl Acad Sci U S A. 2002;99:14694–14699. doi: 10.1073/pnas.232562899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Henry EC, Bemis JC, Henry O, Kende AS, Gasiewicz TA. A potential endogenous ligand for the aryl hydrocarbon receptor has potent agonist activity in vitro and in vivo. Arch Biochem Biophys. 2006;450:67–77. doi: 10.1016/j.abb.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 108.Jinno A, Maruyama Y, Ishizuka M, Kazusaka A, Nakamura A, Fujita S. Induction of cytochrome P450-1A by the equine estrogen equilenin, a new endogenous aryl hydrocarbon receptor ligand. J Steroid Biochem Mol Biol. 2006;98:48–55. doi: 10.1016/j.jsbmb.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 109.Ema M, Ohe N, Suzuki M, Mimura J, Sogawa K, Ikawa I, Fujii-Kuriyama Y. Dioxin binding activities of polymorphic forms of mouse and human aryl hydrocarbon receptors. J. Biol. Chem. 1994;269:27337–27343. [PubMed] [Google Scholar]
- 110.Kroetz DL, Zeldin DC. Cytochrome P450 pathways of arachidonic acid metabolism. Curr Opin Lipidol. 2002;13:273–283. doi: 10.1097/00041433-200206000-00007. [DOI] [PubMed] [Google Scholar]
- 111.Seeds MC, Bass DA. Regulation and metabolism of arachidonic acid. Clin Rev Allergy Immunol. 1999;17:5–26. doi: 10.1007/BF02737594. [DOI] [PubMed] [Google Scholar]
- 112.Puga A, Hoffer A, Zhou S, Bohm JM, Leikauf GD, Shertzer HG. Sustained increase in intracellular free calcium and activation of cyclooxygenase-2 expression in mouse hepatoma cells treated with dioxin. Biochemical Pharmacology. 1997;54:1287–1296. doi: 10.1016/s0006-2952(97)00417-6. [DOI] [PubMed] [Google Scholar]
- 113.Mufti NA, Shuler ML. Possible role of arachidonic acid in stress-induced cytochrome P450IA1 activity. Biotechnol Prog. 1996;12:847–854. doi: 10.1021/bp960067j. [DOI] [PubMed] [Google Scholar]
- 114.Schaldach CM, Riby J, Bjeldanes LF. Lipoxin A4: a new class of ligand for the Ah receptor. Biochemistry. 1999;38:7594–7600. doi: 10.1021/bi982861e. [DOI] [PubMed] [Google Scholar]
- 115.Seidel SD, Winters GM, Rogers WJ, Ziccardi MH, Li V, Keser B, Denison MS. Activation of the Ah receptor signaling pathway by prostaglandins. J Biochem Mol Toxicol. 2001;15:187–196. doi: 10.1002/jbt.16. [DOI] [PubMed] [Google Scholar]
- 116.Kapitulnik J, Gonzalez FJ. Marked endogenous activation of the CYP1A1 and CYP1A2 genes in the congenitally jaundiced Gunn rat. Mol Pharmacol. 1993;43:722–725. [PubMed] [Google Scholar]
- 117.Sinal CJ, Bend JR. Aryl hydrocarbon receptor-dependent induction of cyp1a1 by bilirubin in mouse hepatoma hepa 1c1c7 cells. Mol Pharmacol. 1997;52:590–599. doi: 10.1124/mol.52.4.590. [DOI] [PubMed] [Google Scholar]
- 118.Phelan D, Winter GM, Rogers WJ, Lam JC, Denison MS. Activation of the Ah receptor signal transduction pathway by bilirubin and biliverdin. Arch Biochem Biophys. 1998;357:155–163. doi: 10.1006/abbi.1998.0814. [DOI] [PubMed] [Google Scholar]
- 119.Heath-Pagliuso S, Rogers WJ, Tullis K, Seidel SD, Cenijn PH, Brouwer A, Denison MS. Activation of the Ah receptor by tryptophan and tryptophan metabolites. Biochemistry. 1998;37:11508–11515. doi: 10.1021/bi980087p. [DOI] [PubMed] [Google Scholar]
- 120.Miller CA., 3rd Expression of the human aryl hydrocarbon receptor complex in yeast. Activation of transcription by indole compounds. J Biol Chem. 1997;272:32824–32829. doi: 10.1074/jbc.272.52.32824. [DOI] [PubMed] [Google Scholar]
- 121.Rannug A, Rannug U, Rosenkranz HS, Winqvist L, Westerholm R, Agurell E, Grafstrom AK. Certain photooxidized derivatives of tryptophan bind with very high affinity to the Ah receptor and are likely to be endogenous signal substances. J Biol Chem. 1987;262:15422–15427. [PubMed] [Google Scholar]
- 122.Helferich WG, Denison MS. Ultraviolet photoproducts of tryptophan can act as dioxin agonists. Mol Pharmacol. 1991;40:674–678. [PubMed] [Google Scholar]
- 123.Rannug U, Rannug A, Sjoberg U, Li H, Westerholm R, Bergman J. Structure elucidation of two tryptophan-derived, high affinity Ah receptor ligands. Chem Biol. 1995;2:841–845. doi: 10.1016/1074-5521(95)90090-x. [DOI] [PubMed] [Google Scholar]
- 124.Bergander L, Wincent E, Rannug A, Foroozesh M, Alworth W, Rannug U. Metabolic fate of the Ah receptor ligand 6-formylindolo[3,2-b]carbazole. Chem Biol Interact. 2004;149:151–164. doi: 10.1016/j.cbi.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 125.Wei YD, Helleberg H, Rannug U, Rannug A. Rapid and transient induction of CYP1A1 gene expression in human cells by the tryptophan photoproduct 6-formylindolo[3,2-b]carbazole. Chem Biol Interact. 1998;110:39–55. doi: 10.1016/s0009-2797(97)00111-7. [DOI] [PubMed] [Google Scholar]
- 126.Wei YD, Bergander L, Rannug U, Rannug A. Regulation of CYP1A1 transcription via the metabolism of the tryptophan- derived 6-formylindolo[3,2-b]carbazole. Arch Biochem Biophys. 2000;383:99–107. doi: 10.1006/abbi.2000.2037. [DOI] [PubMed] [Google Scholar]
- 127.Oberg M, Bergander L, Hakansson H, Rannug U, Rannug A. Identification of the tryptophan photoproduct 6-formylindolo[3,2-b]carbazole, in cell culture medium, as a factor that controls the background aryl hydrocarbon receptor activity. Toxicol Sci. 2005;85:935–943. doi: 10.1093/toxsci/kfi154. [DOI] [PubMed] [Google Scholar]
- 128.Katiyar SK, Matsui MS, Mukhtar H. Ultraviolet-B exposure of human skin induces cytochromes P450 1A1 and 1B1. J Invest Dermatol. 2000;114:328–333. doi: 10.1046/j.1523-1747.2000.00876.x. [DOI] [PubMed] [Google Scholar]
- 129.Goerz G, Merk H, Bolsen K, Tsambaos D, Berger H. Influence of chronic UV-light exposure on hepatic and cutaneous monooxygenases. Experientia. 1983;39:385–386. doi: 10.1007/BF01963137. [DOI] [PubMed] [Google Scholar]
- 130.Mukhtar H, DelTito BJ, Jr, Matgouranis PM, Das M, Asokan P, Bickers DR. Additive effects of ultraviolet B and crude coal tar on cutaneous carcinogen metabolism: possible relevance to the tumorigenicity of the Goeckerman regimen. J Invest Dermatol. 1986;87:348–353. doi: 10.1111/1523-1747.ep12524446. [DOI] [PubMed] [Google Scholar]
- 131.Goerz G, Barnstorf W, Winnekendonk G, Bolsen K, Fritsch C, Kalka K, Tsambaos D. Influence of UVA and UVB irradiation on hepatic and cutaneous P450 isoenzymes. Arch Dermatol Res. 1996;289:46–51. doi: 10.1007/s004030050151. [DOI] [PubMed] [Google Scholar]
- 132.Diani-Moore S, Labitzke E, Brown R, Garvin A, Wong L, Rifkind AB. Sunlight generates multiple tryptophan photoproducts eliciting high efficacy CYP1A induction in chick hepatocytes and in vivo. Toxicol Sci. 2006;90:96–110. doi: 10.1093/toxsci/kfj065. [DOI] [PubMed] [Google Scholar]
- 133.Rannug A, Fritsche E. The aryl hydrocarbon receptor and light. Biol Chem. 2006;387:1149–1157. doi: 10.1515/BC.2006.143. [DOI] [PubMed] [Google Scholar]
- 134.Rogan EG. The natural chemopreventive compound indole-3-carbinol: state of the science. In Vivo. 2006;20:221–228. [PubMed] [Google Scholar]
- 135.Kim YS, Milner JA. Targets for indole-3-carbinol in cancer prevention. J Nutr Biochem. 2005;16:65–73. doi: 10.1016/j.jnutbio.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 136.Aggarwal BB, Ichikawa H. Molecular targets and anticancer potential of indole-3-carbinol and its derivatives. Cell Cycle. 2005;4:1201–1215. doi: 10.4161/cc.4.9.1993. [DOI] [PubMed] [Google Scholar]
- 137.Sarkar FH, Li Y. Indole-3-carbinol and prostate cancer. J Nutr. 2004;134:3493S–3498S. doi: 10.1093/jn/134.12.3493S. [DOI] [PubMed] [Google Scholar]
- 138.Abdelrahim M, Newman K, Vanderlaag K, Samudio I, Safe S. 3,3'-diindolylmethane (DIM) and its derivatives induce apoptosis in pancreatic cancer cells through endoplasmic reticulum stress-dependent upregulation of DR5. Carcinogenesis. 2006;27:717–728. doi: 10.1093/carcin/bgi270. [DOI] [PubMed] [Google Scholar]
- 139.Loub WD, Wattenberg LW, Davis DW. Aryl hydrocarbon hydroxylase induction in rat tissues by naturally occurring indoles of cruciferous plants. J Natl Cancer Inst. 1975;54:985–988. [PubMed] [Google Scholar]
- 140.Bradfield CA, Bjeldanes LF. Structure-activity relationships of dietary indoles: A proposed mechanism of action as modifiers of xenobiotic metabolism. J Toxicol Environ Health. 1987;21:311–323. doi: 10.1080/15287398709531021. [DOI] [PubMed] [Google Scholar]
- 141.Bjeldanes LF, Kim JY, Grose KR, Bartholomew JC, Bradfield CA. Aromatic hydrocarbon responsiveness-receptor agonists generated from indole-3-carbinol in vitro and in vivo: comparisons with 2,3,7,8-tetrachlorodibenzo-p-dioxin. Proc Natl Acad Sci U S A. 1991;88:9543–9547. doi: 10.1073/pnas.88.21.9543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Chen YH, Riby J, Srivastava P, Bartholomew J, Denison M, Bjeldanes L. Regulation of CYP1A1 by indolo[3,2-b]carbazole in murine hepatoma cells. J Biol Chem. 1995;270:22548–22555. doi: 10.1074/jbc.270.38.22548. [DOI] [PubMed] [Google Scholar]
- 143.Chang YC, Riby J, Chang GH, Peng BC, Firestone G, Bjeldanes LF. Cytostatic and antiestrogenic effects of 2-(indol-3-ylmethyl)-3,3'-diindolylmethane, a major in vivo product of dietary indole-3-carbinol. Biochem Pharmacol. 1999;58:825–834. doi: 10.1016/s0006-2952(99)00165-3. [DOI] [PubMed] [Google Scholar]
- 144.Chen I, McDougal A, Wang F, Safe S. Aryl hydrocarbon receptor-mediated antiestrogenic and antitumorigenic activity of diindolylmethane. Carcinogenesis. 1998;19:1631–1639. doi: 10.1093/carcin/19.9.1631. [DOI] [PubMed] [Google Scholar]
- 145.Bjeldanes LF, Kim JY, Grose KR, Bartholemew JC, Bradfield CA. Aromatic hydrocarbon responsiveness-receptor agonists generated from indole-3-carbinol in vitro and in vivo: Comparisons with 2,3,7,8-tetrachlorodibenzo-p-dioxin. Proc. Natl. Acad. Sci. USA. 1991;88:9543–9547. doi: 10.1073/pnas.88.21.9543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Jellinck PH, Forkert PG, Riddick DS, Okey AB, Michnovicz JJ, Bradlow HL. Ah receptor binding properties of indole carbinols and induction of hepatic estradiol hydroxylation. Biochem Pharmacol. 1993;45:1129–1136. doi: 10.1016/0006-2952(93)90258-x. [DOI] [PubMed] [Google Scholar]
- 147.Ross JA, Kasum CM. Dietary flavonoids: bioavailability, metabolic effects, and safety. Annu Rev Nutr. 2002;22:19–34. doi: 10.1146/annurev.nutr.22.111401.144957. [DOI] [PubMed] [Google Scholar]
- 148.Kuhnau J. The flavonoids. A class of semi-essential food components: their role in human nutrition. World Rev Nutr Diet. 1976;24:117–191. [PubMed] [Google Scholar]
- 149.Zhang S, Qin C, Safe SH. Flavonoids as aryl hydrocarbon receptor agonists/antagonists: effects of structure and cell context. Environ Health Perspect. 2003;111:1877–1882. doi: 10.1289/ehp.6322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Ciolino HP, Daschner PJ, Yeh GC. Dietary flavonols quercetin and kaempferol are ligands of the aryl hydrocarbon receptor that affect CYP1A1 transcription differentially. Biochem J. 1999;340(Pt 3):715–722. [PMC free article] [PubMed] [Google Scholar]
- 151.Lyon MF, Hawkes SG. X-linked gene for testicular feminization in the mouse. Nature. 1970;227:1217–1219. doi: 10.1038/2271217a0. [DOI] [PubMed] [Google Scholar]
- 152.Goldstein JL, Wilson JD. Studies on the pathogenesis of the pseudohermaphroditism in the mouse with testicular feminization. J Clin Invest. 1972;51:1647–1658. doi: 10.1172/JCI106966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Gehring U, Tomkins GM. Characterization of a hormone receptor defect in the androgen-insensitivity mutant. Cell. 1974;3:59–64. doi: 10.1016/0092-8674(74)90040-3. [DOI] [PubMed] [Google Scholar]
- 154.Sadek CM, Allen-Hoffmann BL. Cytochrome P450IA1 is rapidly induced in normal human keratinocytes in the absence of xenobiotics. Journal of Biological Chemistry. 1994;269:16067–16074. [PubMed] [Google Scholar]
- 155.Sadek CM, Allen-Hoffmann BL. Suspension-mediated Induction of Hepa 1c1c7 Cypa1a-1 Expression Is Dependent on the Ah Receptor Signal Transduction Pathway. Journal of Biological Chemistry. 1994;269:31505–31509. [PubMed] [Google Scholar]
- 156.Monk SA, Denison MS, Rice RH. Transient expression of CYP1A1 in rat epithelial cells cultured in suspension. Arch Biochem Biophys. 2001;393:154–162. doi: 10.1006/abbi.2001.2475. [DOI] [PubMed] [Google Scholar]
- 157.Cho YC, Zheng W, Jefcoate CR. Disruption of cell-cell contact maximally but transiently activates AhR-mediated transcription in 10T1/2 fibroblasts. Toxicol Appl Pharmacol. 2004;199:220–238. doi: 10.1016/j.taap.2003.12.025. [DOI] [PubMed] [Google Scholar]
- 158.Ikuta T, Kobayashi Y, Kawajiri K. Cell density regulates intracellular localization of aryl hydrocarbon receptor. J Biol Chem. 2004;279:19209–19216. doi: 10.1074/jbc.M310492200. [DOI] [PubMed] [Google Scholar]
- 159.Ma Q, Whitlock JP., Jr The aromatic hydrocarbon receptor modulates the Hepa 1c1c7 cell cycle and differentiated state independently of dioxin. Molecular & Cellular Biology. 1996;16:2144–2150. doi: 10.1128/mcb.16.5.2144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Oesch-Bartlomowicz B, Huelster A, Wiss O, Antoniou-Lipfert P, Dietrich C, Arand M, Weiss C, Bockamp E, Oesch F. Aryl hydrocarbon receptor activation by cAMP vs. dioxin: divergent signaling pathways. Proc Natl Acad Sci U S A. 2005;102:9218–9223. doi: 10.1073/pnas.0503488102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.McMillan BJ, Bradfield CA. The aryl hydrocarbon receptor is activated by modified low-density lipoprotein. Proc Natl Acad Sci U S A. 2007;104:1412–1417. doi: 10.1073/pnas.0607296104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Mufti NA, Shuler ML. Induction of cytochrome P-450IA1 activity in response to sublethal stresses in microcarrier-attached Hep G2 cells. Biotechnol Prog. 1995;11:659–663. doi: 10.1021/bp00036a009. [DOI] [PubMed] [Google Scholar]
- 163.Eskin SG, Turner NA, McIntire LV. Endothelial cell cytochrome P450 1A1 and 1B1: up-regulation by shear stress. Endothelium. 2004;11:1–10. doi: 10.1080/10623320490432434. [DOI] [PubMed] [Google Scholar]
- 164.Mufti NA, Bleckwenn NA, Babish JG, Shuler ML. Possible involvement of the Ah receptor in the induction of cytochrome P-450IA1 under conditions of hydrodynamic shear in microcarrier-attached hepatoma cell lines. Biochem Biophys Res Commun. 1995;208:144–152. doi: 10.1006/bbrc.1995.1316. [DOI] [PubMed] [Google Scholar]
- 165.Diaz D, Fabre I, Daujat M, Saint Aubert B, Bories P, Michel H, Maurel P. Omeprazole is an aryl hydrocarbon-like inducer of human hepatic cytochrome P450. Gastroenterology. 1990;99:737–747. doi: 10.1016/0016-5085(90)90963-2. [DOI] [PubMed] [Google Scholar]
- 166.Aix L, Rey-Grobellet X, Larrieu G, Lesca P, Galtier P. Thiabendazole is an inducer of cytochrome P4501A1 in cultured rabbit hepatocytes. Biochem Biophys Res Commun. 1994;202:1483–1489. doi: 10.1006/bbrc.1994.2098. [DOI] [PubMed] [Google Scholar]
- 167.Quattrochi LC, Tukey RH. Nuclear uptake of the Ah (dioxin) receptor in response to omeprazole: transcriptional activation of the human CYP1A1 gene. Mol Pharmacol. 1993;43:504–508. [PubMed] [Google Scholar]
- 168.Baliharova V, Skalova L, Maas RF, De Vrieze G, Bull S, Fink-Gremmels J. The effects of mebendazole on P4501A activity in rat hepatocytes and HepG2 cells. Comparison with tiabendazole and omeprazole. J Pharm Pharmacol. 2003;55:773–781. doi: 10.1211/002235703765951375. [DOI] [PubMed] [Google Scholar]
- 169.Bapiro TE, Andersson TB, Otter C, Hasler JA, Masimirembwa CM. Cytochrome P450 1A1/2 induction by antiparasitic drugs: dose-dependent increase in ethoxyresorufin O-deethylase activity and mRNA caused by quinine, primaquine and albendazole in HepG2 cells. Eur J Clin Pharmacol. 2002;58:537–542. doi: 10.1007/s00228-002-0512-z. [DOI] [PubMed] [Google Scholar]
- 170.Gleizes C, Eeckhoutte C, Pineau T, Alvinerie M, Galtier P. Inducing effect of oxfendazole on cytochrome P450IA2 in rabbit liver. Consequences on cytochrome P450 dependent monooxygenases. Biochem Pharmacol. 1991;41:1813–1820. doi: 10.1016/0006-2952(91)90119-p. [DOI] [PubMed] [Google Scholar]
- 171.Curi-Pedrosa R, Daujat M, Pichard L, Ourlin JC, Clair P, Gervot L, Lesca P, Domergue J, Joyeux H, Fourtanier G, et al. Omeprazole and lansoprazole are mixed inducers of CYP1A and CYP3A in human hepatocytes in primary culture. J Pharmacol Exp Ther. 1994;269:384–392. [PubMed] [Google Scholar]
- 172.Kikuchi H, Kato H, Mizuno M, Hossain A, Ikawa S, Miyazaki J, Watanabe M. Differences in inducibility of CYP1A1-mRNA by benzimidazole compounds between human and mouse cells: evidences of a human-specific signal transduction pathway for CYP1A1 induction. Arch Biochem Biophys. 1996;334:235–240. doi: 10.1006/abbi.1996.0451. [DOI] [PubMed] [Google Scholar]
- 173.Shih H, Pickwell GV, Guenette DK, Bilir B, Quattrochi LC. Species differences in hepatocyte induction of CYP1A1 and CYP1A2 by omeprazole. Hum Exp Toxicol. 1999;18:95–105. doi: 10.1177/096032719901800206. [DOI] [PubMed] [Google Scholar]
- 174.Lesca P, Peryt B, Larrieu G, Alvinerie M, Galtier P, Daujat M, Maurel P, Hoogenboom L. Evidence for the ligand-independent activation of the AH receptor. Biochem Biophys Res Commun. 1995;209:474–482. doi: 10.1006/bbrc.1995.1526. [DOI] [PubMed] [Google Scholar]
- 175.Daujat M, Peryt B, Lesca P, Fourtanier G, Domergue J, Maurel P. Omeprazole, an inducer of human CYP1A1 and 1A2, is not a ligand for the Ah receptor. Biochem Biophys Res Commun. 1992;188:820–825. doi: 10.1016/0006-291x(92)91130-i. [DOI] [PubMed] [Google Scholar]
- 176.Lemaire G, Delescluse C, Pralavorio M, Ledirac N, Lesca P, Rahmani R. The role of protein tyrosine kinases in CYP1A1 induction by omeprazole and thiabendazole in rat hepatocytes. Life Sci. 2003;74:2265–2278. doi: 10.1016/j.lfs.2003.09.056. [DOI] [PubMed] [Google Scholar]
- 177.Bohonowych JE, Denison MS. Persistent binding of ligands to the aryl hydrocarbon receptor. Toxicol Sci. 2007;98:99–109. doi: 10.1093/toxsci/kfm085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Dzeletovic N, McGuire J, Daujat M, Tholander J, Ema M, Fujii-Kuriyama Y, Bergman J, Maurel P, Poellinger L. Regulation of dioxin receptor function by omeprazole. Journal of Biological Chemistry. 1997;272:12705–12713. doi: 10.1074/jbc.272.19.12705. [DOI] [PubMed] [Google Scholar]
- 179.Kikuchi H, Hossain A, Yoshida H, Kobayashi S. Induction of cytochrome P-450 1A1 by omeprazole in human HepG2 cells is protein tyrosine kinase-dependent and is not inhibited by alpha-naphthoflavone. Arch Biochem Biophys. 1998;358:351–358. doi: 10.1006/abbi.1998.0869. [DOI] [PubMed] [Google Scholar]
- 180.Shibazaki M, Takeuchi T, Ahmed S, Kikuchi H. Suppression by p38 MAP kinase inhibitors (pyridinyl imidazole compounds) of Ah receptor target gene activation by 2,3,7,8-tetrachlorodibenzo-p-dioxin and the possible mechanism. J Biol Chem. 2004;279:3869–3876. doi: 10.1074/jbc.M305880200. [DOI] [PubMed] [Google Scholar]
- 181.Fontaine F, Delescluse C, de Sousa G, Lesca P, Rahmani R. Cytochrome 1A1 induction by primaquine in human hepatocytes and HepG2 cells: absence of binding to the aryl hydrocarbon receptor. Biochem Pharmacol. 1999;57:255–262. doi: 10.1016/s0006-2952(98)00304-9. [DOI] [PubMed] [Google Scholar]
- 182.Werlinder V, Backlund M, Zhukov A, Ingelman-Sundberg M. Transcriptional and post-translational regulation of CYP1A1 by primaquine. J Pharmacol Exp Ther. 2001;297:206–214. [PubMed] [Google Scholar]
- 183.Ciolino HP, Daschner PJ, Wang TT, Yeh GC. Effect of curcumin on the aryl hydrocarbon receptor and cytochrome P450 1A1 in MCF-7 human breast carcinoma cells. Biochem Pharmacol. 1998;56:197–206. doi: 10.1016/s0006-2952(98)00143-9. [DOI] [PubMed] [Google Scholar]
- 184.Dierickx PJ. Cytotoxicity of the dicarboximide fungicides, vinclozolin and iprodione, in rat hepatoma-derived Fa32 cells. Altern Lab Anim. 2004;32:369–373. doi: 10.1177/026119290403200408. [DOI] [PubMed] [Google Scholar]
- 185.Ronis MJ, Ingelman-Sundberg M, Badger TM. Induction, suppression and inhibition of multiple hepatic cytochrome P450 isozymes in the male rat and bobwhite quail (Colinus virginianus) by ergosterol biosynthesis inhibiting fungicides (EBIFs) Biochem Pharmacol. 1994;48:1953–1965. doi: 10.1016/0006-2952(94)90594-0. [DOI] [PubMed] [Google Scholar]
- 186.Wu XJ, Lu WQ, Roos PH, Mersch-Sundermann V. Vinclozolin, a widely used fungizide, enhanced BaP-induced micronucleus formation in human derived hepatoma cells by increasing CYP1A1 expression. Toxicol Lett. 2005;159:83–88. doi: 10.1016/j.toxlet.2005.04.010. [DOI] [PubMed] [Google Scholar]
- 187.Backlund M, Ingelman-Sundberg M. Different structural requirements of the ligand binding domain of the aryl hydrocarbon receptor for high- and low-affinity ligand binding and receptor activation. Mol Pharmacol. 2004;65:416–425. doi: 10.1124/mol.65.2.416. [DOI] [PubMed] [Google Scholar]
- 188.Ledirac N, Delescluse C, de Sousa G, Pralavorio M, Lesca P, Amichot M, Berge JB, Rahmani R. Carbaryl induces CYP1A1 gene expression in HepG2 and HaCaT cells but is not a ligand of the human hepatic Ah receptor. Toxicol Appl Pharmacol. 1997;144:177–182. doi: 10.1006/taap.1997.8120. [DOI] [PubMed] [Google Scholar]
- 189.Sandoz C, Lesca P, Carpy A, Laguionie M, Narbonne J. Effects of carbaryl and naphthalene on rat hepatic CYP1A1/2: Potential binding to Ah receptor and 4S benzo(a)pyrene-binding protein. Int J Mol Med. 1998;2:615–623. doi: 10.3892/ijmm.2.5.615. [DOI] [PubMed] [Google Scholar]
- 190.Sandoz C, Lesca P, Narbonne JF, Carpy A. Molecular characteristics of carbaryl, a CYP1A1 gene inducer. Arch Biochem Biophys. 2000;373:275–280. doi: 10.1006/abbi.1999.1515. [DOI] [PubMed] [Google Scholar]
- 191.Denison MS, Phelan D, Winter GM, Ziccardi MH. Carbaryl, a carbamate insecticide, is a ligand for the hepatic Ah (dioxin) receptor. Toxicol Appl Pharmacol. 1998;152:406–414. doi: 10.1006/taap.1998.9999. [DOI] [PubMed] [Google Scholar]