Abstract
After the implantation of the first pacemakers in the Netherlands, controlling the quality of the implantation procedure and the device became an important issue for cardiologists, technicians and manufacturers. Over the years, the cooperation between the different workers in this field led to the development of new devices and novel features in the pacemakers, ensuring improvement in the quality of life of the pacemaker patient. This article gives an impression of how registration in national and local novel systems contributed to this development. (Neth Heart J 2008;16(Suppl1):S9-S11.)
Keywords: pacemaker, ICD, registration, patient care, information system
In 1976, central registration of all Dutch pacemaker patients was initiated1 in order to:
-
Get an overview of:
patients, grouped according to sex, age, indications, pacing modes, etc.
trends in types of pacemakers and leads
the annual number of implants per clinic and nationwide;
Inform the participating clinics about dubious pacemakers and leads and their recipients;
Exchange information with other European countries;
Increase indirect patient care by furnishing information to clinics about pacemakers implanted elsewhere and to patients about pacemaker centres in other countries.
These objectives led to the introduction of a standardised pacemaker registration card, on which a limited amount of data can be filled in about the patient and his/her pacemaker and lead(s). In 1982, the Netherlands Pacemaker Registry Foundation (Stichting Pacemaker Registratie Nederland, SPRN) was established and the computerised Central Pacemaker Patients Registration (CPPR) was started with the requirements that:
Patient-related data should be quickly accessible in order to update the stored information in a user-friendly way;
The database structure should be flexible because the information per patient can differ substantially and in the future relevant data can change;
The programme should check the data on correctness and plausibility;
Opportunities would be created that permit research, simple as well as more complex statistics and export to other programmes.
Data were reported on a quarterly basis to members of the Dutch Working Group on Cardiac Pacing (DWGCP). In this working group, cardiologists, technicians and manufacturers worked together to improve the medical-technical quality of the pacemakers and quality-of life aspects for the patients. Also the CPPR database was used for scientific research.
In-hospital information systems
Starting in 1987, several information systems to support the pacemaker clinic were developed, either commercially by the pacemaker manufacturers or in-hospital by experts in the field of information technology. Most systems were PC based and used DOS as operating system.
When DOS was succeeded by Windows as platform, all the programmes had to be adjusted. By that time the manufacturers focused again on their core business and asked the SPRN and the DWGCP to investigate the possibilities for setting up a new information system that could be used by all pacemaker clinics in the Netherlands and that is supported by the SPRN. For this purpose GRIT-SPRN was developed using Microsoft Visual Foxpro as developing tool.2
The information stored could be divided in two categories:
-
Patient-related information:
Patient details;
Measurements during implantation as well as follow-up, such as stimulation thresholds, sensing thresholds, battery impedance, etc;
Symptoms, ECG and aetiology before implantation, complaint registration, use of medication, interrogation of the pacemaker, failure;
-
Non-patient-related information:
Medical guidelines, national registration, the pacemaker manual, international failure reports, etc.
Because the nationwide stored information was now already present in local databases, the procedure of duplicating this information on a card and then again typing it into the central database became redundant. A standard file structure was developed which was used to send the centrally needed information by modem to the SPRN where it was checked and imported into the national database. When e-mail became more and more integrated in hospitals, the information was encrypted and then sent electronically to the national registry.
In 2007 about 60 of the 104 hospitals in the Netherlands were using the GRIT-SPRN system in their pacemaker department. The use of the programme varied from only sending the information about the required dataset on the implantation to the national database, to storing of all data about implantation and all follow-up visits, and also retrieving reports, statistics and schedules.
Results
When the SPRN ended its registration activities in 2007, after 25 years, data from 174,405 pacemaker implantations with 204,920 leads had been recorded for 136,342 patients. A validation study in 1997 showed that more then 95% of pacemaker implantations in the Netherlands were correctly recorded in the CPPR.3 Since 1990 the ICD registration was added to the registration, ending in 2007 with a total of 14,131 implantations. Also the database contained information on 1600 pacemakers and ICD models, and 1900 lead models that were implanted in this period.
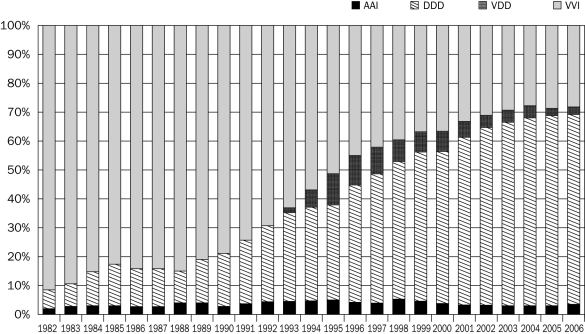
The programming features of the pacemaker changed and more heart rhythm diseases could be treated, changing the implantation mode of the pacemaker from a ‘simple’ VVI (single-chamber) to complex DDD pacemakers and ICDs using two or three leads. Figure 1 shows the change in mode of the pacemakers over this period; for instance, the number of new VDD implantations in 1993 increased rapidly during the first years and decreased when new developments made other options possible.
Figure 1.
Change of mode at implantation in the Netherlands.
In the brochure for pacemaker patients initiated by the Netherlands Heart Foundation, the phone number of the SPRN was mentioned. Patients contacted the foundation about the influence of the use of microwaves, cellular phones, metal detection gates, support abroad when on vacation, information about recall, etc.
Nowadays, most pacemaker departments use their local information system for different purposes. The statistics of the local and the national registration are used to present quantitative data to the hospital management for the purpose of reimbursement, planning of technical equipment and human resources at a time of increasing numbers of device recipients.
The data stored in the information system can be used to show trends in the various pacemaker parameters and of stored data on the pacemaker recipient during the consecutive follow-up visits. This information makes it possible to optimise these parameters to the individual patient and adapt the programming in case of threatening pacing or sensing failures. Also early failure of the pacemaker or lead can be detected.
Reports to cardiologists and general practitioners can be printed immediately after the follow-up visit or sent by e-mail. When using a multi-user programme, such as GRIT-SPRN, the cardiologist is informed on the spot about the functioning of the pacemaker after the patient has been to the pacemaker department.
Each hospital is obliged to keep a record of implantation data on all medical devices, such as data on the patient and date of implantation. The information system within the clinic can be used for this purpose.
All the different manufacturers have their own programming devices to interrogate the pacemaker and to adjust the pacemaker when a change is needed in the settings. Many items are the same for each manufacturer, but not all. Also the output of each programmer is different. A standard protocol used by all pacemaker manufacturers to send data from the programmer to an information system has not yet been realised. The SPRN started an initiative to develop such a protocol with one of the manufacturers but this endeavour was aborted, partly due to the fact that the manufacturers are all international companies.4 A programme was developed by technicians in a local hospital diverting the output not to paper but to a pdf format. These files can be added to the follow-up and in the future recognised and added to the information system.
In 2003, a prospective registration study started to monitor the follow-up of 1500 patients with first pacemaker implantation in 24 implantation centres in the Netherlands using implantation data from the national information system.5 Linking databases in this way meant less administrative work for the participating centres. Of course, more data could be linked and more studies could benefit the data already stored.
Conclusions
In the Netherlands, 10,000 pacemakers and ICDs are implanted yearly, and this number will increase not only because of demographic reasons but also because of expanding indications. Because the device features have become more and more numerous and complex, device technicians and cardiologists are in great need of support in the technical and administrative process. This can be given by linking the hospital (patient and device) information to the information system, as was done in GRIT-SPRN for the patient information.
More uniformity of the device programmers from the different manufacturers and importing these data into an information system can be an important tool to alleviate the task of the professionals. For instance, providing digital information to the hospital about the devices and leads by the manufacturers will decrease the number of errors in serial numbers. Less time on administration leaves more time for attention to the patient.
Because the hospital has to keep track of the implanted devices, their data must always be directly accessible, and therefore these data must be entered into the local database and afterwards can be exported to a national system or used for a device study.
Finally, one should always keep in mind that storage of data supports patient care and can improve the quality of life of pacemaker patients but storage of data must never become a goal in itself.
References
- 1. Dijk WA, Hooijschuur CAM, Dassen WRM. Central Pacemaker Registry in the Netherlands, a 10 year evaluation, Computers in Cardiology, Jerusalem 1989: IEEE Computer Society: 293. [Google Scholar]
- 2. Hooijschuur CAM, Dijk WA. Uniform Pacemaker and ICD Information System in the Netherlands, Conference International Institute of Informatics and Cybernetics I, Atlanta 2003. [Google Scholar]
- 3. Dijk WA, Kingma T, Hooijschuur CAM, et al. Validation of the Netherlands Pacemaker Patient Registry, Computers in Cardiology, Lund Sweden 1997, Volume, Issue, 7-10 Sep: 653-6. [Google Scholar]
- 4. Dijk WA, Hooijschuur CAM, van der Velde W, Dassen WRM. A proposal for a standard communication protocol for pacemaker/ ICD programmers, Computers in Cardiology, 2005 Lyon, Volume, Issue, Sept. 25-28,2005:387-90. [Google Scholar]
- 5. Van Eck JWM, van Hemel, NM, Grobbee, DE, Buskens E, Moons KGM. Followpace study: a prospective study of the cost-effectiveness of routine follow-up visits in patients with a pacemaker, EUROPACE 2006;8:60-4. [DOI] [PubMed] [Google Scholar]