Abstract
The diagnostic programmes of modern pacemakers have increased our knowledge of atrial tachyarrhythmias (ATAs) in chronically paced patients. These programmes also support the evaluation of the effects of pharmacological treatment of ATAs. The success of interruption and/or prevention of ATAs with pacemakers depends strongly on the diagnostic accuracy and the properties of the pacing algorithms, their individual programming and the site and configuration of the pacing leads. Atrial septum pacing can be beneficial in patients with paroxysmal atrial fibrillation and prolonged P wave duration. Recent large-scale studies on preventive and interruptive atrial pacing of ATAs show modestly positive or no results. Therefore, atrial pacing therapy for ATAs should be considered cautiously, serving as an adjuvant to pharmacological treatment rather than as a primary intervention. This also applies for pacing interventions for ATAs in cardiac resynchronisation therapy. The pacemaker algorithms for the detection of ATAs and atrial lead configuration are crucial for the success of pacemaker-mediated prevention or interruption of ATAs. The success of these interventions is dependant on future improvements of pacemaker technology. (Neth Heart J 2008;16(Suppl1):S20-S24.)
Keywords: atrial fibrillation, atrial tachycardia, atrial pacing, pace intervention, pace prevention
In principle, pacemakers (PM) are indicated for the treatment of bradycardia-related symptoms and nowadays also for the resynchronisation of the failing left ventricle. In sick sinus syndrome, atrial tachyarrhythmias (ATAs) frequently emerge. Experience shows that the hybrid approach of standard atrial pacing with AAI(R) or DDD(R) and pharmacological treatment is useful to suppress ATAs in sick sinus syndrome. These results, the growing number of patients with ATAs with different aetiologies and the limited (long-term) success rates of catheter ablative methods of the source of ATAs, recently triggered the technical development of pacing algorithms. These recognise, classify and diagnose ATAs and deliver specific atrial pacing sequences for preventing and/or interrupting ATAs. Recent large-scale studies demonstrate ambiguous results, but all show that additional pharmacological treatment remains indispensable in obtaining benefit. This overview addresses some characteristics of these pacing algorithms, including PM diagnostics and programming as well as discusses the role of the atrial pacing site and configuration of atrial pacing leads.
The contribution of pacemaker diagnostics
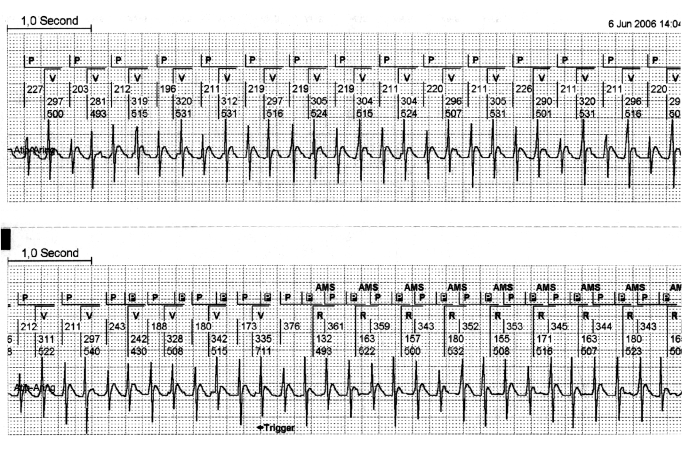
Recent PM diagnostics for ATAs extend our insight towards the variance of the incidence, duration and pattern of ATAs with and without symptoms by permanent monitoring of the intracardiac rhythm. In general, Holter monitoring cannot offer this extensive information because the chest electrodes cannot be tolerated longer than one or two weeks, and no intracardiac information can be obtained.1 With regard to paroxysmal atrial fibrillation, several studies clearly demonstrate that the detection of atrial fibrillation (AF) with modern atrial algorithms is only reliable for the total duration over time of AF but not for the number of episodes of AF.2 The latter are represented to the operator by the number of ‘mode switches’ to the VVI(R) or DDI(R). Dealing with regular atrial tachycardia, the PM diagnostics often fail to establish correctly the duration and number of episodes.2,3 Fully reliable detection of atrial tachycardia is a major target of pacing therapies as these arrhythmias are most likely suitable for interruption by pacing protocols. Their abolition might prevent AF episodes that emerge from degenerating atrial tachycardia (figure 1).
Figure 1 .
Regular atrial tachycardia with a 2:1 lock in. This is a continuous strip from the pacemaker memory. The upper canal of each strip shows the interpretation of the pacemaker of the atrial and ventricular rhythm. The lower canal shows the intracardiac atrial rhythm, derived from the atrial electrode. It is clearly visible that in the beginning of this regular atrial tachycardia, every second atrial depolarisation remains undetected by the pacemaker. This is caused by the programmed postventricular atrial blanking (PVAB). This PVAB (100 msec) was programmed for the prevention of far field R-wave sensing. A slight irregularity of the atrial rhythm in the beginning of the second strip, makes the atrial tachycardia out of the PVAB period and allows the pacemaker to detect all atrial activity. This allows the pacemaker to engage a mode switch to DDI. It is only at this moment that the regular atrial tachycardia can be detected and the window for the implementation of pace interruption is opened. P=sensed atrial activity, P in black box=sensed atrial activity in the refractory period, V=paced ventricular event, R=sensed atrial event. AMS=automatic mode switch.
Failing to detect bouts of atrial tachycardia by a PM results from the postventricular blanking period (PVAB), which is programmed to prevent far field R-wave (FFRW) sensing in the atrial channel. This blanking or ‘closure’ interval of the atrial channel in DDD PM rules out sensing immediately after a ventricular event and can cause the ‘2:1-lock in phenomenon’, where every second atrial depolarisation of the atrial tachycardia is blanked (figure 1).4 This failure to detect every second atrial activation can be avoided by maximal shortening of the blanking interval. The selected time, however, should be counterbalanced towards FFRW sensing in the atrial channel. FFRW sensing, and normal P-wave sensing, will introduce double counting. This double counting has to be avoided as atrial pace intervention protocols can have undesired side effects such as the induction of AF in this situation. An option to prevent FFRW sensing includes the shortened tip-to-ring distance of the atrial electrode.5 Digital signal processing replacing the current analogue signal analysis is also advocated to prevent FFRW sensing but this method is still in its infancy.6 We assume that this novel processing is unnecessary when the FFRW amplitude can be reduced to <0.2 to 0.1 mV by electrode configuration.
Modern pacemakers give the opportunity to diagnose, store and graphically present triggers for AF as atrial premature beats, short-long atrial cycles and patterns of heart rate variability reflecting the condition of the autonomic nervous system. This data can be used in a stepwise diagnostic approach using the PM memory, and to collect information revealing the influence of pharmacological treatments on various AF triggers as well as shifts in the incidence and pattern of ATAs.
Prevention of atrial fibrillation by pacing algorithms
By controlling mechanisms responsible for the initiation of AF, such as atrial ectopic beats, long-short cycles, increased temporal dispersion of conduction and/or refractoriness, atrial overdrive pacing seems to become a promising method to prevent or suppress the induction of persistent or permanent AF by reducing the incidence or duration of paroxysmal atrial fibrillation. Sufficiently short- or long-lasting rapid atrial pacing can eliminate pauses following ectopic beats, and atrial overdrive can suppress atrial ectopy by constant reset, thereby diminishing the degree of dispersion of conduction and/or refractoriness. This approach results in maintenance of control of both rate and rhythm.
It is conceivable that trigger mechanisms for the initiation of AF will differ greatly. A PM study on the mechanisms of onset of AF in 83 patients resulted in different mechanisms of AF onset, even in a single patient.7 Therefore different preventive algorithms are needed to react to different AF triggers: this requires intelligent and flexible software for fully automated pacing interventions. Although current diagnostic programmes are still primitive, they can support the cardiologist in selecting the proper algorithm to switch on, in the prevention of paroxysmal AF or atrial tachy-cardia and can guide the additional pharmacological treatment.
Interruptive pacing to treat atrial fibrillation
The early interruption of bouts of atrial tachycardia to prevent AF is likely to be more successful than late intervention as short periods of a few seconds of regular atrial tachycardia often precede and degenerate into AF. When paroxysmal AF turns to persistent or permanent AF, it is doubtful whether any pacing intervention will be successful in the long run as structural and electro-physiological changes at a cellular level will take place. In addition, the relationship between AF and atrial flutter (AFL) has long been appreciated and patients who initially start with paroxysmal AF commonly also manifest bouts of AFL and vice versa.8 The intervals of atrial tachycardia and AFL constitute the window of opportunity for interruptive pacing strategies. Although burst pacing in AFL is effective in the electrophysiology lab and in some PM patients, the success of fixed burst protocols is limited. As different reentrant circuits can exist in one individual, different pacing protocols are desirable. Some kind of a learning algorithm has to be designed for the termination of different ATAs in a single patient. Although burst pacing may lead to termination of atrial tachycardia, it may also introduce AF, when fine tuning is lacking (figures 2 and 3). Finally, when pacemaker diagnostics are used and/or pace prevention or interruption for ATAs is considered, the operator needs to be fully informed of brand-specific PM properties of these algorithms and protocols.
Figure 2 .
Termination of rapid atrial flutter with overdrive. The tracing is taken during pacemaker follow-up and shows from top to bottom: (1) top channel surface ECG; (2) the marker channel of the pacemaker (AMS=automated mode switch, A=atrial paced, P=atrial sensed event, P in black box=sensed atrial activity in the ventricular refractory period, V=ventricular paced, R=ventricular sensed activity), s=stimulus of the atrial overdrive; (3) the atrial electrogram sensed between the ring and tip of the atrial lead. The figure depicts an example of a successful manually programmed, fast overdrive pacing by an implanted pacemaker in a patient with a regular atrial tachycardia. The burst shows a 1:1 atrial capture overdriving the regular atrial tachycardia. After the burst, the first beat is a spontaneous atrial activity with intrinsic ventricular activity followed by two paced atrial ventricular sequential beats.
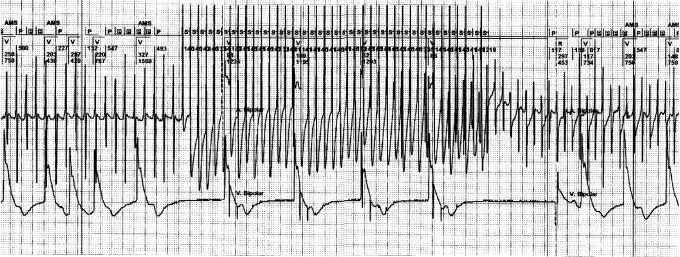
Figure 3 .
Overdrive pacing modifying atrial tachycardia into atrial fibrillation. The tracing shows from top to bottom: (1) the marker channel of the pacemaker (AMS=automatic mode switch, A=atrial paced, P=atrial sensed event, Pin black box=sensed atrial activity in the refractory period, V=ventricular paced, R=ventricular sensed activity), s=stimulus of the atrial overdrive; (2) the atrial electrogram sensed between the ring and tip of the atrial lead; (3) the ventricular electrogram sensed between the ring and tip of the ventricular lead. The tracing depicts an example of an unsuccessful manually programmed, fast overdrive pacing by an implanted pacemaker in a patient with a regular atrial tachycardia. After the burst of the fast overdrive pacing, atrial fibrillation emerges and mode switch (AMS) to DDIpacing arises. In this case AF is introduced by fast overdrive pacing, stressing its potential risk for initiation ofundesired arrhythmias. Note the irregularity of the first paced ventricular beats prior to onset of overdrive pacing. This pattern is caused by the DDI mode where the pacemaker is sensing some of the atrial activity outside the blanking period, thus causing the irregular ventricular response.
Can the most appropriate atrial pacing site in paroysmal AF be selected today?
The right atrial appendage (RAA) is the most commonly used site for atrial lead insertion because this position is easily accessible and shows excellent sensing properties. When pacing and not sensing is the primary objective, several other sites in the right atrium have been proposed for atrial lead placement.9,10 Although more complicated, several techniques make these alternative sites applicable in the human heart.9,11 The principal reason for alternative site pacing in the atrium is the prevention of AF. However, no large-scale trials have reported the results of an alternative atrial pacing site for the electrical treatment of paroxysmal AF.
When a prolonged total atrial activation time contributes to the AF substrate, simultaneous pacing of both atria can support the prevention of ATAs by shortening of this interval.12 Atrial septum pacing promoting a short activation time, either by Bachman bundle pacing or by low atrial septum pacing, has been explored in short-term trials with few patients involved, and looked promising.9,10 However larger trials were unable to confirm the initially positive results.13,14 This difference can be explained by the ‘mixed bag’ of patients included in the larger trials who mostly had a short P-wave duration. Until now, atrial septum pacing could only be recommended in patients with a clearly prolonged P-wave duration (>120 msec), where shortening of the total atrial activation time is a clear component in the prevention of ATAs. It should be stressed that the benefit from this pace prevention can be only obtained when the atrium is paced most, preferably all the time. This can either be achieved by pharmacological drugs that reduce the intrinsic heart rate, and/or an atrial overdrive algorithm.
AF in heart failure patients
In heart failure patients, the elevated atrial wall stress due either to the impaired ventricular function or to considerable mitral or tricuspid valve regurgitation is the principal mechanism for the occurrence of AF. Cardiac resynchronisation therapy (CRT) responders, demonstrated shorter mean AF duration and lower incidence of persistent AF compared with non-responders.15 In heart failure patients with a CRT device, the preservation of sinus rhythm or atrial based pacing is often crucial in optimising haemodynamics. The haemodynamics obtained with CRT is not altered by the atrial pacing site. Only in patients with a prolonged P-wave duration could atrial septum pacing contribute in the prevention of AF, as electrical remodelling can play a role in the initiation or continuation of AF in these patients. Trials designed to evaluate the influence of the atrial pacing site and atrial pacing algorithms in heart failure patients with CRT devices implanted, are ongoing.16
Conclusions
The current PM offers advanced diagnostic tools for the detection and interpretation of various atrial tachyarrhythmias, but the diagnostic power is not yet Clinical application of pacemakers in atrial tachyarrhythmias perfect, particularly in paroxysmal atrial tachycardias. Thus the outcome of these diagnostic and pacing algorithms might be misleading and can promote atrial arrhythmias instead of their suppression. New leads with more appropriate sensing facilities, better signal processing and classification in the atrial channel can improve the diagnostic performance of the new generation of PM. The clinical results of atrial pacing programmes to interrupt or prevent paroxysmal atrial tachyarrhythmias have been equivocal to date. As yet, primary PM implantation for electrical treatment of atrial tachyarrhythmias does not seem justified. When pacemaker diagnostics show opportunities for preventive or interruptive pacing, a stepwise application of the various pacing protocols can be applied in conjunction with antiarrhythmic drugs. Atrial septum overdrive pacing should be restricted to patients with a prolonged atrial depolarisation time.
CRT as such can contribute to AF suppression but the role of AF preventive pacing protocols deserve further studies.
References
- 1.Israel CW, Barold SS. Pacemaker systems as implantable cardiac rhythm monitors. Am J Cardio l2001;88:442-5. [DOI] [PubMed] [Google Scholar]
- 2.de Voogt WG, van Hemel NM, van de Bos AA, Koistinen J, Fast JH. Verification of pacemaker automatic mode switching for the detection of atrial fibrillation and atrial tachycardia with Holter recording. Europace 2006;8:950-61. [DOI] [PubMed] [Google Scholar]
- 3.Israel CW, Barold SS. Failure of atrial flutter detection by a pacemaker with a dedicated atrial flutter detection algorithm. Pacing Clin Electrophysiol 2002;25:1274-7. [DOI] [PubMed] [Google Scholar]
- 4.Goethals M, Timmermans W, Geelen P, Backers J, Brugada P. Mode switching failure during atrial flutter: the ‘2:1 lock-in’ phenomenon. Europace 2003;5:95-102. [DOI] [PubMed] [Google Scholar]
- 5.de Voogt W, van Hemel N, Willems A, Visser J, Chitre Y, Bornzin G, et al. Far-field R-wave reduction with a novel lead design: experimental and human results. Pacing Clin Electrophysiol 2005;28:782-8. [DOI] [PubMed] [Google Scholar]
- 6.van Hemel NM, Wohlgemuth P, Engbers JG, Lawo T, Nebaznivy J, Taborsky M, et al. Form analysis using digital signal processing reliably discriminates far-field R waves from P waves. Pacing Clin Electrophysiol 2004;27:1615-24. [DOI] [PubMed] [Google Scholar]
- 7.Guyomar Y, Thomas O, Marquie C, Jarwe M, Klug D, Kacet S, et al. Mechanisms of onset of atrial fibrillation: a multicenter, prospective, pacemaker-based study. Pacing Clin Electrophysiol 2003;26:1336-41. [DOI] [PubMed] [Google Scholar]
- 8.Waldo AL, Feld GK. Inter-relationships of atrial fibrillation and atrial flutter mechanisms and clinical implications. J Am Coll Cardiol 2008;51:779-86. [DOI] [PubMed] [Google Scholar]
- 9.Bailin SJ, Adler S, Giudici M. Prevention of chronic atrial fibrillation by pacing in the region of Bachmann's bundle: results of a multicenter randomized trial. J Cardiovasc Electrophysiol 2001;12:912-7. [DOI] [PubMed] [Google Scholar]
- 10.Padeletti L, Pieragnoli P, Ciapetti C, Colella A, Musilli N, Porciani MC, et al. Randomized crossover comparison of right atrial appendage pacing versus interatrial septum pacing for prevention of paroxysmal atrial fibrillation in patients with sinus bradycardia. Am Heart J 2001;142:1047-55. [DOI] [PubMed] [Google Scholar]
- 11.de Voogt WG, Van Mechelen R, Van Den BA, Scheffer M, van Hemel NM, Koistinen J. A technique of lead insertion for low atrial septal pacing. Pacing Clin Electrophysiol 2005;28:639-46. [DOI] [PubMed] [Google Scholar]
- 12.Padeletti L, Santini M, Boriani G, Botto G, Ricci R, Spampinato A, et al. Duration of P-wave is associated with atrial fibrillation hospitalizations in patients with atrial fibrillation and paced for bradycardia. Pacing Clin Electrophysiol 2007;30:961-9. [DOI] [PubMed] [Google Scholar]
- 13.Padeletti L, Purerfellner H, Adler SW, Waller TJ, Harvey M, Horvitz L, et al. Combined efficacy of atrial septal lead placement and atrial pacing algorithms for prevention of paroxysmal atrial tachyarrhythmia. J Cardiovasc Electrophysiol 2003;14:1189-95. [DOI] [PubMed] [Google Scholar]
- 14.De Voogt W, Van Hemel N, de Vusser P, Mairesse GH, Van Mechelen R, Koistinen J, et al. No evidence of automatic atrial overdrive pacing efficacy on reduction of paroxysmal atrial fibrillation. Europace 2007;9:798-804. [DOI] [PubMed] [Google Scholar]
- 15.Lellouche N, De Diego C, Vaseghi M, Buch E, Cesario DA, Mahajan A, et al. Cardiac resynchronization therapy response is associated with shorter duration of atrial fibrillation. Pacing Clin Electrophysiol 2007;30:1363-8. [DOI] [PubMed] [Google Scholar]
- 16.Padeletti L, Musilli N, Porciani MC, Colella A, Di Biase L, Ricciardi G, et al. Atrial fibrillation and cardiac resynchronization therapy: the MASCOT study. Europace 2004;5(Suppl 1):S49-S54. [DOI] [PubMed] [Google Scholar]