Abstract
We have developed an assay that quantifies the potential of macrophages to regulate proliferation of endothelial cells. We show that young mice macrophages can be distinguished from old mice macrophages by their ability to inhibit vascular endothelial cell proliferation. While young mice macrophages robustly inhibit proliferation, old mice macrophages fail to do so and actually promote the proliferation of endothelial cells. In this report, we outline a technique that directly assesses the effect of macrophages on modulation of endothelial cell proliferation. This assay will help us in understanding the mechanisms of macrophage function in several disease states characterized by abnormal angiogenesis including cancers, angiogenic eye disease and atherosclerotic heart disease.
Keywords: angiogenesis, endothelial, immunity, innate, macrophage, vascular
Introduction
Angiogenesis and neo-vascularization is subject to various levels of control, including regulation of endothelial cell (EC) proliferation. EC proliferation can be regulated by soluble mediators like VEGF, BDNF and angiopoietin and it can also be suppressed by cytotoxicity (Adamis et al., 1996; Witmer et al., 2003). Cell-mediated cytotoxicity is a major effector pathway of immune protection against intracellular pathogens or tumors and a fundamental mechanism to maintain homeostasis of the immune system. The cytotoxic response is mediated by different cell types and involves several different mechanisms of death induction (Berke, 1991; Russell and Ley, 2002). Cytotoxic cells secrete granules containing various lytic enzymes which then act on the target cells (Shiver et al., 1992). Macrophages play an important role in host immune defense against bacterial infections, tumors and disease states characterized by abnormal angiogenesis (Apte et al., 2006; Blood and Zetter, 1990; Dace and Apte, 2008; Gordon, 1998; Kelly et al., 2007). An important component of inflammatory reactions and subsequent repair and remodeling processes is angiogenesis or neovascularization– the formation of new capillaries from preexisting blood vessels (Folkman and Shing, 1992). Macrophages have been shown to be pro- and anti-angiogenic depending on the type of stimulation given, alternatively activated macrophages are pro-angiogenic and classically activated macrophages act as anti-angiogenic cells (Ruszczak et al., 1990; Rutherford et al., 1993; Schreiber et al., 1986; Sullivan et al., 1983). Endothelial cell proliferation and migration leads to the development of new blood vessels, a process that is important in cancers and angiogenic eye diseases that impair vision. We wanted to develop an assay which would quantitatively determine the direct effect of macrophages on vascular endothelial cell proliferation.
Materials and Methods
Animals
C57BL6 mice were purchased from Jackson Laboratories; they were either used as young mice (<3 months old) or old mice (derived by aging them in the barrier animal facility at Washington University to >18 months of age). Human micro vascular endothelial cells (HMVEC) were purchased from Lonza (Walkersville, MD formerly Cambrex) and cultured in specific media purchased from Lonza. Thymidine was purchased from Amersham (Piscataway, New Jersey). All work was carried out in accordance with Association for Research in Vision and Ophthalmology (ARVO) guidelines for the Use of Animals in Ophthalmic and Vision Research.
Proliferation assay
Effector cell preparation
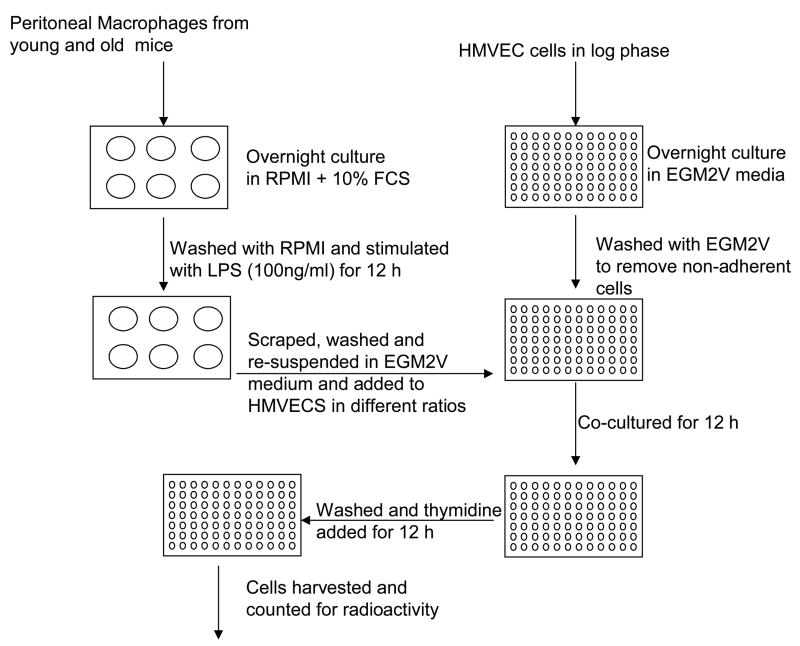
Young B6 (<3 months) and old (>18 months) B6 mice were injected intraperitoneally with 3% thioglycollate for five days. Macrophages were harvested with sterile PBS from the peritoneum of these mice and cultured in RPMI-1640 + 10% FCS overnight. Macrophages were then washed with RPMI-1640 to remove non-adherent cells. Cells were stimulated with LPS (100 ng/ml) for 12 h. Macrophages were washed with RPMI + 10% FCS at 1200 r.p.m. for 8 min (Fig. 1).
Fig. 1.
Schematic representation of vascular endothelial proliferation assay.
Target cell preparation
HMVEC in log phase were washed with PBS and incubated with trypsin/EDTA at 37°C. Trypsin/EDTA was neutralized with Trypsin neutralizing solution (TNS) from Lonza. Cells were then spun down at 1000 r.p.m. for 8 min. Cells were counted and plated in 96-well round bottom plate at a concentration of 5 × 104 for adherence to the plate.
Co-culture
Macrophages from effector cell preparation were resuspended in EGM2V (Lonza, Walkersville, MD), a HMVEC specific media. Media was removed from HMVEC (EC) culture and macrophages (M) were added at various target: effector ratios to HMVEC (1:1 EC/M, 1:10 EC/M and 1:25 EC/M). Cultures were continued for an additional 12 h, then the tritiated thymidine (40 μCi/ml) (TRA61- GE Health Care, Piscataway, NJ) was added to the cultures for 12–18 h. Plate was harvested and read using a Topcount harvester and micro plate reader ((Packard, Meriden, CT).
Results
Macrophages from young mice inhibit vascular endothelial cell proliferation
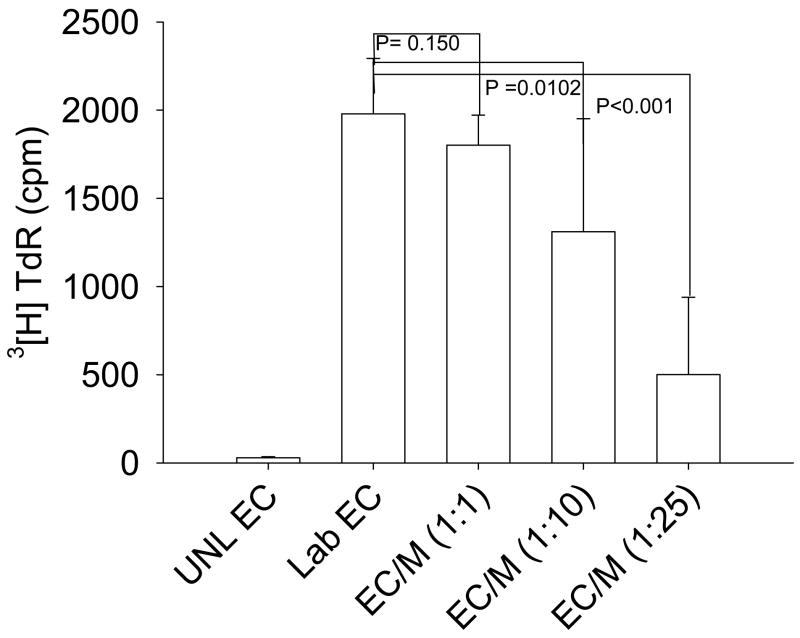
As described above, macrophages have been shown to be important in regulating angiogenesis. We harvested the macrophages from peritoneum exudates of thioglycollate-injected C57BL6 mice in order to assess whether these cells can directly inhibit vascular endothelial cell proliferation. Cells were then plated in 6-well plates to let them adhere to the plate for 12 h and were then gently washed to remove non-adherent cells. Macrophages were stimulated with LPS for 12 h and co-cultured with endothelial cells in various ratios of ECs/Macrophages (1:1, 1:10 and 1:25) for 12 h. Tritiated [3H] thymidine was then added to the culture for an additional 12–18 h. Plates were harvested and read. We found that macrophages from young mice inhibited the proliferation of endothelial cells in a dose dependent manner (Fig. 2). Since thymidine incorporation is a measure for proliferation of cells, we also incubated macrophages by themselves in order to assess the thymidine incorporation in to macrophages. The thymidine incorporation in macrophages was subtracted from co-culture. Incorporation into macrophages was less than 1% of the incorporation into EC cultures.
Fig. 2.
Macrophages from young mice inhibit the proliferation of ECs. Thioglycollate-elicited macrophages were stimulated with LPS (100 ng/ml) for 12 h. Simultaneously, ECs were plated in round bottom 96-well plates. Macrophages were then co-cultured with endothelial cells for 12 h; tritiated thymidine was then added to the culture for 12–18 h. The figure is representative of three independent experiments.
The formula used to calculate the actual thymidine incorporation by endothelial cells was:
Macrophages from old mice induce endothelial cell proliferation
Macrophages were harvested from thioglycollate injected old mice. Cells were then plated in 6-well plates to let them adhere to the plate for 12 h or overnight and were washed to remove non-adherent cells. Macrophages were stimulated with LPS for 12 h and co-cultured with endothelial cells in different ratios such as 1:1 (EC/Macrophage), 1:10 (EC/Macrophages) and 1:25 (EC/Macrophages) for 12 h. Tritiated [3H] thymidine was then added to the culture for further 12–18 h. Plates were harvested and read. We found that not only did old mice macrophages fail to inhibit proliferation of endothelial cells, but, they significantly induced proliferation of ECs in a dose dependent manner (Fig. 3).
Fig. 3.
Macrophages from old mice induce proliferation of EC. Thioglycollate-elicited macrophages were stimulated with LPS (100 ng/ml) for 12 h. Simultaneously, EC were plated in round bottom 96-well plates to let them adhere to the plate. Macrophages were then co-cultured with endothelial cells for 12 h; tritiated thymidine was then added to the culture for 12–18 h. The figure is representative of three independent experiments.
Discussion
The cytotoxic response is mediated by different cell types and involves several different mechanisms of death induction (Russell and Ley, 2002; Berke, 1991). To measure these responses the JAM assay is used, which is based on the DNA-fragmentation which occurs as the “hallmark of apoptosis” in the majority of apoptotic cells (Usharauli et al., 2006). JAM assay is applicable only for analysis of floating (non-adherent) cells, but not for adherent cells. For adherent cells, modified JAM assay is used which is known pJAM assay or post JAM assay (Usharauli et al., 2006). In pJAM assay, target cells are labeled only after the incubation with effector cells unlike JAM assay in which target cells are labeled before incubation with effector cells. In this report we have described an assay that shows the direct effect of macrophages on endothelial cell proliferation. Although it is a modification of pJAM assay, the pJAM assay uses the fact that effector + target cells incorporate less thymidine than target cells alone. If the target + effector cells have more thymidine incorporation than target cells alone, then pJAM assay would show negative killing, which is a non-biological measure. As such, pJAM assay can only show anti-angiogenic effects but not pro-angiogenic effects. We describe a new assay to measure proliferation and not killing i.e. if thymidine incorporation in target + effector cells is less than the target cells alone then effect is anti-angiogenic while increased incorporation shows a pro-angiogenic effect, which correlates very well with biological phenomena. Macrophages are important components of innate immunity that influence angiogenesis and subsets of macrophages exhibit diverse effects on aberrant angiogenesis. Our study facilitates better understanding and quantification of the role of macrophages in regulation of angiogenesis.
Acknowledgments
Funding for this research came from NIH Grant K08EY016139 (RSA), a Carl Marshall Reeves and Mildred Almen Reeves Foundation Inc. Award (RSA), a RPB Career Development Award (RSA) and an American Federation for Aging Research Grant (RSA).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adamis AP, Shima DT, Tolentino MJ, Gragoudas ES, Ferrara N, Folkman J, D’Amore PA, Miller JW. Inhibition of vascular endothelial growth factor prevents retinal ischemia-associated iris neovascularization in a nonhuman primate. Arch Ophthalmol. 1996;114:66–71. doi: 10.1001/archopht.1996.01100130062010. [DOI] [PubMed] [Google Scholar]
- Apte RS, Richter J, Herndon J, Ferguson TA. Macrophages inhibit neovascularization in a murine model of Age-related macular degeneration. PLoS Med. 2006;3:310–320. doi: 10.1371/journal.pmed.0030310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berke G. Debate: the mechanism of lymphocyte-mediated killing. Lymphocyte-triggered internal target disintegration. Immunol Today. 1991;12:396–399. doi: 10.1016/0167-5699(91)90138-j. discussion 402. [DOI] [PubMed] [Google Scholar]
- Blood CH, Zetter BR. Tumor interactions with the vasculature: angiogenesis and tumor metastasis. Biochim Biophys Acta. 1990;1032:89–118. doi: 10.1016/0304-419x(90)90014-r. [DOI] [PubMed] [Google Scholar]
- Dace DS, Apte RS. Effect of senescence on macrophage polarization and angiogenesis. Rejuvenation Res. 2008;11:177–185. doi: 10.1089/rej.2007.0614. [DOI] [PubMed] [Google Scholar]
- Folkman J, Shing Y. Angiogenesis. J Biol Chem. 1992;267:10931–10934. [PubMed] [Google Scholar]
- Gordon S. The role of the macrophage in immune regulation. Res Immunol. 1998;149:685–688. doi: 10.1016/s0923-2494(99)80039-x. [DOI] [PubMed] [Google Scholar]
- Kelly J, Khan AA, Yin J, Ferguson TA, Apte RS. Senescence regulates macrophage activation and angiogenic fate at sites of tissue injury in mice. J Clin Invest. 2007;117:3421–3426. doi: 10.1172/JCI32430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell JH, Ley TJ. Lymphocyte-mediated cytotoxicity. Annu Rev Immunol. 2002;20:323–370. doi: 10.1146/annurev.immunol.20.100201.131730. [DOI] [PubMed] [Google Scholar]
- Ruszczak Z, Detmar M, Imcke E, Orfanos CE. Effects of rIFN alpha, beta, and gamma on the morphology, proliferation, and cell surface antigen expression of human dermal microvascular endothelial cells in vitro. J Invest Dermatol. 1990;95:693–699. doi: 10.1111/1523-1747.ep12514496. [DOI] [PubMed] [Google Scholar]
- Rutherford MS, Witsell A, Schook LB. Mechanisms generating functionally heterogeneous macrophages: chaos revisited. J Leukoc Biol. 1993;53:602–618. doi: 10.1002/jlb.53.5.602. [DOI] [PubMed] [Google Scholar]
- Schreiber AB, Winkler ME, Derynck R. Transforming growth factor-alpha: a more potent angiogenic mediator than epidermal growth factor. Science. 1986;232:1250–1253. doi: 10.1126/science.2422759. [DOI] [PubMed] [Google Scholar]
- Shiver JW, Su L, Henkart PA. Cytotoxicity with target DNA breakdown by rat basophilic leukemia cells expressing both cytolysin and granzyme A. Cell. 1992;71:315–322. doi: 10.1016/0092-8674(92)90359-k. [DOI] [PubMed] [Google Scholar]
- Sullivan R, Gans PJ, McCarroll LA. The synthesis and secretion of granulocyte-monocyte colony-stimulating activity (CSA) by isolated human monocytes: kinetics of the response to bacterial endotoxin. J Immunol. 1983;130:800–807. [PubMed] [Google Scholar]
- Usharauli D, Perez-Diez A, Matzinger P. The JAM test and its daughter P-JAM: simple tests of DNA fragmentation to measure cell death and stasis. Nat Protoc. 2006;1:672–682. doi: 10.1038/nprot.2006.107. [DOI] [PubMed] [Google Scholar]
- Witmer AN, Vrensen GF, Van Noorden CJ, Schlingemann RO. Vascular endothelial growth factors and angiogenesis in eye disease. Prog Retin Eye Res. 2003;22:1–29. doi: 10.1016/s1350-9462(02)00043-5. [DOI] [PubMed] [Google Scholar]