Abstract
Magainin is a naturally occurring, pore-forming peptide that has recently been shown to increase skin permeability. This study tested the hypothesis that electrostatic forces between magainin peptides and drugs mediate drug transport across the skin. Electrostatic interaction between positively charged magainin and a negatively charged model drug, fluorescein, was attractive at pH 7.4 and resulted in a 35 fold increase in delivery across human epidermis in vitro when formulated with 2% N-lauroylsarcosine in 50% ethanol. Increasing to pH 10 or 11 largely neutralized magainin’s charge, which eliminated enhancement due to magainin. Shielding electrostatic interactions with 1–2 M NaCl solution similarly eliminated enhancement. Showing the opposite dependence on pH, electrostatic interaction between magainin and a positively charged anti-nausea drug, granisetron, was largely neutralized at pH 10 and resulted in a 59 fold increase in transdermal delivery. Decreasing to pH 5 increased magainin’s positive charge, which repelled granisetron and progressively decreased transdermal flux. Circular dichroism analysis, multi-photon microscopy, and FTIR spectroscopy showed no significant pH effect on magainin secondary structure, magainin deposition in stratum corneum, or stratum corneum lipid order, respectively. We conclude that magainin increases transdermal delivery by a mechanism involving electrostatic interaction between magainin peptides and drugs.
Keywords: electrostatic interaction, granisetron, magainin pore forming peptide, pH effect, skin permeability enhancement, transdermal drug delivery
1. Introduction
Traditional approaches to increase transdermal drug delivery have involved synthetic chemical enhancers, such as surfactants, fatty acids, and solvents (Williams and Barry, 2004), and physical enhancers, such as electric fields, ultrasound, heat, and microneedles (Cross and Roberts, 2004). Recent work has considered the use of biochemical enhancers, such as an 11-amino acid synthetic peptide identified by phage display screening (Chen et al., 2006), a polyarginine heptamer covalently bonded to a drug using a prodrug approach (Rothbard et al., 2000), and a naturally occurring pore-forming peptide called magainin (Kim et al., 2007), which is the subject of this study.
Magainin is a 23-residue peptide (Sequence: GIGKFLHSAKKFGKAFVGEIMNS) that is naturally produced on the skin of African clawed frogs and exhibits a broad spectrum of antimicrobial activity properties (Zasloff, 1987). It has a net charge up to +4 and binds to negatively charged lipid membranes by way of electrostatic interactions. Its secondary structure is not well-defined in a neutral aqueous environment, however it forms an alpha-helical structure when adsorbed onto a negatively charged membrane such as the surface of a bacterium (Matsuzaki et al., 1997). Magainins can then self-assemble into transmembrane pores that make the cell membrane leaky and can also lead to cell lysis preferentially in bacterial cells. The size of pores formed by magainins in lipid bilayers is estimated to be approximately 1 nm diameter (Matsuzaki et al., 1994, Matsuzaki, 1998).
We previously hypothesized that magainin could be used to make lipid bilayers in the skin’s stratum corneum leaky as well, and showed that magainin can increase skin permeability when delivered from a formulation including an anionic surfactant, N-lauroyl sarcosine, in a 50% ethanol-PBS solution (Kim et al., 2007). Initial tests showed that magainin alone had no effect on skin permeability, probably because the relatively large magainin molecule had difficulty penetrating throughout the stratum corneum to make continuous transdermal pathways. Addition of the anionic surfactant increased skin permeability and thereby facilitated magainin penetration throughout the stratum corneum, which provided a synergistic enhancement. This enhancement was accompanied by increased stratum corneum lipid fluidity, as shown through differential scanning calorimetry, infrared spectroscopy and X-ray diffraction measurements (Kim et al., 2007). These results were consistent with the hypothesis that magainin makes stratum corneum lipid layers leaky.
Building off these observations that magainin, in the presence of surfactant, interacts with stratum corneum to increase permeability, this study seeks to investigate the role of interactions between magainin and a drug as it diffuses through magainin-mediated pathways in the stratum corneum. We expect that the charge properties of magainin play a role in these interactions, especially with charged drugs, and have therefore investigated this effect by changing the magainin charge state by changing pH. For this analysis, we have used two model drugs - fluoresein and granisetron – which are of similar molecular weight, but carry apposite charge. We hypothesize that electrostatic forces between magainin peptides and drugs mediate drug transport across the skin.
Previous studies have shown that changing pH can alter the structure and properties of antimicrobial peptides in various ways, such as switching between the nematic and isotropic phase of a β-sheet peptide (Aggeli et al., 2003), changing the charge of a transmembrane α-helical peptide (Subczynski et al., 2005), enhancing antimicrobial activity (Lee et al., 1997), controlling haemolytic activity (Moser, 1992), and altering pore-formation properties (Shai et al., 1991).
The effect of pH on transdermal delivery has also been widely investigated. For example, indomethacin delivery was enhanced under acidic conditions due to increased drug solubility (Chiang et al., 1991), the dependence of skin permeability to cephalexin on pH was described by a U-shaped curve (Hatanaka et al., 1995), the effect of ionization of 5-fluorouracil on transdermal flux was determined by pH (Singh et al., 2005), and pH had a significant role in enhancing skin permeation of acidic drugs (Katayama et al., 2001) and lidocaine (Kushla and Zatz, 1991).
2. Materials and methods
2.1 Skin preparation
Human cadaver skin was obtained from Emory University School of Medicine (Atlanta, GA, USA) with approval from the Georgia Institute of Technology Institutional Review Board and stored at −75°C. Immediately prior to an experiment, whole skin was thawed in deionized water at 30°C for 1 h. Intact epidermis was isolated from dermis using the heat separation method (Kligman and Christophel, 1963), in which thawed whole skin was immersed in deionized water for 2 min at 60°C and the epidermis was carefully peeled away with a spatula. Stratum corneum sheets were isolated from the epidermis by trypsin digestion in which the epidermis was incubated in phosphate-buffered saline containing 0.25% trypsin and 0.01% gentamicin at 32 °C for 24 h and the resulting sheet of isolated stratum corneum was rinsed with distilled water three times and stored on polymer-coated paper under vacuum overnight at 25°C.
2.2 Skin permeability measurement
Skin permeability measurements were carried out in three steps. As a first step, skin pretreatment was performed using epidermis (0.7 cm2 skin surface area) mounted in a vertical, glass Franz diffusion cell (PermeGear, Bethlehem, PA, USA). A solution of 1 mM magainin peptide (Microchemical and Proteomics Facility, Emory University School of Medicine) and 2% (w/v) N-lauroylsarcosine (NLS, 98%, Fluka, Buchs, Switzerland) in 50% (v/v) aqueous ethanol solution was placed in the donor chamber and phosphate-buffered saline (PBS, Sigma Aldrich, St. Louis, MO, USA) was placed in the receiver chamber for 12 h at 4 °C to minimize skin degradation. The magainin solution had a pH of 3.5 and the PBS had a pH of 7.4.
As a second step, the Franz cell was removed from refrigeration and placed in a heater/stirrer block (PermeGear) that warmed the receiver chamber at 37 °C and maintained stirring at 455 rpm.
As a third step, fresh PBS (pH from 5 to 11) was placed in the receiver chamber and 0.3 mL of 1 mM fluorescein (Sigma Aldrich, St. Louis, MO, USA) solution in PBS (pH from 7.4 to 11) or 1 wt% granisetron hydrochloride (Ultratech SPC PVT Ltd., Mumbai, India) solution in PBS (pH from 5 to 10) was placed in the donor chamber, which replaced the magainin/NLS solution. Every hour for 5 h, the receiver solution was removed for sampling and then replaced with fresh PBS (pH from 5 to 11). The pH of sample solution was adjusted to pH 7.4 by adding 1 N nitric acid to decrease pH and 1 N NaOH solution to increase pH. Samples containing fluorescein were analyzed by spectrofluorimetry (Photon Technologies International, Birmingham, NJ, USA) to determine fluorescein concentration, from which transdermal fluorescein flux was calculated. Samples containing granisetron were analyzed by HPLC, as described below.
2.3 HPLC analysis
HPLC analysis was carried out using an Alliance HPLC system (Waters, Milford, MA, USA) with Empower software, a fluorescence detector (Waters 2475, 305 nm excitation, 350 nm emission gain setting of 1) and a Spherisorb Cyano column (4.6 × 250 mm, Waters). Samples were injected directly into the column and eluted in a 72:28 mixture of acetate buffer (25 mM) and acetonitrile at a flow rate of 1 mL/min. Calibration curves were prepared at a concentration range of 10 – 100 ng/mL granisetron. The interday and intraday variation was less than 6.0 %. The LOQ was 1 ng/ml.
2.4 Multi-photon excitation microscopy
To image magainin and fluorescein distribution within the skin, epidermis was treated as described above for skin permeability experiments, except that sulphorhodamine-tagged magainin (Microchemical and Proteomics Facility, Emory University School of Medicine) was used instead of un-labeled magainin in the first step and the experiment was terminated after 3 h exposure to fluorescein in the third step, after which the skin sample was removed from the Franz cell and placed on a glass cover-slip.
Imaging was carried out using a Zeiss LSM/NLO 510 Confocal/Multi-Photon Microscope (Zeiss, Oberkochen, Germany) with a 40× oil-immersion objective used in conjunction with an oil having an index of refraction of 1.51, which is similar to that of the stratum corneum (Corcuff et al., 1993, Knuttel and Boehlau-Godau, 2000).
2.5 Circular dichroism spectra
Solutions were prepared containing 50 μM magainin in 50% (v/v) aqueous ethanol. pH was adjusted to a value between 7.4 and 11. Solutions were placed in a capped quartz optical cell (1 mm path length; Starna cells, Atascadero, CA, USA) and circular dichroism spectra were acquired on a JASCO J-720 CD spectropolarimeter (JASCO, Easton, MD). Spectra were obtained at room temperature by scanning five times from 250 nm to 190 nm at a rate of 500 nm/min in continuous scanning mode.
2.6 Fourier transform infrared spectroscopy
Stratum corneum was exposed to magainin-NLS solution for 15 h at 4 °C. Then stratum corneum samples were transferred to different pH buffer solutions for 5 h and washed with PBS solution. Fourier-transform infrared spectra were then obtained using a Magma-IR 560 FTIR spectrometer (Nicolet, Thermo Electron Corporation, Waltham, MA, USA). Reported spectra represent the average of 64 scans over the frequency range of 4000 – 1000 cm−1. OMNIC professional software (Thermo Electron Corporation) was used to measure the peak position. Although the FTIR had a data collection spacing of 4 cm−1, interpolation between points is reliable because the noise level is so low and the reproducibility of FTIR spectra is so high. This permits one to determine the location of a peak, even if it exists between data points that were actually collected. This is well established in the spectroscopy literature (Cameron et al., 1982) and is consistent with many previous studies involving FTIR analysis of skin, where peak shifts much smaller than the data collection spacing are reported (Anigbogu et al., 1995, Naik et al., 1995).
2.7 Statistical Analysis
Transdermal penetration of fluorescein and granisetron and FTIR spectra were measured using at least three skin specimens, from which the mean and standard error of the mean were calculated. A two-tailed Student’s t-test (α=0.05) was performed when comparing two different conditions. When comparing three or more conditions, a one-way analysis of variance (ANOVA; α=0.05) was performed. In all cases, a value p<0.05 was considered statistically significant.
3. Results and discussion
3.1 Effect of pH on transdermal flux of fluorescein
We hypothesize that electrostatic forces between magainin peptides and drugs mediate drug transport across the skin. To address this issue, we measured skin permeability to fluorescein over a pH range of 7.4 to 11. Over this range, the charge of the skin (Marro et al., 2001), NLS surfactant, and fluorescein should all remain strongly negative. However, magainin has an isoelectric point at pH 10.5, such that magainin changes from a +2 positive charge at pH 7.4 to a neutral charge at pH 10.5 and a negative charge at pH 11 (Skoog and Wichman, 1986).
We first carried out a control experiment to assess the effect of pH on skin permeability to fluorescein after pretreatment with NLS (without magainin). At pH 7.4, transdermal delivery was increased 16 fold by the NLS control formulation (i.e., from an average of 0.037 μg to 0.599 μg of fluorescein), as shown in Fig. 1. Increasing to pH 11 had no additional effect (ANOVA, p = 0.73), which is consistent with the expectation that the charge of skin, NLS and fluorescein should be unaffected by increased pH. It is also consistent with previous observations that skin permeability is not generally changed at pH values up to 11 (Sznitowska et al., 2001).
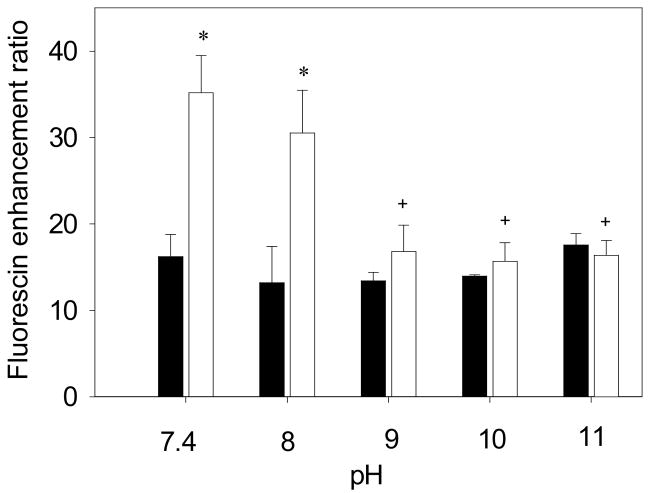
Figure 1.
Enhancement of transdermal fluorescein delivery as a function of pH. Skin was pre-treated with NLS (■) or magainin + NLS (□) in 50% ethanol. Enhancement ratio represents the increase in transdermal fluorescein transported across skin over 5 h at various pH values compared to delivery under identical conditions using a formulation of fluorescein in PBS. The * symbol identifies enhancement ratios for magainin + NLS that are significantly different from NLS at the same pH (Student’s t-test, p < 0.05). The † symbol identifies enhancement ratios at a given pH that are significantly different from the same formulation at pH 7.4 (Student’s t-test, p < 0.05). Data represent averages of n ≥ 3 samples ± standard error of the mean.
Transdermal permeation of fluorescein after treatment with magainin and NLS was increased by a factor of 35 (i.e., from and average of 0.037μg to 1.302 μg of fluorescein) at pH 7.4, as shown in Fig. 1. This increase is significantly larger than for NLS without magainin (Student’s t-test, p < 0.01). However, raising the pH and thereby reducing magainin charge, progressively removed magainin’s enhancement until pH 11 (ANOVA, p < 0.05). This suggests that a positively charged magainin facilitated transdermal transport of negatively charged fluorescein due to electrostatic attraction at pH 7.4, but as the attraction decreased with increasing pH, the skin permeability enhancement decreased as well.
3.2 Effect of pH on transdermal flux of granisetron
The effect of pH on skin permeability might be mediated by changes in the electrostatic interactions between magainin and fluorescein, but might also be mediated by changes in the interactions between magainin and skin. To address this uncertainty, we measured the effect of pH on skin permeability to granisetron, which differs from fluorescein in that it carries a positive charge, but is similar in that its molecular weight (349 Da) is similar to fluorescein (332 Da).
The control formulation of NLS without magainin at pH 7.4 enhanced transdermal granisetron delivery 59 fold (i.e., from and average of 2.23 μg to 131.44 μg of granisetron), as shown in Fig. 2. Neither decreasing to pH 5 nor increasing to pH 10 had any additional effect (ANOVA, p = 0.84). Unfortunately, we could not test pH 11 due to precipitation of granisetron at high pH.
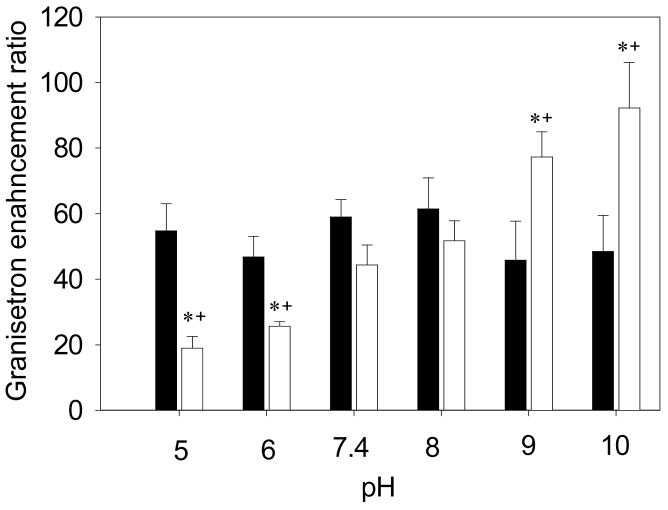
Figure 2.
Enhancement of transdermal granisetron delivery as a function of pH. Skin was pre-treated with NLS (■) or magainin + NLS (□) in 50% ethanol. Enhancement ratio represents the increase in transdermal granisetron transported across skin over 5 h at various pH values compared to delivery under identical conditions using a formulation of granisetron in PBS. The * symbol identifies enhancement ratios for magainin + NLS that are significantly different from NLS at the same pH (Student’s t-test, p < 0.05). The † symbol identifies enhancement ratios at a given pH that are significantly different from the same formulation at pH 7.4 (Student’s t-test, p < 0.05). Data represent averages of n ≥ 3 samples ± standard error of the mean.
Unlike for fluorescein, the addition of magainin at pH 7.4 had no additional effect on skin permeability to granisetron (Student’s t-test, p = 0.167), as shown in Fig. 2, perhaps because of repulsion between the positively charged magainin and positively charged granisetron. Decreasing the pH to 5, which increased magainin charge to a value of +4, resulted in a reduction of granisetron delivery (Student’s t-test, p <0.05) and raising the pH to 10, which almost eliminated magainin charge, increased granisetron delivery to a peak value of 92–fold (i.e., from and average of 2.23 μg to 205.55 μg of granisetron) enhancement (Student’s t-test, p <0.05) due to removal of the electrostatic repulsion.
The opposite effects of pH on skin permeability to fluorescein versus granisetron suggest that the pH changes influenced interactions between magainin and each model drug and did not primarily influence interactions between magainin and skin.
3.3 Effect of salt concentration on transdermal flux of fluorescein
To further test whether the effect of pH on skin permeability is mediated by electrostatic interactions between magainin and the model drug, we changed salt concentration rather than changing pH to alter electrostatic interactions. An increase of salt concentration screens out electrostatic effects between charged species (Kandasamy and Larson, 2006). Thus, increased salt concentration should effectively neutralize charge-charge interactions in a way similar to increasing pH to the magainin isoelectric point.
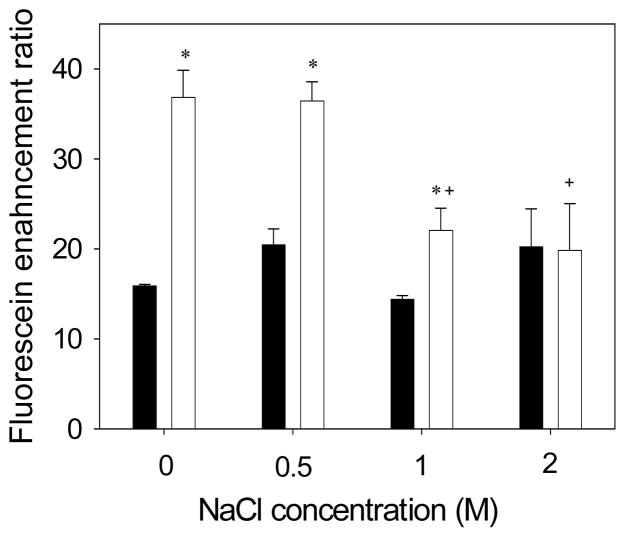
For the NLS control formulation, skin permeability to fluorescein was unaffected by salt concentration, as shown in Figure 3 (ANOVA, p = 0.51). This is consistent with the expectation that skin permeability enhancement by NLS is not mediated by electrostatic interactions. For skin treated with NLS and magainin peptide, skin permeability was increased by magainin at salt concentrations of 0 M and 0.5 M (Student’s t test, p <0.01). However, increasing the salt concentration to 1 M or 2 M eliminated magainin’s enhancement effect, such that skin permeability was indistinguishable from the NLS control (Student’s t test, p >0.05). This result further confirms the interpretation that magainin increases transdermal transport when there is an attractive electrostatic interaction with a fluorescein and loses its ability to enhance skin permeability to fluorescein if that electrostatic interaction is blocked.
Figure 3.
Enhancement of transdermal fluorescein delivery as a function of NaCl concentration. Skin was pre-treated with NLS (■) or magainin + NLS (□) in 50% ethanol at pH 7.4. Enhancement ratio represents the increase in transdermal fluorescein transported across skin over 5 h at various salt concentrations compared to delivery under identical conditions using a formulation of fluorescein in PBS. The * symbol identifies enhancement ratios for magainin + NLS that are significantly different from NLS at the same salt concentration (Student’s t-test, p < 0.05). The † symbol identifies enhancement ratios at a given pH that are significantly different from the same formulation at 0 M NaCl (Student’s t-test, p < 0.05). Data represent averages of n ≥ 3 samples ± standard error of the mean.
3.4 Circular dichroism analysis of magainin structure
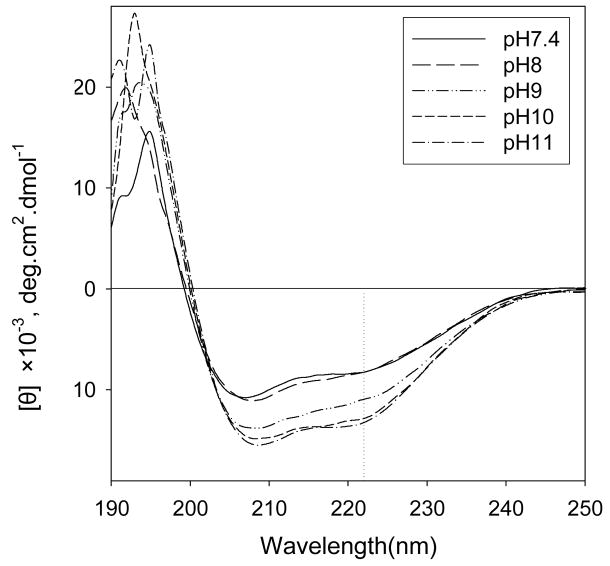
Changing the pH could alter magainin structure, which could additionally affect skin permeability enhancement. To assess the effects of pH on magainin secondary structure, we collected circular dichroism spectra of magainin solutions over a range of pH from 7.4 to 11. These spectra showed that magainin retained an α–helix structure over the pH range studied, as indicated by the maximum at 192 nm and the two minima at 208 and 222 nm (Fig. 4). The mean residue ellipticity at 222 nm, commonly considered an indicator of α–helicity, is more negative for magainin at higher pH, which suggest that higher pH enhanced α–helicity of magainin peptide. Although this experiment was done using magainin in solution, rather than within the skin, these results suggest that magainin structure was not significantly altered by pH.
Figure 4.
Circular dichroism spectra of magainin as a function of pH. Magainin was dissolved in 50 % ethanol at various pH values.
3.5 Microscopy analysis of magainin and fluorescein delivery into stratum corneum
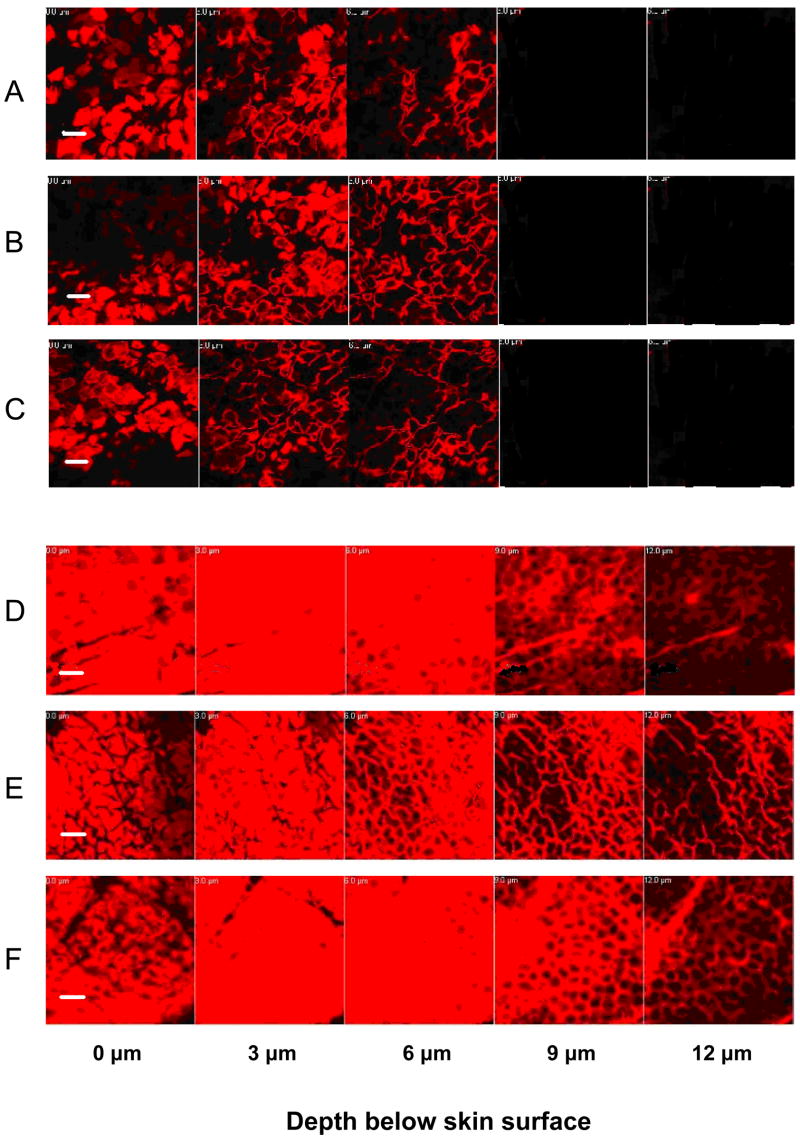
Changing pH could alter the amount of magainin in the stratum corneum, which could thereby alter skin permeability. To assess the effects of pH on magainin content in the stratum corneum, we imaged the amount of sulforhodamine-labelled magainin inside stratum corneum using multi-photon excitation microscopy at various pH values. Magainin delivered to skin in the absence of NLS surfactant had limited penetration into the skin and showed no significant dependence on pH, as shown in Figs. 5A – 5C. Magainin formulated with NLS was delivered to a much greater extent than in the absence of NLS, but also did not show significant dependence on pH, as shown in Figs. 5D – 5F. These data indicate that the effects of pH on skin permeability cannot be explained by changes in the amount of magainin peptide present in the stratum corneum.
Figure 5.
Penetration of sulforhodamine-tagged magainin peptide into human epidermis imaged by multi-photon microscopy. Skin was pre-treated with magainin formulated (A–C) without NLS and (D–F) with NLS for 15 h. Skin was exposed PBS solution for 5 h at (A, D) pH 5, (B, E) pH 7.4, (C, F) pH 10. Optical sections were taken at 5 μm increments starting at the stratum corneum surface on the left and proceeding deeper on the right. Scale bar is 50 μm.
To provide additional insight into the flux data presented in Fig. 1, we imaged the amount of fluorescein delivered into the skin using multi-photon excitation microscopy at various pH values. Consistent with the flux data, optical sections through the epidermis show that the smallest amount of fluorescein was seen in the skin at pH 10, when magainin charge was almost neutralized (Fig. 6B); a greater amount was seen at pH 7.4, when magainin carried a +2 charge that attracted negatively charged fluorescein (Fig. 6A). Z-stack images showing cross-sectional views of the epidermis in Figs. 6C and 6D provide similar, complimentary results. Transdermal transport was not imaged at lower pH values (e.g., pH 5) due to extensive fluorescein bleaching at acidic pH (Yoshida et al., 2000).
Figure 6.
Penetration of fluorescein into human epidermis imaged by multi-photon microscopy. Skin was treated with magainin + NLS. Fluorescein was delivered to skin for 5 h at (A, C) pH 7.4, (B, D) pH 10. (A–B) Optical sections were taken at 5 μm increments starting at the stratum corneum surface on the left and proceeding deeper on the right. Scale bar is 100 μm. (C–D) Cross-sectional images were reconstructed as z-stacks with the stratum corneum surface on top and deeper tissue below. Scale bar is 20 μm.
3.6 Fourier transform infrared spectroscopy
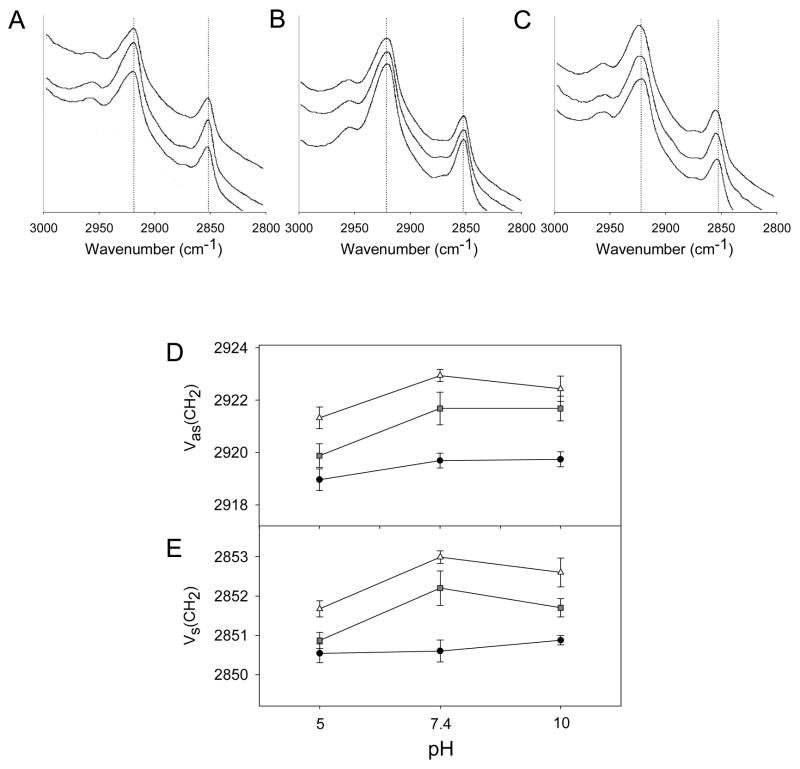
Changing pH could directly alter stratum corneum lipid structure or could change the way that magainin alters stratum corneum lipid structure. To assess these effects, we used Fourier-transform infrared spectroscopy to measure the degree of stratum corneum lipid disruption. Representative spectra are shown for untreated skin (Fig. 7A), skin treated with NLS (Fig. 7B) and skin treated with magainin and NLS (Fig. 7C) as a function of pH. Characteristic peaks can be seen in these spectra near 2920 cm−1, which corresponds to asymmetric C-H stretching, and near 2850 cm−1, which corresponds to symmetric C-H stretching. The frequencies of these C-H stretching bands are related to lipid order in stratum corneum and are significantly influenced by the degree of conformational order, the freedom of alkyl chain motion, and possible incorporation of chemical enhancers, such as NLS (Naik and Guy, 1997).
Figure 7.
Fourier-transform infrared spectroscopy analysis of human stratum corneum treated with different formulations as a function of pH. Representative spectra highlighting wavenumbers characteristic of C-H stretching in stratum corneum lipids after pre-treatment with (A) PBS, (B) NLS, and (C) magainin + NLS for 15 H and soaked in PBS at pH 5, 7.4, and 10 (shown from top to bottom). Dashed lines identify peaks of interest. Change of (D) CH2 asymmetric stretching frequency and (E) CH2 symmetric stretching frequency for stratum corneum pre-treated with PBS (●), NLS (■), and magainin + NLS (△) determined from graphs like (A), (B) and (C), respectively. Data represent averages of n ≥ 3 samples ± standard error of the mean. The * symbol identifies wavenumbers significantly different from the corresponding wavenumber at pH 7.4 (Student’s t-test, p<0.05).
To address the effect of pH alone on skin, the frequency of the two characteristic peaks showed no dependence on pH (ANOVA; Fig 7D, black circles, p = 0.26; Fig. 7E, black circles, p = 0.89).
After treatment with formulations containing NLS or magainin + NLS, there was no statistically significant difference between pH 7 and 10 for skin treated with NLS (ANOVA; Fig. 7D, gray squares, p = 0.54; Fig. 7E, gray squares, p = 0.12) and with magainin + NLS (ANOVA; fig. 7D, white triangles. p = 0.29; Fig. 7E, white triangles, p = 0.09). However, exposure at pH 5 significantly decreased the peak frequency (i.e., increased lipid order) for skin treated with NLS (ANOVA; Fig. 7D, gray squares, p < 0.01; Fig. 7E, gray squares, p < 0.01) and with magainin + NLS (ANOVA; fig. 7D, white triangles, p < 0.05; Fig. 7E, white triangles, p < 0.05), when considered over the full range of pH 5 to 10.
Although the lowest pH had an effect of lipid order, there was no effect over the pH range of 7.4 to 10, which is the range over which pH was shown to have a dramatic effect on skin permeability to fluorescein and granisetron in Figs. 1 and 2. These data suggest that the effects of pH on skin permeability cannot be explained by changes in stratum corneum lipid order.
3.7. Interpretation of the data
Our previous studies showed that NLS surfactant is needed to drive magainin into the skin. This finding was confirmed by Fig. 5, which showed little magainin penetration into the stratum corneum in the absence of NLS and extensive magainin penetration with NLS. Our previous studies also showed that the presence of magainin increased skin permeability, which is consistent with Figs. 1 and 2, and that increased skin permeability was associated with increased stratum corneum lipid disorder, which is consistent with Fig. 7.
For the first time, this study examined the effects of pH on skin permeability and other properties. Our data support the hypothesis that electrostatic forces between magainin peptides and drugs mediate drug transport across the skin. We found that transdermal transport of negatively charged fluorescein decreased as the positive charge of magainin was reduced by increasing pH. We also found that transdermal transport of positively charge granisetron increased as the positive charge of magainin was reduced by increasing pH. Finally, we found that transdermal transport of fluorescein also decreased as the screening of electrostatic interactions with magainin increased by increasing salt concentration.
These three observations are all consistent with the proposed hypothesis and are not consistent with changes in magainin conformation, magainin content in the skin or magainin interaction with the skin being directly responsible for increased transdermal transport. This is because these effects should not depend on the charge of the molecule being delivered across the skin and should not be affected by changing the pH and by increasing salt concentration.
In addition, CD measurements demonstrated that pH did not change the secondary structure of magainin in solution, microscopy indicated that pH did not affect the magainin content in the stratum corneum, and FTIR showed that pH did not affect lipid order, except at the lowest pH. Altogether, these data suggest that electrostatic interactions between magainin peptides and drugs provide the most plausible explanation for the observed effects.
We believe that these electrostatic interactions occur between magainins loaded into the stratum corneum and drug molecules diffusing through transport pathways created, at least in part, by these magainins. We do not believe that magainins and drugs molecules bind and diffuse together through the stratum corneum for a number of reasons.
First, magainins were added as a pretreatment and then removed from the donor compartment of the diffusion cell before fluorescein or granisetron was added. Thus, there was no opportunity for magainin-drug binding in the donor compartment.
Second, Fig. 5 shows magainin localized to the stratum corneum (i.e., upper 10μm of skin), whereas Fig. 6 shows fluorescein penetrating deeply across the epidermis (i.e., >40μm). Fig. 1 and 2 further show fluorescein and granisetron diffusing across the full epidermis and into the receiver compartment of the diffusion cell.
Finally, fluorescein has a molecular weight of 332 Da and magainin has a molecular weight of 2504 Da. Thus, a fluorescein-magainin complex would have molecular weight of at least 2836 Da. Magainin’s large size precludes it from diffusing across skin to an appreciable extent; it can only penetrate and remain within stratum corneum lipid bilayers due to its special physicochemical properties as a pore-forming peptide. Given the large size of a fluorescein-magainin complex, it is unlikely that such as large complex would cross the skin well. A similar analysis applies to a possible granisetron-magainin complex.
3.8 Debye length calculations
The effect of salt concentration on skin permeability provides an opportunity to further evaluate the transport pathways created by magainin peptides. As salt concentration increases, the Debye length decreases. The Debye length is a measure of the distance over which the electric field induced by charge separation is felt. As greater numbers of salt ions in solution surround the charge on a magainin peptide, they shield its electric field.
Debye length, λD, can be calculated at the conditions used in this study by (Goldston and Rutherford, 1995).
| (1) |
where ε0 is the permittivity of an electrical field in free space (8.8542 × 10−12 F m−1), εr is the dielectric constant (66.89 for 0.5 M saline and 59.89 for 1.0 M saline at 32°C and 1 atm (Lane and Saxton, 1952, Malmberg and Maryott, 1956, Stogryn, 1971)), R is the gas constant (8.314 N m K−1 mol−1), T is the absolute temperature (310.15 K), F is the Faraday constant (96,485.34 C mol−1), and C is the salt concentration (0.5 M or 1.0 M). This analysis assumes that the pathways created by magainin are aqueous and has the dielectric constant of saline. If the pathway, in fact, has lipid components in it, which would reduce the dielectric constant, then the estimates of Debye length reported below would be smaller.
At a salt concentration of 0.5 M, the Debye length is 4.02 Å. At this salt concentration, the electrostatic interactions between magainin peptides and fluorescein molecules were still effective. At a salt concentration of 1 M, the Debye length is 2.69 Å. At this salt concentration, the electrostatic interactions between magainin peptides and fluorescein molecules were blocked.
Fluorescein molecules have a radius of approximately 5 Å (Prausnitz and Noonan, 1998). The fact that the Debye length at which magainin – fluorescein interactions are shielded is smaller than the radius of the fluorescein molecule indicates that the pathways created by magainins in the skin are just slightly larger than a fluorescein molecule. The screening of the attractive electrostatic interaction by such a small change in the Debye length implies that the fluorescein molecule must be close to the edge of this pathway. This is consistent with our previous finding that magainin did not enhance transdermal transport of molecules larger than fluorescein (Kim et al.,2008), such as calcein (623 Da, r = 6 Å (Edwards et al., 1995) and dextran (3,000 Da, r = 16 Å (Oliver et al., 1992). This is also consistent with previous studies of magainin in simpler lipid bilayer systems, in which the pores are believed to be of Angstrom dimensions (Ludtke et al., 1995, Matsuzaki et al., 1996).
3.9 Implications for drug delivery
Biochemical enhancers are a novel approach to increasing skin permeability for transdermal drug delivery. However, the use of peptides to increase transdermal transport has been addressed in just a small number of studies. This study provides insight into the mechanism by which magainin peptides increases skin permeability, which may have broader relevance to other peptide-based enhancement strategies.
The use of magainin peptide in combination with NLS surfactant increased transdermal transport of fluorescein 35 fold at pH 7.4. Magainin with NLS also increased transdermal transport of granisetron 59 fold at pH 7.4 and up to 92 fold at pH 10. These large increases in skin permeability may be useful for transdermal drug delivery. Although fluorescein is a model compound, granisetron is a drug in clinical use to prevent nausea and is in late-stage development as a transdermal patch (ProStrakan, 2007). There are many chemical and physical enhancers available for transdermal drug delivery (Asbill et al., 2000). It is not yet clear in which specific scenarios magainins will be useful for drug delivering applications when compared with other methods.
The dependence of transdermal transport on pH, as well as on salt concentration, presents the opportunity to modulate or trigger transdermal delivery rates by increasing or decreasing pH or salt. Although we do not yet know the speed with which changing pH or salt concentration can alter transdermal transport, novel approaches to initiate, terminate or otherwise modulate transdermal delivery rates could be achieved by changing pH or salt concentration.
4. Conclusion
This study supported the hypothesis that electrostatic forces between magainin peptides and drugs mediate drug transport across the skin. Mechanisms of transport were studied by measuring rates of transdermal transport, fluorescence microscopy, and CD and FTIR spectroscopy,
Transdermal delivery of negatively charged fluorescein was shown to be increased 35 fold using a formulation containing magainin peptide that carried a +2 charge at pH 7.4 and thereby provided an attractive electrostatic interaction with fluorescein. Increasing pH to 10 or 11, which neutralized the charge on magainin and thereby removed the electrostatic attraction, eliminated the enhancement due to magainin. Blocking electrostatic interactions at high salt concentration similarly eliminated the enhancement due to magainin.
Transdermal delivery of positively granisetron was shown to be increased 92 fold using the same formulation containing magainin peptide that was neutralized at pH 10. Decreasing pH to 7.4, which gave magainin a +2 charge and thereby provided a repulsive electrostatic interaction with granisetron, eliminated the enhancement due to magainin. Decreasing pH further to 5, which gave magainin a +4 charge, inhibited granisetron flux.
These observations are consistent with the stated hypothesis and are not consistent with changes in magainin conformation, magainin content in the skin, magainin interaction with the skin or diffusion of drug-magainin complexes across the skin as being directly responsible for increased transdermal transport. CD analysis, fluorescence microscopy and FTIR spectroscopy, respectively, provided further evidence to rule out these alternative mechanisms.
Overall, magainin peptides represent a novel class of biochemical enhancers of transdermal transport. Their ability to increase skin permeability and to be modulated by pH and salt concentration suggest applications for transdermal drug delivery.
Acknowledgments
This work was supported in part by the National Institutes of Health. Mark Prausnitz is the Emerson-Lewis Faculty Fellow. This work was carried out in the Center for Drug Design, Development and Delivery and the Institute for Bioengineering and Bioscience at the Georgia Institute of Technology and at the Center for Drug Delivery Research at the College of Pharmacy and Health Sciences at Mercer University. Yeu-Chun Kim carried out the studies presented here. Sameer Late conducted HPLC analysis under the guidance of Ajay Banga. Mark Prausnitz and Peter Ludovice served as the principal investigators directing the project.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aggeli A, Bell M, Carrick LM, Fishwick CWG, Harding R, Mawer PJ, Radford SE, Strong AE, Boden N. Ph as a trigger of peptide beta-sheet self-assembly and reversible switching between nematic and isotropic phases. Journal of the American Chemical Society. 2003;125:9619–9628. doi: 10.1021/ja021047i. [DOI] [PubMed] [Google Scholar]
- Anigbogu ANC, Williams AC, Barry BW, Edwards HGM. Fourier-transform raman-spectroscopy of interactions between the penetration enhancer dimethyl-sulfoxide and human stratum-corneum. International Journal of Pharmaceutics. 1995;125:265–282. [Google Scholar]
- Asbill CS, El-Kattan AF, Michniak B. Enhancement of transdermal drug delivery: Chemical and physical approaches. Critical Reviews in Therapeutic Drug Carrier Systems. 2000;17:621–658. [PubMed] [Google Scholar]
- Cameron DG, Kauppinen JK, Moffatt DJ, Mantsch HH. Precision in condensed phase vibrational spectroscopy. Applied Spectroscopy. 1982;36:245–250. [Google Scholar]
- Chen YP, Shen YY, Guo X, Zhang CS, Yang WJ, Ma ML, Liu S, Zhang MB, Wen LP. Transdermal protein delivery by a coadministered peptide identified via phage display. Nature Biotechnology. 2006;24:455–460. doi: 10.1038/nbt1193. [DOI] [PubMed] [Google Scholar]
- Chiang CH, Lai JS, Yang KH. The effects of ph and chemical enhancers on the percutaneous-absorption of indomethacin. Drug Development and Industrial Pharmacy. 1991;17:91–111. [Google Scholar]
- Corcuff P, Bertrand C, Leveque JL. Morphometry of human epidermis in-vivo by real-time confocal microscopy. Archives of Dermatological Research. 1993;285:475–481. doi: 10.1007/BF00376820. [DOI] [PubMed] [Google Scholar]
- Cross SE, Roberts MS. Physical enhancement of transdermal drug application: Is delivery technology keeping up with pharmaceutical development? Current Drug Delivery. 2004;1:81–92. doi: 10.2174/1567201043480045. [DOI] [PubMed] [Google Scholar]
- Edwards DA, Prausnitz MR, Langer R, Weaver JC. Analysis of enhanced transdermal transport by skin electroporation. Journal of Controlled Release. 1995;34:211–221. [Google Scholar]
- Goldston RJ, Rutherford PH. Introduction to plasma physics. Institute of Physics Publishing; Philadelphia: 1995. [Google Scholar]
- Hatanaka T, Morigaki S, Aiba T, Katayama K, Koizumi T. Effect of ph on the skin permeability of a zwitterionic drug, cephalexin. International Journal of Pharmaceutics. 1995;125:195–203. [Google Scholar]
- Kandasamy SK, Larson RG. Effect of salt on the interactions of antimicrobial peptides with zwitterionic lipid bilayers. Biochimica Et Biophysica Acta-Biomembranes. 2006;1758:1274–1284. doi: 10.1016/j.bbamem.2006.02.030. [DOI] [PubMed] [Google Scholar]
- Katayama K, Matsui R, Hatanaka T, Koizumi T. Effect of ph on skin permeation enhancement of acidic drugs by 1-menthol-ethanol system. International Journal of Pharmaceutics. 2001;226:69–80. doi: 10.1016/s0378-5173(01)00778-5. [DOI] [PubMed] [Google Scholar]
- Kim Y, Ludovice PJ, Prausnitz MR. Transdermal delivery enhanced by magainin pore-forming peptide. Journal of Controlled Release. 2007;122:375–383. doi: 10.1016/j.jconrel.2007.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y, Ludovice PJ, Prausnitz MR. Optimization of transdermal delivery using magainin pore-forming peptide. Journal of Physics and Chemistry of Solids. 2008;69:1560–1563. doi: 10.1016/j.jpcs.2007.10.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kligman AM, Christophel E. Preparation of isolated sheets of human stratum corneum. Archives of Dermatology. 1963;88:702–705. doi: 10.1001/archderm.1963.01590240026005. [DOI] [PubMed] [Google Scholar]
- Knuttel A, Boehlau-Godau M. Spatially confined and temporally resolved refractive index and scattering evaluation in human skin performed with optical coherence tomography. Journal of Biomedical Optics. 2000;5:83–92. doi: 10.1117/1.429972. [DOI] [PubMed] [Google Scholar]
- Kushla GP, Zatz JL. Influence of ph on lidocaine penetration through human and hairless mouse skin invitro. International Journal of Pharmaceutics. 1991;71:167–173. [Google Scholar]
- Lane JA, Saxton JA. Dielectric dispersion in pure polar liquids at very high radio frequencies.3. The effect of electrolytes in solution. Proceedings of the Royal Society of London Series a-Mathematical and Physical Sciences. 1952;214:531–545. [Google Scholar]
- Lee IH, Cho Y, Lehrer RI. Effects of ph and salinity on the antimicrobial properties of clavanins. Infection and Immunity. 1997;65:2898–2903. doi: 10.1128/iai.65.7.2898-2903.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludtke S, He K, Huang H. Membrane thinning caused by magainin 2. Biochemistry. 1995;34:16764–16769. doi: 10.1021/bi00051a026. [DOI] [PubMed] [Google Scholar]
- Malmberg CG, Maryott AA. Dielectric constant of water from 0-degrees-c to 100-degrees-c. Journal of Research of the National Bureau of Standards. 1956;56:1–8. [Google Scholar]
- Marro D, Guy RH, Delgado-Charro MB. Characterization of the iontophoretic permselectivity properties of human and pig skin. Journal of Controlled Release. 2001;70:213–217. doi: 10.1016/s0168-3659(00)00350-3. [DOI] [PubMed] [Google Scholar]
- Matsuzaki K, Murase O, Tokuda H, Funakoshi S, Fujii N, Miyajima K. Orientational and aggregational states of magainin-2 in phospholipid-bilayers. Biochemistry. 1994;33:3342–3349. doi: 10.1021/bi00177a027. [DOI] [PubMed] [Google Scholar]
- Matsuzaki K, Murase O, Fujii N, Miyajima K. An antimicrobial peptide, magainin 2, induced rapid flip-flop of phospholipids coupled with pore formation and peptide translocation. Biochemistry. 1996;35:11361–11368. doi: 10.1021/bi960016v. [DOI] [PubMed] [Google Scholar]
- Matsuzaki K, Nakamura A, Murase O, Sugishita K, Fujii N, Miyajima K. Modulation of magainin 2-lipid bilayer interactions by peptide charge. Biochemistry. 1997;36:2104–2111. doi: 10.1021/bi961870p. [DOI] [PubMed] [Google Scholar]
- Matsuzaki K. Magainins as paradigm for the mode of action of pore forming polypeptides. Biochimica Et Biophysica Acta-Reviews on Biomembranes. 1998;1376:391–400. doi: 10.1016/s0304-4157(98)00014-8. [DOI] [PubMed] [Google Scholar]
- Moser R. Design, synthesis and structure of an amphipathic peptide with ph-inducible hemolytic-activity. Protein Engineering. 1992;5:323–331. doi: 10.1093/protein/5.4.323. [DOI] [PubMed] [Google Scholar]
- Naik A, Pechtold L, Potts RO, Guy RH. Mechanism of oleic acid-induced skin penetration enhancement in vivo in humans. Journal of Controlled Release. 1995;37:299–306. [Google Scholar]
- Naik A, Guy RH. Infrared spectroscopic and differential scanning calorimetric investigations of stratum corneum barrier function. Marcel Dekker; New York: 1997. [Google Scholar]
- Oliver JD, Anderson S, Troy JL, Brenner BM, Deen WM. Determination of glomerular size-selectivity in the normal rat with ficoll. Journal of the American Society of Nephrology. 1992;3:214–228. doi: 10.1681/ASN.V32214. [DOI] [PubMed] [Google Scholar]
- Prausnitz MR, Noonan JS. Permeability of cornea, sclera, and conjunctiva: A literature analysis for drug delivery to the eye. Journal of Pharmaceutical Sciences. 1998;87:1479–1488. doi: 10.1021/js9802594. [DOI] [PubMed] [Google Scholar]
- ProStrakan Group plc. ProStrakan announces positive results from pivotal Phase 3 study on SancusoTM (transdermal granisetron patch) 2007 December 17; http://www.prostrakan.com/uploads/RapinylUSTrialInterimResultsPressRelease171207.pdf.
- Rothbard JB, Garlington S, Lin Q, Kirschberg T, Kreider E, McGrane PL, Wender PA, Khavari PA. Conjugation of arginine oligomers to cyclosporin a facilitates topical delivery and inhibition of inflammation. Nature Medicine. 2000;6:1253–1257. doi: 10.1038/81359. [DOI] [PubMed] [Google Scholar]
- Shai Y, Hadari YR, Finkels A. Ph-dependent pore formation properties of pardaxin analogs. Journal of Biological Chemistry. 1991;266:22346–22354. [PubMed] [Google Scholar]
- Singh BN, Singh RB, Singh J. Effects of ionization and penetration enhancers on the transdermal delivery of 5-fluorouracil through excised human stratum corneum. International Journal of Pharmaceutics. 2005;298:98–107. doi: 10.1016/j.ijpharm.2005.04.004. [DOI] [PubMed] [Google Scholar]
- Skoog B, Wichman A. Calculation of the isoelectric points of polypeptides from the amino-acid-composition. Trac-Trends in Analytical Chemistry. 1986;5:82–83. [Google Scholar]
- Stogryn A. Equations for calculating dielectric constant of saline water. IEEE Transactions on Microwave Theory and Techniques. 1971:733–736. [Google Scholar]
- Subczynski WK, Wisniewska A, Kusumi A, McElhaney RN. Effects of ph-induced variations of the charge of the transmembrane alpha-helical peptide ac-k-2(la)(12)k-2-amide on the organization and dynamics of the host dimyristoylphosphatidylcholine bilayer membrane. Biochimica Et Biophysica Acta - Biomembranes. 2005;1720:99–109. doi: 10.1016/j.bbamem.2005.11.014. [DOI] [PubMed] [Google Scholar]
- Sznitowska M, Janicki S, Baczek A. Studies on the effect of ph on the lipoidal route of penetration across stratum corneum. Journal of Controlled Release. 2001;76:327–335. doi: 10.1016/s0168-3659(01)00443-6. [DOI] [PubMed] [Google Scholar]
- Williams AC, Barry BW. Penetration enhancers. Advanced Drug Delivery Reviews. 2004;56:603–618. doi: 10.1016/j.addr.2003.10.025. [DOI] [PubMed] [Google Scholar]
- Yoshida N, Tamura M, Kinjo M. Fluorescence correlation spectroscopy: A new tool for probing the microenvironment of the internal space of organelles. Single Molecules. 2000;1:279–283. [Google Scholar]
- Zasloff M. Magainins, a class of antimicrobial peptides from xenopus skin-isolation, characterization of 2 active forms, and partial cdna sequence of a precursor. Proceedings of the National Academy of Sciences of the United States of America. 1987;84:5449–5453. doi: 10.1073/pnas.84.15.5449. [DOI] [PMC free article] [PubMed] [Google Scholar]