Abstract
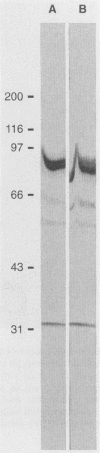
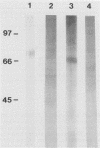
An extract of the cell wall and cell membrane from Histoplasma capsulatum yeast cells was assayed by Western blot (immunoblot) for reactivity with two monoclonal antibodies to heat shock protein 70. Four bands with molecular masses of 80, 66, 54, and 32 kDa bound both antibodies. The 80-kDa protein was isolated, analyzed for homology to heat shock protein 70, and tested for antigenicity and immunogenicity in C57BL/6 mice. The 80-kDa protein reacted with monoclonal antibody to heat shock protein 70. Sera from mice immunized with the antigen recognized H. capsulatum heat shock protein 70. Moreover, the amino-terminal sequence of the 80-kDa protein revealed substantial homology with heat shock protein 70 from several species. The 80-kDa protein induced delayed-type hypersensitivity responses in mice immunized with either viable yeast cells or antigen. Splenocytes from mice immunized with yeast cells or with antigen responded in vitro to the 80-kDa antigen. Immunization of mice with the antigen enhanced host resistance against a sublethal inoculum of H. capsulatum yeast cells, but it did not reduce the mortality of mice given a lethal challenge of yeast cells. Thus, this antigen manifests homology with members of the heat shock protein 70 family. Furthermore, the 80-kDa protein elicits cellular immune responses to H. capsulatum, and it mediates protective immunity.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ardeshir F., Flint J. E., Richman S. J., Reese R. T. A 75 kd merozoite surface protein of Plasmodium falciparum which is related to the 70 kd heat-shock proteins. EMBO J. 1987 Feb;6(2):493–499. doi: 10.1002/j.1460-2075.1987.tb04780.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianco A. E., Favaloro J. M., Burkot T. R., Culvenor J. G., Crewther P. E., Brown G. V., Anders R. F., Coppel R. L., Kemp D. J. A repetitive antigen of Plasmodium falciparum that is homologous to heat shock protein 70 of Drosophila melanogaster. Proc Natl Acad Sci U S A. 1986 Nov;83(22):8713–8717. doi: 10.1073/pnas.83.22.8713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Britton W. J., Hellqvist L., Basten A., Inglis A. S. Immunoreactivity of a 70 kD protein purified from Mycobacterium bovis Bacillus Calmette-Guerin by monoclonal antibody affinity chromatography. J Exp Med. 1986 Sep 1;164(3):695–708. doi: 10.1084/jem.164.3.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caruso M., Sacco M., Medoff G., Maresca B. Heat shock 70 gene is differentially expressed in Histoplasma capsulatum strains with different levels of thermotolerance and pathogenicity. Mol Microbiol. 1987 Sep;1(2):151–158. doi: 10.1111/j.1365-2958.1987.tb00507.x. [DOI] [PubMed] [Google Scholar]
- Chappell T. G., Welch W. J., Schlossman D. M., Palter K. B., Schlesinger M. J., Rothman J. E. Uncoating ATPase is a member of the 70 kilodalton family of stress proteins. Cell. 1986 Apr 11;45(1):3–13. doi: 10.1016/0092-8674(86)90532-5. [DOI] [PubMed] [Google Scholar]
- Danilition S. L., Maclean I. W., Peeling R., Winston S., Brunham R. C. The 75-kilodalton protein of Chlamydia trachomatis: a member of the heat shock protein 70 family? Infect Immun. 1990 Jan;58(1):189–196. doi: 10.1128/iai.58.1.189-196.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshaies R. J., Koch B. D., Werner-Washburne M., Craig E. A., Schekman R. A subfamily of stress proteins facilitates translocation of secretory and mitochondrial precursor polypeptides. Nature. 1988 Apr 28;332(6167):800–805. doi: 10.1038/332800a0. [DOI] [PubMed] [Google Scholar]
- Domer J. E. In vivo and in virto cellular responses to cytoplasmic and cell wall antigens of Histoplasma capsulatum in artificially immunized or infected guinea pigs. Infect Immun. 1976 Mar;13(3):790–799. doi: 10.1128/iai.13.3.790-799.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finley D., Ciechanover A., Varshavsky A. Thermolability of ubiquitin-activating enzyme from the mammalian cell cycle mutant ts85. Cell. 1984 May;37(1):43–55. doi: 10.1016/0092-8674(84)90299-x. [DOI] [PubMed] [Google Scholar]
- Garcia J. P., Howard D. H. Characterization of antigens from the yeast phase of Histoplasma capsulatum. Infect Immun. 1971 Aug;4(2):116–125. doi: 10.1128/iai.4.2.116-125.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez F. J., Gomez A. M., Deepe G. S., Jr Protective efficacy of a 62-kilodalton antigen, HIS-62, from the cell wall and cell membrane of Histoplasma capsulatum yeast cells. Infect Immun. 1991 Dec;59(12):4459–4464. doi: 10.1128/iai.59.12.4459-4464.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gómez A. M., Rhodes J. C., Deepe G. S., Jr Antigenicity and immunogenicity of an extract from the cell wall and cell membrane of Histoplasma capsulatum yeast cells. Infect Immun. 1991 Jan;59(1):330–336. doi: 10.1128/iai.59.1.330-336.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedstrom R., Culpepper J., Harrison R. A., Agabian N., Newport G. A major immunogen in Schistosoma mansoni infections is homologous to the heat-shock protein Hsp70. J Exp Med. 1987 May 1;165(5):1430–1435. doi: 10.1084/jem.165.5.1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunkapiller M. W., Lujan E., Ostrander F., Hood L. E. Isolation of microgram quantities of proteins from polyacrylamide gels for amino acid sequence analysis. Methods Enzymol. 1983;91:227–236. doi: 10.1016/s0076-6879(83)91019-4. [DOI] [PubMed] [Google Scholar]
- Konigsberg W. H., Henderson L. Removal of sodium dodecyl sulfate from proteins by ion-pair extraction. Methods Enzymol. 1983;91:254–259. doi: 10.1016/s0076-6879(83)91022-4. [DOI] [PubMed] [Google Scholar]
- Kurtz S., Rossi J., Petko L., Lindquist S. An ancient developmental induction: heat-shock proteins induced in sporulation and oogenesis. Science. 1986 Mar 7;231(4742):1154–1157. doi: 10.1126/science.3511530. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lindquist S., Craig E. A. The heat-shock proteins. Annu Rev Genet. 1988;22:631–677. doi: 10.1146/annurev.ge.22.120188.003215. [DOI] [PubMed] [Google Scholar]
- Lindquist S. The heat-shock response. Annu Rev Biochem. 1986;55:1151–1191. doi: 10.1146/annurev.bi.55.070186.005443. [DOI] [PubMed] [Google Scholar]
- Matsudaira P. Sequence from picomole quantities of proteins electroblotted onto polyvinylidene difluoride membranes. J Biol Chem. 1987 Jul 25;262(21):10035–10038. [PubMed] [Google Scholar]
- Pelham H. R. Speculations on the functions of the major heat shock and glucose-regulated proteins. Cell. 1986 Sep 26;46(7):959–961. doi: 10.1016/0092-8674(86)90693-8. [DOI] [PubMed] [Google Scholar]
- Peto R., Pike M. C., Armitage P., Breslow N. E., Cox D. R., Howard S. V., Mantel N., McPherson K., Peto J., Smith P. G. Design and analysis of randomized clinical trials requiring prolonged observation of each patient. II. analysis and examples. Br J Cancer. 1977 Jan;35(1):1–39. doi: 10.1038/bjc.1977.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rees A., Scoging A., Mehlert A., Young D. B., Ivanyi J. Specificity of proliferative response of human CD8 clones to mycobacterial antigens. Eur J Immunol. 1988 Dec;18(12):1881–1887. doi: 10.1002/eji.1830181203. [DOI] [PubMed] [Google Scholar]
- Selkirk M. E., Denham D. A., Partono F., Maizels R. M. Heat shock cognate 70 is a prominent immunogen in Brugian filariasis. J Immunol. 1989 Jul 1;143(1):299–308. [PubMed] [Google Scholar]
- Shearer G., Jr, Birge C. H., Yuckenberg P. D., Kobayashi G. S., Medoff G. Heat-shock proteins induced during the mycelial-to-yeast transitions of strains of Histoplasma capsulatum. J Gen Microbiol. 1987 Dec;133(12):3375–3382. doi: 10.1099/00221287-133-12-3375. [DOI] [PubMed] [Google Scholar]
- Tsang V. C., Hancock K., Maddison S. E., Beatty A. L., Moss D. M. Demonstration of species-specific and cross-reactive components of the adult microsomal antigens from Schistosoma mansoni and S. japonicum (MAMA and JAMA). J Immunol. 1984 May;132(5):2607–2613. [PubMed] [Google Scholar]
- Vanbuskirk A., Crump B. L., Margoliash E., Pierce S. K. A peptide binding protein having a role in antigen presentation is a member of the HSP70 heat shock family. J Exp Med. 1989 Dec 1;170(6):1799–1809. doi: 10.1084/jem.170.6.1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vass K., Welch W. J., Nowak T. S., Jr Localization of 70-kDa stress protein induction in gerbil brain after ischemia. Acta Neuropathol. 1988;77(2):128–135. doi: 10.1007/BF00687422. [DOI] [PubMed] [Google Scholar]
- Velazquez J. M., Lindquist S. hsp70: nuclear concentration during environmental stress and cytoplasmic storage during recovery. Cell. 1984 Mar;36(3):655–662. doi: 10.1016/0092-8674(84)90345-3. [DOI] [PubMed] [Google Scholar]
- Welch W. J., Feramisco J. R. Rapid purification of mammalian 70,000-dalton stress proteins: affinity of the proteins for nucleotides. Mol Cell Biol. 1985 Jun;5(6):1229–1237. doi: 10.1128/mcb.5.6.1229. [DOI] [PMC free article] [PubMed] [Google Scholar]