Abstract
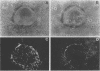
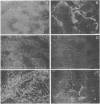
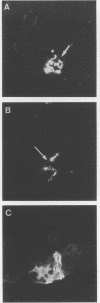
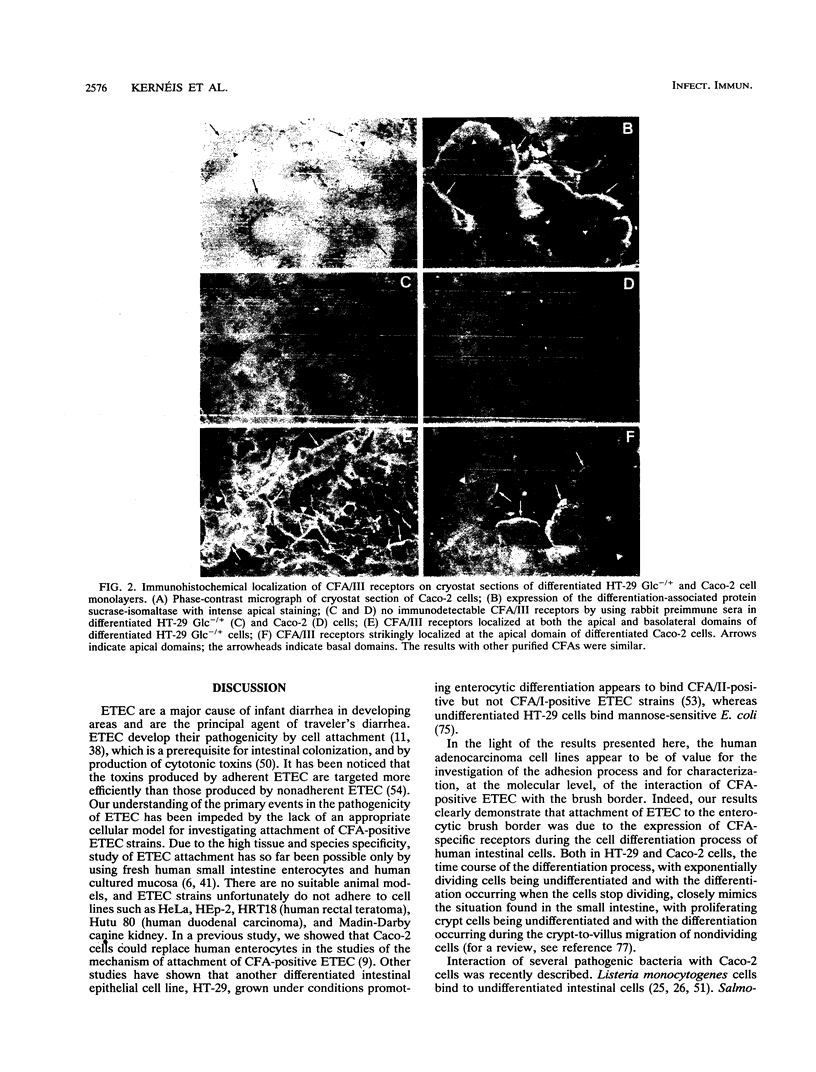
To study the expression of human intestinal receptors for enterotoxigenic Escherichia coli (ETEC), the human polarized intestinal epithelial cell line Caco-2 in culture and several subpopulations of HT-29 cells in culture--parental (mainly undifferentiated) HT-29 cells (HT-29 Std), an enterocytelike subpopulation obtained by selection through glucose deprivation (HT-29 Glc-), and an enterocytelike subpopulation obtained by selection through glucose deprivation which maintains its differentiation characteristics when switched back to standard glucose-containing medium (HT-29 Glc-/+)--were used. Since Caco-2 spontaneously differentiated in culture under standard culture conditions (in the presence of glucose) and HT-29 cells were undifferentiated when cultured under standard conditions (HT-29 Std) and differentiated when grown in a glucose-free medium (HT-29 Glc-), we studied the expression of the receptors for colonization factor antigens (CFA) I, II, and III and the 2230 antigen of ETEC in relation to enterocytic differentiation. We provide evidence that expression of ETEC CFA receptors develops in parallel with other differentiation functions of the cultured cells. The expression of ETEC-specific brush border receptors was studied by indirect immunofluorescence using antibodies raised against purified ETEC CFA. No ETEC receptors were detected in HT-29 Std or short-term-cultured Caco-2 cells. However, among the population of HT-29 Std cells, 2 to 4% of the cells were found to bind ETEC, and these cells expressed positive carcinoembryonic antigen immunoreactivity. This indicated that among the population of undifferentiated HT-29 cells, clusters of differentiated cells were present. ETEC CFA receptors were expressed in the apical and basolateral domains of differentiated HT-29 cells, whereas in differentiated Caco-2 cells only apical expression was observed. Both in HT-29 cells (HT-29 Glc-/+) and in Caco-2 cells cultured under standard conditions, ETEC CFA receptors develop as a function of day in culture. This indicated that the expression of the ETEC CFA receptors was a growth-related event. Indeed, ETEC CFA receptors developed in step with the apical expression of differentiation-associated proteins.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ambler L., Mackay M. Reovirus 1 and 3 bind and internalise at the apical surface of intestinal epithelial cells. Virology. 1991 Sep;184(1):162–169. doi: 10.1016/0042-6822(91)90832-v. [DOI] [PubMed] [Google Scholar]
- Anderson J. M., Van Itallie C. M., Peterson M. D., Stevenson B. R., Carew E. A., Mooseker M. S. ZO-1 mRNA and protein expression during tight junction assembly in Caco-2 cells. J Cell Biol. 1989 Sep;109(3):1047–1056. doi: 10.1083/jcb.109.3.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aubel D., Darfeuille-Michaud A., Joly B. New adhesive factor (antigen 8786) on a human enterotoxigenic Escherichia coli O117:H4 strain isolated in Africa. Infect Immun. 1991 Apr;59(4):1290–1299. doi: 10.1128/iai.59.4.1290-1299.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Augeron C., Laboisse C. L. Emergence of permanently differentiated cell clones in a human colonic cancer cell line in culture after treatment with sodium butyrate. Cancer Res. 1984 Sep;44(9):3961–3969. [PubMed] [Google Scholar]
- Buraud M., Forget E., Favennec L., Bizet J., Gobert J. G., Deluol A. M. Sexual stage development of cryptosporidia in the Caco-2 cell line. Infect Immun. 1991 Dec;59(12):4610–4613. doi: 10.1128/iai.59.12.4610-4613.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheney C. P., Boedeker E. C. Adherence of an enterotoxigenic Escherichia coli strain, serotype O78:H11, to purified human intestinal brush borders. Infect Immun. 1983 Mar;39(3):1280–1284. doi: 10.1128/iai.39.3.1280-1284.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Citi S., Sabanay H., Jakes R., Geiger B., Kendrick-Jones J. Cingulin, a new peripheral component of tight junctions. Nature. 1988 May 19;333(6170):272–276. doi: 10.1038/333272a0. [DOI] [PubMed] [Google Scholar]
- Darfeuille-Michaud A., Aubel D., Chauviere G., Rich C., Bourges M., Servin A., Joly B. Adhesion of enterotoxigenic Escherichia coli to the human colon carcinoma cell line Caco-2 in culture. Infect Immun. 1990 Apr;58(4):893–902. doi: 10.1128/iai.58.4.893-902.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darfeuille-Michaud A., Forestier C., Joly B., Cluzel R. Identification of a nonfimbrial adhesive factor of an enterotoxigenic Escherichia coli strain. Infect Immun. 1986 May;52(2):468–475. doi: 10.1128/iai.52.2.468-475.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darfeuille A., Lafeuille B., Joly B., Cluzel R. A new colonization factor antigen (CFA/III) produced by enteropathogenic Escherichia coli O128:B12. Ann Microbiol (Paris) 1983 Jan-Feb;134A(1):53–64. doi: 10.1016/0769-2609(83)90103-5. [DOI] [PubMed] [Google Scholar]
- De Graaf F. K., Mooi F. R. The fimbrial adhesins of Escherichia coli. Adv Microb Physiol. 1986;28:65–143. doi: 10.1016/s0065-2911(08)60237-4. [DOI] [PubMed] [Google Scholar]
- Elsinghorst E. A., Baron L. S., Kopecko D. J. Penetration of human intestinal epithelial cells by Salmonella: molecular cloning and expression of Salmonella typhi invasion determinants in Escherichia coli. Proc Natl Acad Sci U S A. 1989 Jul;86(13):5173–5177. doi: 10.1073/pnas.86.13.5173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans D. G., Evans D. J., Jr New surface-associated heat-labile colonization factor antigen (CFA/II) produced by enterotoxigenic Escherichia coli of serogroups O6 and O8. Infect Immun. 1978 Aug;21(2):638–647. doi: 10.1128/iai.21.2.638-647.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans D. G., Silver R. P., Evans D. J., Jr, Chase D. G., Gorbach S. L. Plasmid-controlled colonization factor associated with virulence in Esherichia coli enterotoxigenic for humans. Infect Immun. 1975 Sep;12(3):656–667. doi: 10.1128/iai.12.3.656-667.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falkow S. Bacterial entry into eukaryotic cells. Cell. 1991 Jun 28;65(7):1099–1102. doi: 10.1016/0092-8674(91)90003-h. [DOI] [PubMed] [Google Scholar]
- Finlay B. B., Falkow S. Common themes in microbial pathogenicity. Microbiol Rev. 1989 Jun;53(2):210–230. doi: 10.1128/mr.53.2.210-230.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finlay B. B., Falkow S. Salmonella interactions with polarized human intestinal Caco-2 epithelial cells. J Infect Dis. 1990 Nov;162(5):1096–1106. doi: 10.1093/infdis/162.5.1096. [DOI] [PubMed] [Google Scholar]
- Finlay B. B., Gumbiner B., Falkow S. Penetration of Salmonella through a polarized Madin-Darby canine kidney epithelial cell monolayer. J Cell Biol. 1988 Jul;107(1):221–230. doi: 10.1083/jcb.107.1.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flanigan T. P., Aji T., Marshall R., Soave R., Aikawa M., Kaetzel C. Asexual development of Cryptosporidium parvum within a differentiated human enterocyte cell line. Infect Immun. 1991 Jan;59(1):234–239. doi: 10.1128/iai.59.1.234-239.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fogh J., Fogh J. M., Orfeo T. One hundred and twenty-seven cultured human tumor cell lines producing tumors in nude mice. J Natl Cancer Inst. 1977 Jul;59(1):221–226. doi: 10.1093/jnci/59.1.221. [DOI] [PubMed] [Google Scholar]
- Francis C. L., Jerse A. E., Kaper J. B., Falkow S. Characterization of interactions of enteropathogenic Escherichia coli O127:H6 with mammalian cells in vitro. J Infect Dis. 1991 Oct;164(4):693–703. doi: 10.1093/infdis/164.4.693. [DOI] [PubMed] [Google Scholar]
- Gahring L. C., Heffron F., Finlay B. B., Falkow S. Invasion and replication of Salmonella typhimurium in animal cells. Infect Immun. 1990 Feb;58(2):443–448. doi: 10.1128/iai.58.2.443-448.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaillard J. L., Berche P., Frehel C., Gouin E., Cossart P. Entry of L. monocytogenes into cells is mediated by internalin, a repeat protein reminiscent of surface antigens from gram-positive cocci. Cell. 1991 Jun 28;65(7):1127–1141. doi: 10.1016/0092-8674(91)90009-n. [DOI] [PubMed] [Google Scholar]
- Gaillard J. L., Berche P., Mounier J., Richard S., Sansonetti P. In vitro model of penetration and intracellular growth of Listeria monocytogenes in the human enterocyte-like cell line Caco-2. Infect Immun. 1987 Nov;55(11):2822–2829. doi: 10.1128/iai.55.11.2822-2829.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert T., Le Bivic A., Quaroni A., Rodriguez-Boulan E. Microtubular organization and its involvement in the biogenetic pathways of plasma membrane proteins in Caco-2 intestinal epithelial cells. J Cell Biol. 1991 Apr;113(2):275–288. doi: 10.1083/jcb.113.2.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauri H. P. Biogenesis and intracellular transport of intestinal brush border membrane hydrolases. Use of antibody probes and tissue culture. Subcell Biochem. 1988;12:155–219. doi: 10.1007/978-1-4899-1681-5_5. [DOI] [PubMed] [Google Scholar]
- Hauri H. P., Sterchi E. E., Bienz D., Fransen J. A., Marxer A. Expression and intracellular transport of microvillus membrane hydrolases in human intestinal epithelial cells. J Cell Biol. 1985 Sep;101(3):838–851. doi: 10.1083/jcb.101.3.838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huet C., Sahuquillo-Merino C., Coudrier E., Louvard D. Absorptive and mucus-secreting subclones isolated from a multipotent intestinal cell line (HT-29) provide new models for cell polarity and terminal differentiation. J Cell Biol. 1987 Jul;105(1):345–357. doi: 10.1083/jcb.105.1.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isberg R. R. Discrimination between intracellular uptake and surface adhesion of bacterial pathogens. Science. 1991 May 17;252(5008):934–938. doi: 10.1126/science.1674624. [DOI] [PubMed] [Google Scholar]
- Isberg R. R., Leong J. M. Multiple beta 1 chain integrins are receptors for invasin, a protein that promotes bacterial penetration into mammalian cells. Cell. 1990 Mar 9;60(5):861–871. doi: 10.1016/0092-8674(90)90099-z. [DOI] [PubMed] [Google Scholar]
- Isberg R. R., Voorhis D. L., Falkow S. Identification of invasin: a protein that allows enteric bacteria to penetrate cultured mammalian cells. Cell. 1987 Aug 28;50(5):769–778. doi: 10.1016/0092-8674(87)90335-7. [DOI] [PubMed] [Google Scholar]
- Jerse A. E., Gicquelais K. G., Kaper J. B. Plasmid and chromosomal elements involved in the pathogenesis of attaching and effacing Escherichia coli. Infect Immun. 1991 Nov;59(11):3869–3875. doi: 10.1128/iai.59.11.3869-3875.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerse A. E., Yu J., Tall B. D., Kaper J. B. A genetic locus of enteropathogenic Escherichia coli necessary for the production of attaching and effacing lesions on tissue culture cells. Proc Natl Acad Sci U S A. 1990 Oct;87(20):7839–7843. doi: 10.1073/pnas.87.20.7839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerneis S., Bilge S. S., Fourel V., Chauviere G., Coconnier M. H., Servin A. L. Use of purified F1845 fimbrial adhesin to study localization and expression of receptors for diffusely adhering Escherichia coli during enterocytic differentiation of human colon carcinoma cell lines HT-29 and Caco-2 in culture. Infect Immun. 1991 Nov;59(11):4013–4018. doi: 10.1128/iai.59.11.4013-4018.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutton S., Baldwin T., Williams P. H., McNeish A. S. Actin accumulation at sites of bacterial adhesion to tissue culture cells: basis of a new diagnostic test for enteropathogenic and enterohemorrhagic Escherichia coli. Infect Immun. 1989 Apr;57(4):1290–1298. doi: 10.1128/iai.57.4.1290-1298.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krogfelt K. A. Bacterial adhesion: genetics, biogenesis, and role in pathogenesis of fimbrial adhesins of Escherichia coli. Rev Infect Dis. 1991 Jul-Aug;13(4):721–735. doi: 10.1093/clinids/13.4.721. [DOI] [PubMed] [Google Scholar]
- Kuhn M., Prévost M. C., Mounier J., Sansonetti P. J. A nonvirulent mutant of Listeria monocytogenes does not move intracellularly but still induces polymerization of actin. Infect Immun. 1990 Nov;58(11):3477–3486. doi: 10.1128/iai.58.11.3477-3486.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lafeuille B., Darfeuille A., Petit S., Joly B., Cluzel R. Etude de l'attachement aux entérocytes humains in vitro de Escherichia coli isolés de selles diarrhéiques chez l'enfant. Ann Microbiol (Paris) 1981 Jul-Aug;132B(1):57–67. [PubMed] [Google Scholar]
- Le Bivic A., Real F. X., Rodriguez-Boulan E. Vectorial targeting of apical and basolateral plasma membrane proteins in a human adenocarcinoma epithelial cell line. Proc Natl Acad Sci U S A. 1989 Dec;86(23):9313–9317. doi: 10.1073/pnas.86.23.9313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesuffleur T., Barbat A., Dussaulx E., Zweibaum A. Growth adaptation to methotrexate of HT-29 human colon carcinoma cells is associated with their ability to differentiate into columnar absorptive and mucus-secreting cells. Cancer Res. 1990 Oct 1;50(19):6334–6343. [PubMed] [Google Scholar]
- Lesuffleur T., Kornowski A., Augeron C., Dussaulx E., Barbat A., Laboisse C., Zweibaum A. Increased growth adaptability to 5-fluorouracil and methotrexate of HT-29 sub-populations selected for their commitment to differentiation. Int J Cancer. 1991 Nov 11;49(5):731–737. doi: 10.1002/ijc.2910490517. [DOI] [PubMed] [Google Scholar]
- Leusch H. G., Drzeniek Z., Markos-Pusztai Z., Wagener C. Binding of Escherichia coli and Salmonella strains to members of the carcinoembryonic antigen family: differential binding inhibition by aromatic alpha-glycosides of mannose. Infect Immun. 1991 Jun;59(6):2051–2057. doi: 10.1128/iai.59.6.2051-2057.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathewson J. J., Cravioto A. HEp-2 cell adherence as an assay for virulence among diarrheagenic Escherichia coli. J Infect Dis. 1989 Jun;159(6):1057–1060. doi: 10.1093/infdis/159.6.1057. [DOI] [PubMed] [Google Scholar]
- Matter K., Brauchbar M., Bucher K., Hauri H. P. Sorting of endogenous plasma membrane proteins occurs from two sites in cultured human intestinal epithelial cells (Caco-2). Cell. 1990 Feb 9;60(3):429–437. doi: 10.1016/0092-8674(90)90594-5. [DOI] [PubMed] [Google Scholar]
- Matter K., Bucher K., Hauri H. P. Microtubule perturbation retards both the direct and the indirect apical pathway but does not affect sorting of plasma membrane proteins in intestinal epithelial cells (Caco-2). EMBO J. 1990 Oct;9(10):3163–3170. doi: 10.1002/j.1460-2075.1990.tb07514.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurelli A. T., Sansonetti P. J. Genetic determinants of Shigella pathogenicity. Annu Rev Microbiol. 1988;42:127–150. doi: 10.1146/annurev.mi.42.100188.001015. [DOI] [PubMed] [Google Scholar]
- Middlebrook J. L., Dorland R. B. Bacterial toxins: cellular mechanisms of action. Microbiol Rev. 1984 Sep;48(3):199–221. doi: 10.1128/mr.48.3.199-221.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mounier J., Ryter A., Coquis-Rondon M., Sansonetti P. J. Intracellular and cell-to-cell spread of Listeria monocytogenes involves interaction with F-actin in the enterocytelike cell line Caco-2. Infect Immun. 1990 Apr;58(4):1048–1058. doi: 10.1128/iai.58.4.1048-1058.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mounier J., Vasselon T., Hellio R., Lesourd M., Sansonetti P. J. Shigella flexneri enters human colonic Caco-2 epithelial cells through the basolateral pole. Infect Immun. 1992 Jan;60(1):237–248. doi: 10.1128/iai.60.1.237-248.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neeser J. R., Chambaz A., Golliard M., Link-Amster H., Fryder V., Kolodziejczyk E. Adhesion of colonization factor antigen II-positive enterotoxigenic Escherichia coli strains to human enterocytelike differentiated HT-29 cells: a basis for host-pathogen interactions in the gut. Infect Immun. 1989 Dec;57(12):3727–3734. doi: 10.1128/iai.57.12.3727-3734.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ofek I., Zafriri D., Goldhar J., Eisenstein B. I. Inability of toxin inhibitors to neutralize enhanced toxicity caused by bacteria adherent to tissue culture cells. Infect Immun. 1990 Nov;58(11):3737–3742. doi: 10.1128/iai.58.11.3737-3742.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogier-Denis E., Codogno P., Chantret I., Trugnan G. The processing of asparagine-linked oligosaccharides in HT-29 cells is a function of their state of enterocytic differentiation. An accumulation of Man9,8-GlcNAc2-Asn species is indicative of an impaired N-glycan trimming in undifferentiated cells. J Biol Chem. 1988 May 5;263(13):6031–6037. [PubMed] [Google Scholar]
- Panigrahi P., Tall B. D., Russell R. G., Detolla L. J., Morris J. G., Jr Development of an in vitro model for study of non-O1 Vibrio cholerae virulence using Caco-2 cells. Infect Immun. 1990 Oct;58(10):3415–3424. doi: 10.1128/iai.58.10.3415-3424.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rafiee P., Leffler H., Byrd J. C., Cassels F. J., Boedeker E. C., Kim Y. S. A sialoglycoprotein complex linked to the microvillus cytoskeleton acts as a receptor for pilus (AF/R1) mediated adhesion of enteropathogenic Escherichia coli (RDEC-1) in rabbit small intestine. J Cell Biol. 1991 Nov;115(4):1021–1029. doi: 10.1083/jcb.115.4.1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Relman D., Tuomanen E., Falkow S., Golenbock D. T., Saukkonen K., Wright S. D. Recognition of a bacterial adhesion by an integrin: macrophage CR3 (alpha M beta 2, CD11b/CD18) binds filamentous hemagglutinin of Bordetella pertussis. Cell. 1990 Jun 29;61(7):1375–1382. doi: 10.1016/0092-8674(90)90701-f. [DOI] [PubMed] [Google Scholar]
- Rigothier M. C., Coconnier M. H., Servin A. L., Gayral P. A new in vitro model of Entamoeba histolytica adhesion, using the human colon carcinoma cell line Caco-2: scanning electron microscopic study. Infect Immun. 1991 Nov;59(11):4142–4146. doi: 10.1128/iai.59.11.4142-4146.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rousset M., Chantret I., Darmoul D., Trugnan G., Sapin C., Green F., Swallow D., Zweibaum A. Reversible forskolin-induced impairment of sucrase-isomaltase mRNA levels, biosynthesis, and transport to the brush border membrane in Caco-2 cells. J Cell Physiol. 1989 Dec;141(3):627–635. doi: 10.1002/jcp.1041410322. [DOI] [PubMed] [Google Scholar]
- Rousset M., Laburthe M., Pinto M., Chevalier G., Rouyer-Fessard C., Dussaulx E., Trugnan G., Boige N., Brun J. L., Zweibaum A. Enterocytic differentiation and glucose utilization in the human colon tumor cell line Caco-2: modulation by forskolin. J Cell Physiol. 1985 Jun;123(3):377–385. doi: 10.1002/jcp.1041230313. [DOI] [PubMed] [Google Scholar]
- Semenza G. Anchoring and biosynthesis of stalked brush border membrane proteins: glycosidases and peptidases of enterocytes and renal tubuli. Annu Rev Cell Biol. 1986;2:255–313. doi: 10.1146/annurev.cb.02.110186.001351. [DOI] [PubMed] [Google Scholar]
- Simons K., Fuller S. D. Cell surface polarity in epithelia. Annu Rev Cell Biol. 1985;1:243–288. doi: 10.1146/annurev.cb.01.110185.001331. [DOI] [PubMed] [Google Scholar]
- Stieger B., Matter K., Baur B., Bucher K., Höchli M., Hauri H. P. Dissection of the asynchronous transport of intestinal microvillar hydrolases to the cell surface. J Cell Biol. 1988 Jun;106(6):1853–1861. doi: 10.1083/jcb.106.6.1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svensson L., Finlay B. B., Bass D., von Bonsdorff C. H., Greenberg H. B. Symmetric infection of rotavirus on polarized human intestinal epithelial (Caco-2) cells. J Virol. 1991 Aug;65(8):4190–4197. doi: 10.1128/jvi.65.8.4190-4197.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasselon T., Mounier J., Hellio R., Sansonetti P. J. Movement along actin filaments of the perijunctional area and de novo polymerization of cellular actin are required for Shigella flexneri colonization of epithelial Caco-2 cell monolayers. Infect Immun. 1992 Mar;60(3):1031–1040. doi: 10.1128/iai.60.3.1031-1040.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viallard V., Denis C., Trocheris V., Murat J. C. Effect of glutamine deprivation and glutamate or ammonium chloride addition on growth rate, metabolism and differentiation of human colon cancer cell-line HT29. Int J Biochem. 1986;18(3):263–269. doi: 10.1016/0020-711x(86)90116-3. [DOI] [PubMed] [Google Scholar]
- Wennerås C., Holmgren J., Svennerholm A. M. The binding of colonization factor antigens of enterotoxigenic Escherichia coli to intestinal cell membrane proteins. FEMS Microbiol Lett. 1990 Jan 1;54(1-3):107–112. doi: 10.1016/0378-1097(90)90266-s. [DOI] [PubMed] [Google Scholar]
- Wice B. M., Trugnan G., Pinto M., Rousset M., Chevalier G., Dussaulx E., Lacroix B., Zweibaum A. The intracellular accumulation of UDP-N-acetylhexosamines is concomitant with the inability of human colon cancer cells to differentiate. J Biol Chem. 1985 Jan 10;260(1):139–146. [PubMed] [Google Scholar]
- Willcocks M. M., Carter M. J., Laidler F. R., Madeley C. R. Growth and characterisation of human faecal astrovirus in a continuous cell line. Arch Virol. 1990;113(1-2):73–81. doi: 10.1007/BF01318354. [DOI] [PubMed] [Google Scholar]
- Wold A. E., Thorssén M., Hull S., Edén C. S. Attachment of Escherichia coli via mannose- or Gal alpha 1----4Gal beta-containing receptors to human colonic epithelial cells. Infect Immun. 1988 Oct;56(10):2531–2537. doi: 10.1128/iai.56.10.2531-2537.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young V. B., Falkow S., Schoolnik G. K. The invasin protein of Yersinia enterocolitica: internalization of invasin-bearing bacteria by eukaryotic cells is associated with reorganization of the cytoskeleton. J Cell Biol. 1992 Jan;116(1):197–207. doi: 10.1083/jcb.116.1.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zweibaum A., Pinto M., Chevalier G., Dussaulx E., Triadou N., Lacroix B., Haffen K., Brun J. L., Rousset M. Enterocytic differentiation of a subpopulation of the human colon tumor cell line HT-29 selected for growth in sugar-free medium and its inhibition by glucose. J Cell Physiol. 1985 Jan;122(1):21–29. doi: 10.1002/jcp.1041220105. [DOI] [PubMed] [Google Scholar]