Abstract
One of the major limitations of unrelated umbilical cord blood transplantation (UCBT) is the lack of donor cells available for post-transplant donor leukocyte infusions (DLI) to boost immunity or induce GVL. Starting from ~5% fraction of a UCB graft we report the feasibility and biological characteristics of ex vivo expansion of frozen/thawed cord blood T cells by anti-CD3 and anti-CD28 antibody-coated Dynal beads in the presence of IL-2. We postulated that while undergoing expansion, CB T cells may mature towards a Th1/Tc1 phenotype and acquire the potential for cytotoxicity. While an almost 2 log expansion also led to the acquisition of IL-12Rα and increase in Th1 characteristics, post-expansion lymphocytes produced less IFNγ, TNFα, Granzyme B, stored almost no perforin, and lacked cytotoxicity against allogeneic targets. Collectively, these suggest relative safety from acute/hyperacute GVHD. CD8+ T cells expanded preferentially while a higher rate of apoptosis among CD4+ T cells promoted an inverted CD4/CD8 ratio. Most expanded T cells retained expression of CD27, CD28, L-selectin, however, the majority down regulated CCR-7. In sum, CB T cell proliferation sustained by CD3/CD28 costimulatory beads and IL-2 can lead to clinically relevant doses of DLI from a very small fraction of the UCB graft, although future strategies to reduce apoptosis may enhance their clinical potential.
INTRODUCTION
Unrelated cord blood transplantation (UCBT) is a life-saving form of hematopoietic cell transplantation (HCT) for patients who lack HLA-matched sibling or living unrelated donors. However, UCBT is limited by high incidence of opportunistic infections (OI) within the first 100 days, most of which are viral. OI is the major cause of death during the first 6 months after transplant and is caused by delays in immune reconstitution, reviewed in [1]. Protective antiviral immunity resides primarily in the T cell compartment of the adaptive immune system. For several months post UCBT, until recovery of the thymus is restored to support de novo T cell generation, protective antiviral immunity depends on the activity of post-thymic T cells that are infused within the cord blood (CB) grafts. However, CB T cells are antigen inexperienced (naïve) lymphocytes that have been in utero biased by anti-inflammatory placental factors that are Th2 trophic and/or Th1 suppressive (IL-4, IL-10, TGFβ, prostaglandin E2, etc) [2] that together protect pregnancy, reviewed in [3]. CB T cells have been coined by some as ‘immature’ due to their impaired capacity for Th1/Tc1 cytokine production [4] and diminished cytolytic activity [5] compared to adult T cells, reviewed [6].
The relative cytolytic deficiency of CB T cells is associated with absent expression of Granzymes and Perforin [7], essential for eradicating viral pathogens. The exuberant production of the Th2 cytokine IL-13 by CD8+ CB T cells [8] is paired with poor IFNγ production by CD4+ CB T cells as a consequence of hypermethylation of the IFNγ promoter [9]. [2] Not surprisingly, cord blood T cells infused into transplant recipients need to undergo in vivo priming, maturation, and peripheral expansion before they can afford immunologic protection. By analyzing the reconstitution of the T cell and dendritic cell compartment within the first 50 days after UCBT we have previously demonstrated that lower infused T cell dose/kg and lower absolute numbers of CD4+ T cells in the circulation are independently associated with an increased risk for opportunistic infections (OI) and death related to OI [1].
Bulk donor leukocyte infusions (DLI) that contain antigens-specific memory cells have proven efficacy in the post-BMT setting to control and eradicate EBV-associated lymphomas and adenovirus infection [10, 11]. However, in the cord blood transplant setting there is no obvious available source for adoptive cell therapy strategies. Ex vivo T cell expansion from a small fraction of the HCT graft would be an ideal approach to generate T cells for DLI purposes. In vitro T cell expansion has been attainable by various methods but it has gained particular clinical relevance once artificial antigen-presenting cells (APC) became available. The clinical applicability of anti-CD3 plus anti-CD28 stimulatory antibody coated paramagnetic Dynal M-450 tosylactivated beads have been amply demonstrated [12–15]. These artificial APC beads provide simultaneous TCR-agonist and co-stimulating signals to trigger T cell activation and eventual blastogenesis [16]. Recently, CD3/CD28 beads were also shown to be capable to expand CB T cells up to 2 logs over the course of 12–17 days [17]. Bead-selected and expanded CB T cells expressed an almost even mixture of ‘naïve’, ‘central memory’, and ‘effector memory’ phenotype and retained the pre-expansion polyclonal TCR diversity, similar to what had been described by expanding adult T cells [17].
In this study we have evaluated the feasibility and biological consequences of ex vivo expansion of frozen/thawed cord blood T cells on anti-CD3 plus anti-CD28 antibody coated Dynal beads in the presence of interleukin 2 (IL-2) from a small (~5%) fraction of the UCB graft. We hypothesized, that by removing such small aliquot the infused 95% fraction’s engraftment potential will not be hampered and successful expansion from the 5% fraction could generate a clinically relevant cellular product. We also postulated that while undergoing expansion, CB T cells may also mature towards a Th1/Tc1 phenotype and such would acquire the potential for cytotoxic effector function as assessed by expression of essential cytokines and de novo expression of members of the granzyme-perforin pathway. Here we confirm the expansion potential of CB T cells and report that during expansion, CB T cells undergo partial maturation towards Th1 phenotype, though this is offset by preferential apoptosis among CD4+ T cells.
MATERIALS AND METHODS
Specimens
Collected umbilical cord blood (UCB) samples were obtained from frozen research units that were processed at the Duke University Stem Cell Laboratory and the Carolinas Cord Blood Bank at Duke University. These anonymous specimens were not eligible for clinical use due to insufficient volume.
T cell enrichment and expansion system
UBC samples with a leukocyte content <5% of an average UCB graft were thawed in 5% human albumin and 10% Dextran 40 in 0.9% sodium chloride solution and rested for 1 hour. Mononuclear cells (MNCs) were obtained by density gradient centrifugation over Ficoll (Pharmacia Fine Chemicals, Uppsala, Sweden) and washed. CD3+ T cells were enriched by negative immunomagnetic selection “EasySep T Cell Enrichment Cocktail” (StemCell Technology) depleting CD14, CD16, CD19, CD56, and glycophorin A positive cells following manufacturer’s instructions. By FACS analysis the purity of the negatively selected T cells was ~75–90%. Expansion was started at 6–8*105 T cells/ml density in gas permeable VueLife® Teflon bags (American Fluoroseal Corporation, Gaithesburg, MD). T cells were incubated with “CD3/28 T cell Expander” artificial antigen presenting cell beads (Dynal/Invitrogen Corp, Sammamish, WA,) at a ratio of 3:1 in X Vivo-15 (BioWhittacker, Walkersville, MD,) + 5.5 × 10−5M of BME, 10mM Hepes, 200u/ml IL-2 (Proleukin, Novartis, East Hanover, NJ) and 5% heat-inactivated pooled human AB serum (PHS) (NABI, Miami, FL). The starting absolute T cell numbers ranged from ~1–2 ×106 CD3+ T cells. Medium and cytokines were replenished × 3/week to maintain a concentration of ~ 1*106 cells/ml based on nucleated cell count, obtained by an automated hematology analyzer (Sysmex K-1000, GMI Inc., Ramsey, MN). After 12–14 days the cells were harvested, washed, and the beads were removed on a magnetic particle concentrator MPC-2 (Dynal). T cells were assessed for viability manually by Trypan Blue staining besides flow cytometric characterization to determine proliferation, apoptosis, changes affecting their surface and intracellular phenotype, homing receptors, and cytokine secretion.
Immunophenotypic characterization of the expanded T cells
Surface and intracellular (ic) T cell immunophenotyping and T cell enumeration was performed by 4-color FACS staining as previously described[18, 19]. One fluorescent channel (FL-3) was dedicated to gate only on CD3+ T cells while the FL-4 channel was dedicated for identifying subsets based on either CD4 or CD8 staining. Acquisition was performed on a FACSCalibur (BD Biosciences, San Jose, CA) and typically >5,000 CD3+ events in FL-3 were acquired to perform subset analysis. Lymphocyte subset specific surface markers were monitored in FL-1 and FL-2 fluorescent channels to analyze antigen expression over isotype controls, while intracellular cytokine production was monitored in FL-1, FL-2, and FL-4 channels. All antibodies including isotype-specific negative controls were purchased from BD Biosciences except anti-Granzyme B that was purchased from Serotec (Raleigh, NC), and anti-CCR-9 from R&D Systems (Minneapolis, MN)
Statistical Analysis
A 2-tailed t-test was employed for statistical comparisons between test and control conditions. Statistical significance was set at P values less than 0.05.
RESULTS
Differential T cell expansion and apoptosis among CD4+ and CD8+ cells
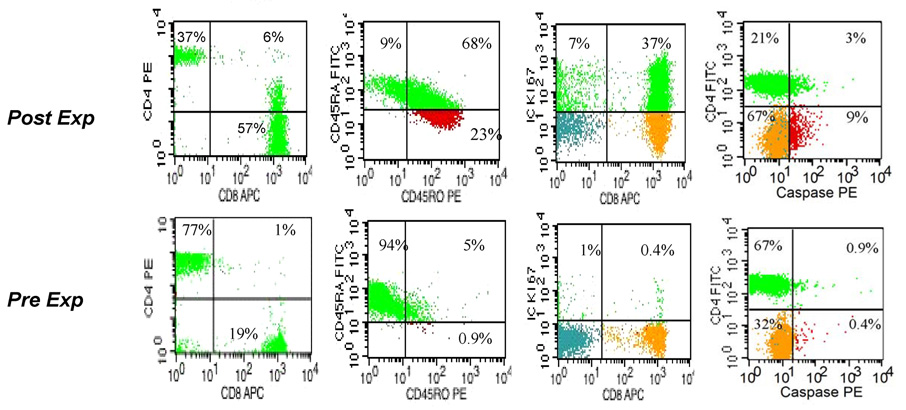
One of the major limitations of UCBT is the lack of donor cells available for post-transplant donor leukocyte infusions (DLI) to boost immunity or induce GVL. We have evaluated the feasibility of T cell expansion from thawed cord blood and analyzed the biological effects of T cell proliferation stimulated by CD3/CD28 costimulatory beads+ IL-2 in clinical grade gas permeable bags. At the end of 14 days the ex vivo cultured UCB T Cells expanded 76 fold ± 42, n=10, with up to 40% of T cells in cell cycle as measured by intracellular Ki-67 expression, Figure 1, Table 1. Expansion was inferior if starting cultures were impure, at least partially due to phagocytosis of the beads by monocytes/macrophages, data not shown. Significant evolution in phenotype was noted after the 2 weeks in culture, see Figure 1 and Table 1. Notably, expansion on CD3/28 APC beads in the presence of IL-2 led to inversion of the CD4/CD8 ratio, with a median frequency of 40% CD4+ T cells at day 14 compared with 74% at day 0 [18]. This relative CD8+ dominance is likely the result of two parallel processes. At the end of expansion a median of 46% of CD8+ T cells were still in cell cycle in contrast with 37% of CD4+ T cells as measured by intracellular expression of Ki-67, p=0.05, Table 1. The expansion advantage of CD8+ T cells was accompanied by a significantly higher rate of apoptosis among CD4+ T cells (p=0.002), as measured by the ic expression of activated caspase-3, see Table 1 and Figure 2. It is important to note that apoptosis was scored in T cells that otherwise deemed viable by their surface CD3 expression and “healthy” forward and side scatter flow cytometric characteristics. To better monitor cell death, we also monitored the fraction of viable CD3+ cells among all CD45+ cellular events detectable by 4-color flow cytometry. According to this FACS criteria, at the end of expansion, viable CD3+ cells represented 54% ± 16% of all CD45+ cellular events that is in contrast with assessment by Trypan Blue where viability remained typically >85%, data not shown. Monitoring by flow cytometry as a more sensitive and objective tool we can demonstrate that over the course of expansion, ongoing apoptosis at a rate of ~15% (see table 1) provides a constant influx of T cells into the non-viable fraction. Termination of expansion cultures at earlier time points, e.g.: at day 10–12 rather than day 14 have yielded inferior overall expansion. Similarly, extending cultures beyond day 14 up to day 18 in the hope that ongoing proliferation may still override apoptosis, have also yielded inferior expansion results as compared to day 12–14, data not shown.
Figure 1. Phenotypic evolution, proliferation and apoptosis in the expanded T cell progeny.
4-color FACS dotplot profile of viable T lymphocytes contrasting the starting day 0 and post-expansion day 14 progeny. Surface detection of indicated antibodies is presented except for Ki-67 and Activated Caspase-3 which were detected following permeabilization and intracellular staining. The relative size of the indicated T cell subsets in a quadrant is expressed as the percentage of total viable T cells. Representative experiment, n=10
Table 1. Maturational changes of CD3/CD28 Bead –Expanded CB T Cells, n=10,
4-color surface and intracellular (ic) FACS immunophenotype of lymphocytes after 14 days of culture by anti-CD3 plus anti-CD28 antibody coated Dynal® beads in the presence of IL-2.
| Variable | mean | ± SD |
|---|---|---|
| % CD3+ | 99 | 3 |
| % CD4+ | 39 | 9 |
| % CD28+/CD3+ | 96 | 7 |
| % CD27+/CD3+ | 98 | 1 |
| % CD45RA+/RO− | 16 | 14 |
| % CD45RA−/RO+ | 39 | 26 |
| % CD25+/ CD3+ | 57 | 19 |
| % CD25+/CD4+ | 85 | 10 |
| % CD45RA+/CD62L+ | 58 | 23 |
| % CD62L+/CD3+ | 92 | 6 |
| % CCR7+/CD3+ | 32 | 14 |
| % CD212+/CD3+ | 49 | 22 |
| % CD45RA+/CD27+/CD8+ “naïve CD8+” | 73 | 14 |
| % CD45RA-/CD27+/CD8+ “central memory CD8+” | 24 | 14 |
| % CD45RA+/CD27-/CD8+ “EMRA” | 2 | 1 |
| % CD57+/CD28-/CD8+ “effector CTL” | 0 | 0 |
| % Ki67+/CD8+ | 39 | 14 |
| % KI-67+/CD4+ | 35 | 12 |
| % HLA- DR+/CD3+ | 41 | 16 |
| % HLA- DR+/CD4+ | 36 | 18 |
| % HLA-DR+/CD8+ | 51 | 18 |
| % Granzyme A+/CD8+ | 53 | 17 |
| % Granzyme B+/CD8+ | 9 | 5 |
| % Perforin+/CD8+ | 2 | 1 |
| % INFγ+/CD3+ | 4 | 5 |
| % TNFα+/CD3+ | 27 | 24 |
| % IL2+/CD4+ | 21 | 12 |
| % IL4+/CD3+ | 0 | 0 |
| % Activated-Caspase 3+/CD4+ | 16 | 9 |
| % Activated-Caspase 3+/CD8+ | 13 | 10 |
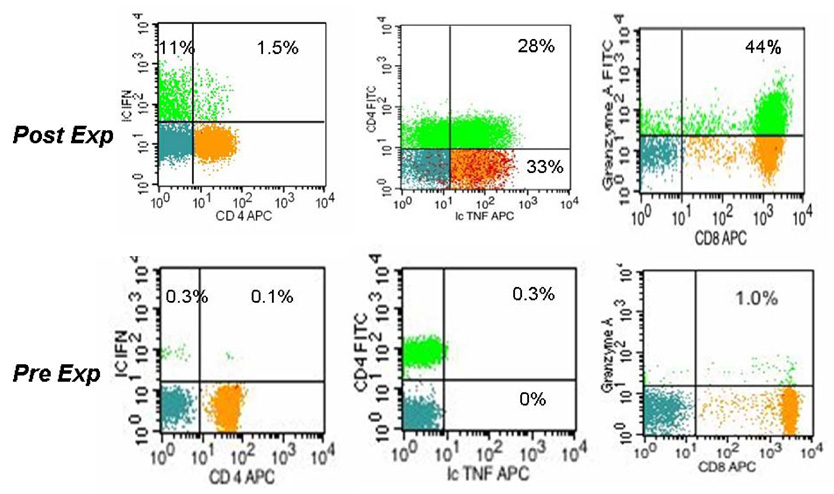
Figure 2. Th1/Tc1 cytokine secretion profile and Granzyme A expression in the expanded T cell progeny.
4-color FACS dotplot profile of viable T lymphocytes contrasting the starting day 0 and post-expansion day 14 progeny. Surface detection of indicated antibodies is presented except for IL-2, TNFα and Granzyme B which were detected following permeabilization and intracellular staining. The relative size of the indicated T cell subsets in a quadrant is expressed as the percentage of total viable T cells. Representative experiment, n=10
Proliferation induced by CD3/CD28 costimulatory beads results in an activated surface phenotype and a more pronounced Th1/Tc1 profile
Compared to the starting population of ‘resting’ CB T cells[18], the majority of expanded cells have acquired the surface phenotype of activated (HLA-DR+/ CD45RO+, CD25+ T cells) ‘memory like’ cells, at the expense of naive/recent thymic emigrants that co-express CD45RA and CD62L. Notably, after 12–14 days of expansion the majority of T cells coexpressed CD45RA and CD45RO very similar to what had been described within a few days following PHA stimulation [20–22]. Despite the proliferation induced evolution of immunophenotype, >90% of expanded T cells retained expression of CD62L, while CD27 and CD28 expression remained present in >97% of CD3+ T cells post-expansion. While the maintenance of L-selectin expression (CD62L) suggests preserved capability to migrate to secondary peripheral lymphoid organs for antigenic encounter, CCR7 expression remained detectable on only a third of the expanded progeny, see Table 1. This may result in diminished lymph node retention if expanded cells were adoptively transferred. Tissue homing molecules that are notably absent on the surface of fresh unmanipulated cord blood,[18] were expressed to varying degrees, with “skin homing” CCR4 and CLA detectable on ~5–15% of CD3+ cells, while the presumably ‘gut homing’ CCR-9 remained mostly undetectable, data not shown.
Significant Th1/Tc1 cell maturation was observed after expansion. Post-expansion T cells have acquired the capacity to secrete IFNγ, Figure 2, compared to minimal if any secretion by fresh uncultured CB T cells. Similarly, over half of the T cells expressed IL-12Rα (CD212) at a median 56% post-expansion, Table 1. IL-2 and TNFα̣ secretion was also enhanced, see Table 1, and the majority of TNFα secreting T cells were simultaneously secreting IL-2, data not shown. Despite these maturational events, a much larger fraction of unstimulated adult peripheral blood T cells are capable to secrete these cytokines as demonstrated by others [4] and us, (data not shown), suggesting only partial maturation towards the Th1/Tc1 direction among the post-expansion CB T cells.
Low expression levels of perforin and Granzyme B correspond with the absence of “effector CTL” population and lack of cytotoxicity post-expansion
Since cytotoxicity of fresh cord blood T cells is virtually absent [6] coinciding with the absence of intracellularly stored granzymes A, B, and perforin, post-expansion we monitored for the de novo expression of these critical mediators of cytotoxicity. While Granzyme A was stored in many post-expansion CD8+ T cells, see Fig 2 and Table 1, ic Granzyme B expression, was detected at significantly lower frequency (9 ±5%) than in normal adult peripheral blood [23] (35±15% in our laboratory, p>0.001), data not shown. There were also very few CD8+ T cells with preformed ic Perforin detected, Table 1, which correlates with the paucity of CD57+/CD28−/CD8+ “effector CTL” cells, Table 1. These findings together may explain the complete absence of cytotoxicity post-expansion, even at a high effector : target ratio (60:1), against a highly immunogenic EBV positive (and HLA-DR+, CD40+, CD80+ and CD86+) allogeneic lymphoma cell line (IM9) despite a week of pre-stimulation that promotes adult blood T cells to attain specific killing in excess of 50–60%, data not shown. Nevertheless, the expanded T cell progeny contained significantly more of these effectors of cytotoxicity compared to pre-expansion resting T cells.
DISCUSSION
These experiments above demonstrate significant T cell expansion matched with partial maturation among the proliferating CB T cells. The expansion of viable cord blood T cells has reached clinically relevant numbers. As an example, if one started with 2 ×106 total CB T cells derived from <3% of a typical thawed UCB graft, if this was expanded ~70 fold the progeny could provide a DLI product with up to 2 × 106 T cells/kg for patients weighing 60-kg or less. Although, in our cultures different clones of anti-CD3 and anti-CD28 MoAbs were linked to the Dynal beads as compared to the Xcyte® beads used by Parmar et al [17] we can corroborate that up to 100 fold expansion may be attained. However, our fold expansion may be spuriously lower as we enumerated viable CD3+ T cells by Trucount FACS-based technology while total nucleated cell count (TNC) was invariably higher, typically by 10–30%. Others, focusing on expanding CD25+ regulatory T cells (Treg) from CB, have also demonstrated expansion of non-regulatory T cells. [24, 25] although did not perform similar phenotypic and functional characterization of these cells.
The experiments above reveal significant immunophenotypic evolution among the expanded cells both on the surface and intracellularly. These changes might have clinical relevance if cells expanded by this method were infused as DLI product. In contrast with Parmar et al. [17], we find significantly less if any (<3%) “terminally differentiated” CD8+ T cells ( CD27−/CD28−), and the newly emerging “central memory” subset (CD45RA−/CD27+) represents less than a third of the progeny in our cultures, possibly reflecting again different CD3/CD28 stimulatory beads and different levels of activation. The absence of “effector CTL” is not surprising considering that during expansion CD8+ T cells in our cultures do not receive a “third signal” in the form of IL-12, TLR ligands, or type I IFNs [26]. The majority of the expanded cells in our culture retain several surface markers of the starting pool of “naïve/resting” T cells with >90% of expanded progeny expressing CD62L, CD27 and CD28. However, there is ample evidence that significant activation has occurred in these cultures, based on the high expression of HLA-DR, CD25 and the acquisition of CD212, Table I. Moreover, TNFα,IL-2 and IFNγ secreting cells are more frequent compared to the starting cultures and the Granzyme A expressing CD8+ T cell pool is remarkably reconstituted not dissimilar to what can be seen in normal adult peripheral blood as illustrated in Fig 2. However, compared to resting adult peripheral blood, fewer CD8+ T cells store Granzyme B and no CD57+/ Perforin+ cells could be detected in any of our experiments. These findings may explain the complete absence of cytotoxicity against a highly immunogenic allogeneic target.
While this apparent lack of cytotoxicity against an HLA-mismatched cell line suggests relative safety from acute/hyperacute GVHD, some cautionary findings emerge from the extensive analysis of the expanded T cells if these cells were taken to the clinical arena. While Parmar al. [17], found no change in T cell receptor diversity, the inversion of CD4/CD8 ratio seen in our cultures reflects different degree of proliferation along with a significant degree of apoptosis that can vary among specific T cell subsets. These together may lead to the survival of only a select fraction of the expanded cells. Apoptosis might be even higher once these cells were infused since the level of endogenous IL-2 would be likely ~ 2log less than the ex vivo cytokine-supplemented cultures. The possibility for accelerated apoptosis via “death by neglect” may not be clinically as relevant in most clinical settings so far tested. Clinical trials with CD3/28 expanded DLI products have reported long term T cell survival and even significant lymphocytosis [13, 15, 27] moreover autologous DLI could even correct a skewed T cell receptor diversity [14]. However, the adoptive T cell infusions were almost all performed in the autologous setting and DLI doses at 107–108/kg range were typically infused. These doses are 10–50 fold in excess of what one may be able to provide in the cord blood transplant setting. Moreover, immunosuppressive drugs that are essential in the HLA-mismatched unrelated cord blood transplantation setting to prevent GVHD will certainly pose an additional barrier. A smaller DLI cell dose paired with IS drugs in the circulation together could hamper clinically relevant changes in T cell reconstitution if there is already significant apoptosis occurring in vitro. Supporting evidence for this concern comes from studies that compared adult lymphocytes with cord blood T cells revealing that the latter are more likely to undergo activation induced cell death (AICD) following strong TCR signaling such as allo-priming [28]. While we demonstrate robust expansion potential from a very small starting fraction associated with partial Th1/Tc1 maturation, clearly one of the important other findings of our study is the significant rate of apoptosis in particular among CD4+ T cells that results in an inverted CD4/CD8 ratio. In contrast, the phenotypic evolution of naïve T cell population towards a central memory phenotype creates less of a concern, as these cells remain antigen inexperienced and their future priming in DLI recipients in vivo will require their homing and retention in secondary lymphoid tissues. However, retention may be compromised due to the progressive loss of CCR7 observed after 14 days in culture.
In summary, although the clinical implications of our study is very encouraging along with the report from the group at MD Anderson Cancer Center [17], we believe there are at least three areas that deserve further studies and possible modifications to improve on these encouraging pre-clinical findings; (1) reducing apoptosis among IL-2 and bead activated T cells in particular among CD4+ T cells, (2) increasing the rate of expansion to reach potentially higher DLI dose levels so adults of all sizes may benefit from DLI, and (3) inducing some additional functional maturation towards Th1/Tc1 competence while retaining low/minimal allogeneic cytotoxicity and sufficiently high expression of homing molecules necessary for entry and retention in secondary lymphoid organs. Once these conditions are satisfied, DLI could not only be safe hopefully in the cord blood transplant setting but ex vivo expanded T cells would have the potential for more effective control and/or reduction of opportunistic viral infections, that are so common currently after UCBT[1]. It is also noteworthy that following DLI infusion, the expanded cells may undergo further functionial maturation in vivo and could attain GVL activity as well. However, we believe that some degree of “immaturity” should probably be retained among the expanded CB T cells prior to DLI infusion, as opposed to full adult like cytotoxicity which could lead to severe alloreactivity/GVHD in the unrelated HLA-mismatched setting. It is essential for engineered DLI products generated from cord blood to retain the ability to safely cross the HLA barrier as one of the most significant benefits of unrelated cord blood transplantation.
ACKNOWLEDGEMENT
The authors thank Bruce Levine, Mark Bonyhadi, and Joanne Kurtzberg for helpful discussions, Young-Ah Lee and Melissa Reese for technical assistance, and the staff at the Duke University Stem Cell Laboratory and the Carolinas Cord Blood Bank at Duke University.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Szabolcs P, Niedzwiecki D. Immune reconstitution after unrelated cord blood transplantation. Cytotherapy. 2007;9:111–122. doi: 10.1016/j.bbmt.2007.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Goodwin VJ, Sato TA, Mitchell MD, Keelan JA. Anti-inflammatory effects of interleukin-4, interleukin-10, and transforming growth factor-beta on human placental cells in vitro. Am J Reprod Immunol. 1998;40:319–325. doi: 10.1111/j.1600-0897.1998.tb00060.x. [DOI] [PubMed] [Google Scholar]
- 3.Marchant A, Goldman M. T cell-mediated immune responses in human newborns: ready to learn? Clin Exp Immunol. 2005;141:10–18. doi: 10.1111/j.1365-2249.2005.02799.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chalmers IM, Janossy G, Contreras M, Navarrete C. Intracellular cytokine profile of cord and adult blood lymphocytes. Blood. 1998;92:11–18. [PubMed] [Google Scholar]
- 5.Risdon G, Gaddy J, Stehman FB, Broxmeyer HE. Proliferative and cytotoxic responses of human cord blood T lymphocytes following allogeneic stimulation. Cell Immunol. 1994;154:14–24. doi: 10.1006/cimm.1994.1053. [DOI] [PubMed] [Google Scholar]
- 6.Broxmeyer HE American Association of Blood Banks. Cord blood : biology, immunology, and clinical transplantation. Bethesda, Md.: AABB Press; 2004. [Google Scholar]
- 7.Berthou C, Legros-Maida S, Soulie A, et al. Cord blood T lymphocytes lack constitutive perforin expression in contrast to adult peripheral blood T lymphocytes. Blood. 1995;85:1540–1546. [PubMed] [Google Scholar]
- 8.Ribeiro-do-Couto LM, Boeije LC, Kroon JS, et al. High IL-13 production by human neonatal T cells: neonate immune system regulator? Eur J Immunol. 2001;31:3394–3402. doi: 10.1002/1521-4141(200111)31:11<3394::aid-immu3394>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 9.White GP, Watt PM, Holt BJ, Holt PG. Differential patterns of methylation of the IFN-gamma promoter at CpG and non-CpG sites underlie differences in IFN-gamma gene expression between human neonatal and adult CD45RO- T cells. J Immunol. 2002;168:2820–2827. doi: 10.4049/jimmunol.168.6.2820. [DOI] [PubMed] [Google Scholar]
- 10.Papadopoulos EB, Ladanyi M, Emanuel D, et al. Infusions of donor leukocytes to treat Epstein-Barr virus-associated lymphoproliferative disorders after allogeneic bone marrow transplantation. N Engl J Med. 1994;330:1185–1191. doi: 10.1056/NEJM199404283301703. [DOI] [PubMed] [Google Scholar]
- 11.Hromas R, Cornetta K, Srour E, Blanke C, Broun ER. Donor leukocyte infusion as therapy of life-threatening adenoviral infections after T-cell-depleted bone marrow transplantation. Blood. 1994;84:1689–1690. [PubMed] [Google Scholar]
- 12.Levine BL, Bernstein WB, Aronson NE, et al. Adoptive transfer of costimulated CD4+ T cells induces expansion of peripheral T cells and decreased CCR5 expression in HIV infection. Nat Med. 2002;8:47–53. doi: 10.1038/nm0102-47. [DOI] [PubMed] [Google Scholar]
- 13.Laport GG, Levine BL, Stadtmauer EA, et al. Adoptive transfer of costimulated T cells induces lymphocytosis in patients with relapsed/refractory non-Hodgkin lymphoma following CD34+-selected hematopoietic cell transplantation. Blood. 2003;102:2004–2013. doi: 10.1182/blood-2003-01-0095. [DOI] [PubMed] [Google Scholar]
- 14.Bonyhadi M, Frohlich M, Rasmussen A, et al. In vitro engagement of CD3 and CD28 corrects T cell defects in chronic lymphocytic leukemia. J Immunol. 2005;174:2366–2375. doi: 10.4049/jimmunol.174.4.2366. [DOI] [PubMed] [Google Scholar]
- 15.Porter DL, Levine BL, Bunin N, et al. A phase 1 trial of donor lymphocyte infusions expanded and activated ex vivo via CD3/CD28 costimulation. Blood. 2006;107:1325–1331. doi: 10.1182/blood-2005-08-3373. [DOI] [PubMed] [Google Scholar]
- 16.June CH, Ledbetter JA, Linsley PS, Thompson CB. Role of the CD28 receptor in T-cell activation. Immunol Today. 1990;11:211–216. doi: 10.1016/0167-5699(90)90085-n. [DOI] [PubMed] [Google Scholar]
- 17.Parmar S, Robinson SN, Komanduri K, et al. Ex vivo expanded umbilical cord blood T cells maintain naive phenotype and TCR diversity. Cytotherapy. 2006;8:149–157. doi: 10.1080/14653240600620812. [DOI] [PubMed] [Google Scholar]
- 18.Szabolcs P, Park KD, Reese M, Marti L, Broadwater G, Kurtzberg J. Coexistent naïve phenotype and higher cycling rate of cord blood T cells as compared to adult peripheral blood. Exp Hematol. 2003;31:708–714. doi: 10.1016/s0301-472x(03)00160-7. [DOI] [PubMed] [Google Scholar]
- 19.Szabolcs P, Park KD, Marti L, et al. Superior depletion of alloreactive T cells from peripheral blood stem cell and umbilical cord blood grafts by the combined use of trimetrexate and interleukin-2 immunotoxin. Biol Blood Marrow Transplant. 2004;10:772–783. doi: 10.1016/j.bbmt.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 20.LaSalle JM, Hafler DA. The coexpression of CD45RA and CD45RO isoforms on T cells during the S/G2/M stages of cell cycle. Cell Immunol. 1991;138:197–206. doi: 10.1016/0008-8749(91)90144-z. [DOI] [PubMed] [Google Scholar]
- 21.Maccario R, Chirico G, Mingrat G, et al. Expression of CD45R0 antigen on the surface of resting and activated neonatal T lymphocyte subsets. Biology of the neonate. 1993;64:346–353. doi: 10.1159/000244010. [DOI] [PubMed] [Google Scholar]
- 22.Picker LJ, Treer JR, Ferguson-Darnell B, Collins PA, Buck D, Terstappen LW. Control of lymphocyte recirculation in man. I. Differential regulation of the peripheral lymph node homing receptor L-selection on T cells during the virgin to memory cell transition. J Immunol. 1993;150:1105–1121. [PubMed] [Google Scholar]
- 23.Takata H, Takiguchi M. Three memory subsets of human CD8+ T cells differently expressing three cytolytic effector molecules. J Immunol. 2006;177:4330–4340. doi: 10.4049/jimmunol.177.7.4330. [DOI] [PubMed] [Google Scholar]
- 24.Godfrey WR, Spoden DJ, Ge YG, et al. Cord blood CD4(+)CD25(+)-derived T regulatory cell lines express FoxP3 protein and manifest potent suppressor function. Blood. 2005;105:750–758. doi: 10.1182/blood-2004-06-2467. [DOI] [PubMed] [Google Scholar]
- 25.Li L, Godfrey WR, Porter SB, et al. CD4+CD25+ regulatory T-cell lines from human cord blood have functional and molecular properties of T-cell anergy. Blood. 2005;106:3068–3073. doi: 10.1182/blood-2005-04-1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Williams MA, Bevan MJ. Effector and memory CTL differentiation. Annu Rev Immunol. 2007;25:171–192. doi: 10.1146/annurev.immunol.25.022106.141548. [DOI] [PubMed] [Google Scholar]
- 27.Walker RE, Bechtel CM, Natarajan V, et al. Long-term in vivo survival of receptor-modified syngeneic T cells in patients with human immunodeficiency virus infection. Blood. 2000;96:467–474. [PubMed] [Google Scholar]
- 28.Hagihara M, Chargui J, Gansuvd B, et al. Umbilical cord blood T lymphocytes are induced to apoptosis after being allo-primed in vitro. Bone Marrow Transplant. 1999;24:1229–1233. doi: 10.1038/sj.bmt.1702050. [DOI] [PubMed] [Google Scholar]