Abstract
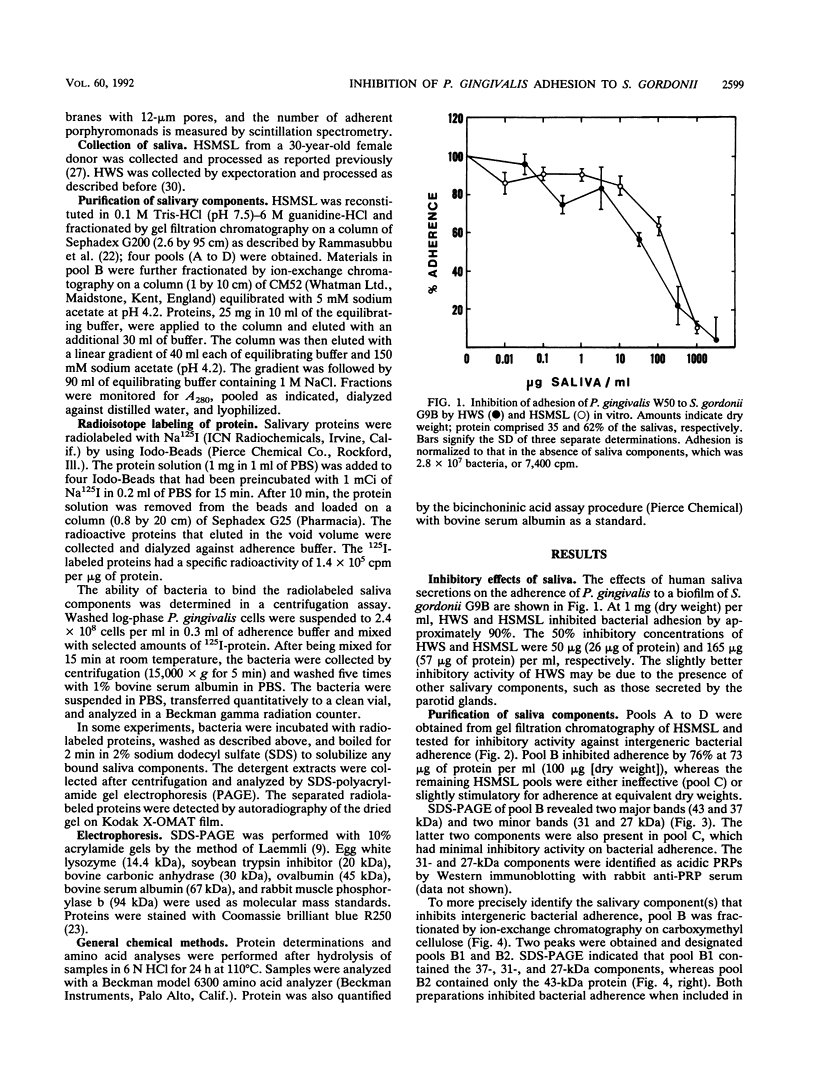
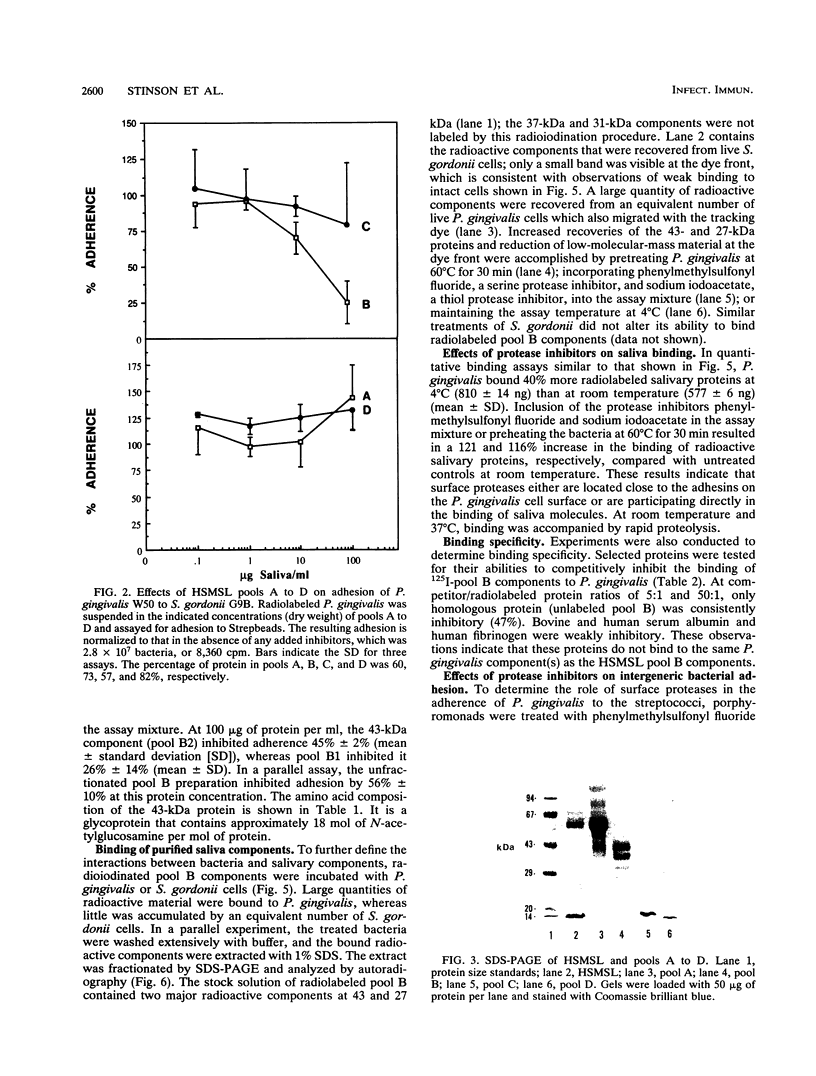
Porphyromonas gingivalis W50 adheres in vitro to biofilms of Streptococcus gordonii G9B. This phenomenon is believed to facilitate the initial colonization of the oral cavity by P. gingivalis and to contribute to the maturation of dental plaque. In this report, we describe the modulating effects of human submandibular-sublingual saliva (HSMSL) on this in vitro model of intergeneric bacterial adhesion (coaggregation). HSMSL inhibited P. gingivalis adhesion to S. gordonii by 50% at a concentration of 57 micrograms of protein per ml. Maximum inhibitory activity was associated with a 43-kDa protein obtained by sequential Sephadex G200 gel filtration and CM52 ion-exchange chromatography of HSMSL. Pools of other column fractions of HSMSL showed no effect or were slightly stimulatory for bacterial adhesion. The binding of radioiodinated column fractions containing the 43-kDa protein by P. gingivalis was accompanied by their rapid enzymatic degradation. Treating P. gingivalis at 60 degrees C for 30 min or with protease inhibitors (phenylmethylsulfonyl fluoride and sodium iodoacetate) reduced adherence to streptococcal biofilms. These treatments did not prevent P. gingivalis from binding soluble HSMSL saliva components, although subsequent proteolysis was nearly eliminated. These observations indicate that surface-associated proteases of P. gingivalis, either independently or in concert with adjacent surface adhesins, interact with surfaces of oral streptococci to facilitate interbacterial adhesion. The adhesion-blocking properties of HSMSL, particularly the 43-kDa protein, may represent an important host defense mechanism in the oral cavity.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Birkedal-Hansen H., Taylor R. E., Zambon J. J., Barwa P. K., Neiders M. E. Characterization of collagenolytic activity from strains of Bacteroides gingivalis. J Periodontal Res. 1988 Jul;23(4):258–264. doi: 10.1111/j.1600-0765.1988.tb01369.x. [DOI] [PubMed] [Google Scholar]
- Grenier D., McBride B. C. Isolation of a membrane-associated Bacteroides gingivalis glycylprolyl protease. Infect Immun. 1987 Dec;55(12):3131–3136. doi: 10.1128/iai.55.12.3131-3136.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilian M., Nyvad B. Ability to bind salivary alpha-amylase discriminates certain viridans group streptococcal species. J Clin Microbiol. 1990 Nov;28(11):2576–2577. doi: 10.1128/jcm.28.11.2576-2577.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolenbrander P. E., Andersen R. N., Holdeman L. V. Coaggregation of oral Bacteroides species with other bacteria: central role in coaggregation bridges and competitions. Infect Immun. 1985 Jun;48(3):741–746. doi: 10.1128/iai.48.3.741-746.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lantz M. S., Allen R. D., Vail T. A., Switalski L. M., Hook M. Specific cell components of Bacteroides gingivalis mediate binding and degradation of human fibrinogen. J Bacteriol. 1991 Jan;173(2):495–504. doi: 10.1128/jb.173.2.495-504.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lantz M. S., Rowland R. W., Switalski L. M., Hök M. Interactions of Bacteroides gingivalis with fibrinogen. Infect Immun. 1986 Dec;54(3):654–658. doi: 10.1128/iai.54.3.654-658.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lev M. Casamino acids enhance growth of Bacteroides melaninogenicus. J Bacteriol. 1977 Jan;129(1):562–563. doi: 10.1128/jb.129.1.562-563.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J., Ellen R. P., Hoover C. I., Felton J. R. Association of proteases of Porphyromonas (Bacteroides) gingivalis with its adhesion to Actinomyces viscosus. J Dent Res. 1991 Feb;70(2):82–86. doi: 10.1177/00220345910700021501. [DOI] [PubMed] [Google Scholar]
- Mayrand D., Grenier D. Detection of collagenase activity in oral bacteria. Can J Microbiol. 1985 Feb;31(2):134–138. doi: 10.1139/m85-026. [DOI] [PubMed] [Google Scholar]
- Murakami H., Sly W. S. Purification and characterization of human salivary carbonic anhydrase. J Biol Chem. 1987 Jan 25;262(3):1382–1388. [PubMed] [Google Scholar]
- Nagata H., Murakami Y., Inoshita E., Shizukuishi S., Tsunemitsu A. Inhibitory effect of human plasma and saliva on co-aggregation between Bacteroides gingivalis and Streptococcus mitis. J Dent Res. 1990 Aug;69(8):1476–1479. doi: 10.1177/00220345900690080501. [DOI] [PubMed] [Google Scholar]
- Naito Y., Gibbons R. J. Attachment of Bacteroides gingivalis to collagenous substrata. J Dent Res. 1988 Aug;67(8):1075–1080. doi: 10.1177/00220345880670080301. [DOI] [PubMed] [Google Scholar]
- Nishikata M., Kanehira T., Oh H., Tani H., Tazaki M., Kuboki Y. Salivary histatin as an inhibitor of a protease produced by the oral bacterium Bacteroides gingivalis. Biochem Biophys Res Commun. 1991 Jan 31;174(2):625–630. doi: 10.1016/0006-291x(91)91463-m. [DOI] [PubMed] [Google Scholar]
- Ono M., Okuda K., Takazoe I. Purification and characterization of a thiol-protease from Bacteroides gingivalis strain 381. Oral Microbiol Immunol. 1987 Jun;2(2):77–81. doi: 10.1111/j.1399-302x.1987.tb00294.x. [DOI] [PubMed] [Google Scholar]
- Ramasubbu N., Reddy M. S., Bergey E. J., Haraszthy G. G., Soni S. D., Levine M. J. Large-scale purification and characterization of the major phosphoproteins and mucins of human submandibular-sublingual saliva. Biochem J. 1991 Dec 1;280(Pt 2):341–352. doi: 10.1042/bj2800341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scannapieco F. A., Bergey E. J., Reddy M. S., Levine M. J. Characterization of salivary alpha-amylase binding to Streptococcus sanguis. Infect Immun. 1989 Sep;57(9):2853–2863. doi: 10.1128/iai.57.9.2853-2863.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shomers J. P., Tabak L. A., Levine M. J., Mandel I. D., Ellison S. A. The isolation of a family of cysteine-containing phosphoproteins from human submandibular-sublingual saliva. J Dent Res. 1982 Aug;61(8):973–977. doi: 10.1177/00220345820610081101. [DOI] [PubMed] [Google Scholar]
- Slots J., Gibbons R. J. Attachment of Bacteroides melaninogenicus subsp. asaccharolyticus to oral surfaces and its possible role in colonization of the mouth and of periodontal pockets. Infect Immun. 1978 Jan;19(1):254–264. doi: 10.1128/iai.19.1.254-264.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorsa T., Uitto V. J., Suomalainen K., Turto H., Lindy S. A trypsin-like protease from Bacteroides gingivalis: partial purification and characterization. J Periodontal Res. 1987 Sep;22(5):375–380. doi: 10.1111/j.1600-0765.1987.tb01602.x. [DOI] [PubMed] [Google Scholar]
- Stinson M. W., Safulko K., Levine M. J. Adherence of Porphyromonas (Bacteroides) gingivalis to Streptococcus sanguis in vitro. Infect Immun. 1991 Jan;59(1):102–108. doi: 10.1128/iai.59.1.102-108.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suido H., Neiders M. E., Barua P. K., Nakamura M., Mashimo P. A., Genco R. J. Characterization of N-CBz-glycyl-glycyl-arginyl peptidase and glycyl-prolyl peptidase of Bacteroides gingivalis. J Periodontal Res. 1987 Sep;22(5):412–418. doi: 10.1111/j.1600-0765.1987.tb01608.x. [DOI] [PubMed] [Google Scholar]
- Tsutsui H., Kinouchi T., Wakano Y., Ohnishi Y. Purification and characterization of a protease from Bacteroides gingivalis 381. Infect Immun. 1987 Feb;55(2):420–427. doi: 10.1128/iai.55.2.420-427.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura F., Nishikata M., Suzuki T., Hoover C. I., Newbrun E. Characterization of a trypsin-like protease from the bacterium Bacteroides gingivalis isolated from human dental plaque. Arch Oral Biol. 1984;29(7):559–564. doi: 10.1016/0003-9969(84)90078-5. [DOI] [PubMed] [Google Scholar]