Abstract
An 8-year-old horse was presented with a submandibular swelling. Biopsy of the lesion indicated granulomatous osteomyelitis due to Halicephalobus gingivalis. In the absence of evidence of involvement of the central nervous system at the time of diagnosis, the horse was treated with ivermectin. Unfortunately, the horse did not survive.
Résumé
Échec du traitement d’une ostéomyélite granulomateuse mandibulaire à Halicephalobus gingivalis chez un cheval. Un cheval âgé de 8 ans a été présenté pour une enflure sous-mandibulaire. Une biopsie de la lésion a révélé une ostéomyélite granulomateuse causée par Halicephalobus gingivalis. En l’absence de signes d’atteinte du système nerveux central au moment du diagnostic, le cheval a été traité à l’ivermectine. Malheureusement, le cheval n’a pas survécu.
(Traduit par Docteur André Blouin)
An 8-year old, Arabian/warmblood gelding was presented on day 1 to Manning Equine Veterinary Services, Ontario, with a submandibular mass of 2 weeks’ duration. The horse had originally been seen by a referring veterinarian, who performed an oral examination at the time of initial presentation. The farm from which the horse originated had a deworming program consisting of the administration of an ivermectin product 3 times per year.
Case description
On presentation, the horse was bright, alert, and responsive. Palpation of the left submandibular area revealed a firm mass, approximately 4 cm in diameter, at the level of the 2nd and 3rd premolar teeth, with associated soft-tissue swelling.
Manning Equine, PO Box 519, Erin, Ontario N0B 1T0 (Ferguson, Manning, Malatestinic); Animal Health Laboratory (van Dreumel), and Department of Pathobiology (Caswell, Peregrine), University of Guelph, Guelph, Ontario N1G 2W1; Department of Medicine, University of Toronto, Centre for Travel and Tropical Medicine, Toronto General Hospital, 200 Elizabeth Street, 13 North Wing-1350 Toronto, Ontario M5G 2C4 (Keystone).
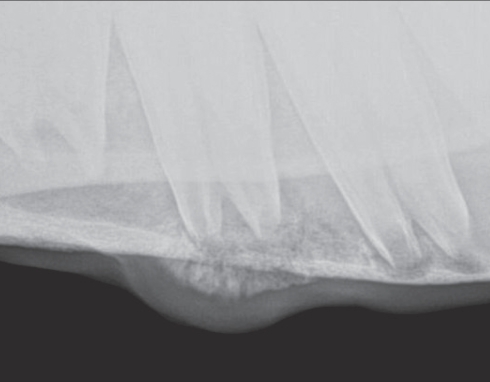
Examination of the oral cavity revealed sharp enamel points on the buccal upper and lingual lower arcades on both sides. Two small buccal erosions were noted at the level of the 2nd mandibular premolar teeth. No other dental abnormalities were present. Digital radiographs (TruDR; Sound Technologies, Carlsbad, California, USA) were taken of the affected area, revealing a mottled appearance of the left mandibular cortical bone associated with the 2nd and 3rd premolar teeth (Figure 1). After consultation with an equine surgeon, the decision was made to drill out the affected area to rule out an atypical tooth root abscess.
Figure 1.
Radiograph of the left mandible showing osteolytic and osteoproductive lesions involving the 2nd and 3rd premolar teeth.
On day 2, the horse appeared mildly uncomfortable and dull. A physical examination indicated a mild decrease in gastrointestinal sounds, a heart rate of 40 beats/min (reference interval: 24 to 40 beats/min), rectal temperature of 38.3°C (reference interval: 37.5°C to 38.5°C), and respiratory rate of 16 breaths/min (reference interval: 8 to 12 breaths/min). Flunixin meglumine (Cronyxin 50 mg/mL; Bioniche Animal Health, Belleville, Ontario), 1.1 mg/kg bodyweight (BW), IV, was administered and the horse was turned out in a round pen for exercise. Over the next hour, the colicky signs improved and the horse returned to normal. Results from a complete blood (cell) count (CBC) taken on the same day revealed a mild, nonregenerative anemia [red blood cells (RBC) = 6.03 × 1012/L; reference interval: 6.50 to 12.50 × 1012/L: hemoglobin = 102 g/L; reference interval: 110 to 190 g/L: hematocrit = 0.26 L/L; reference interval: 0.32 to 0.52 L/L].
On day 4, the horse was sedated with detomidine hydrochloride (Dormosedan 10 mg/mL; Pfizer Animal Health, Kirkland, Quebec), 20 μg/kg BW, IV, and butorphanol tartrate (Torbugesic 10 mg/mL; Wyeth Animal Health, Guelph, Ontario), 0.015 mg/kg BW, IV, and the left mandibular area was clipped, prepared for surgery, and locally anesthetized by using mepivacaine hydrochloride (Carbocaine-V 20 mg/mL; Pfizer Animal Health). Digital radiographs were used to mark placement of the drill bit. A small incision was made in the ventral aspect of the left mandible at the level of the 3rd premolar tooth with a #15 scalpel blade, and a 3/8” bit was used to drill into the mandible. A total depth of approximately 4 cm was reached. The material recovered from the lesion, using Addison forceps, appeared as granulomatous bone, and was submitted for culture and histologic examination. The surgical area was wrapped with gauze and self-adherent bandage (Vetwrap; 3M, London, Ontario), leaving the original incision open to allow for flushing over the following days. The horse was administered metronidazole (Metronidazole paste 450 mg/mL; Summit Veterinary Pharmacy, Aurora, Ontario), 20 mg/kg BW, PO, q8h, for 10 d, and flunixin meglumine, 1.1 mg/kg BW, PO, q12h for 5 d. The metronidazole was administered for activity against potential anaerobic bacterial infections, and the flunixin for postoperative nonsteroidal anti-inflammatory activity. The surgical area was flushed twice daily with saline containing enrofloxacin [1 L saline containing 1 g enrofloxacin (Baytril, 50 mg/mL, Bayer Health Care, Etobicoke, Ontario)], as this drug has broad-spectrum antibacterial activity.
The mandibular biopsy results were received on day 5 and indicated immature woven bone with fibrous tissue in the marrow space. The fibrous tissue contained large numbers of nematode larvae, approximately 15 μm by at least 150 μm, with numerous basophilic granules throughout. Occasionally, an esophagus with a distinct bulb, characteristic of rhabditiform nematodes, was visible. The morphology was typical of Halicephalobus gingivalis. Macrophages and scattered multinucleate giant cells in the fibrous tissue, indicative of granulomatous osteomyelitis, were present around the parasites.
On day 6, a protocol consisting of ivermectin (Eqvalan, 10 mg/mL; Merial Canada, Baie d’Urfé, Quebec), 1.2 mg/kg BW, PO, q14d for 6 wk, was instituted. Examination of fresh feces, using standard gravitational flotation, was negative for parasite eggs.
On day 7, results from a CBC revealed a low hematocrit (0.32 L/L), and from a biochemical profile, a hyperproteinemia characterized by hyperglobulinemia (47 g/L; reference interval: 22 to 40 g/L) and a decreased albumin/globulin (A/G) ratio (0.7; reference interval: 0.8 to 1.4). At this time, due to zoonotic issues associated with H. gingivalis and the fact that 14 people had had direct contact with fluid draining from the incision in the mandible, all 14 individuals were referred to a medical parasitologist and all were treated with ivermectin (Stromectol, 6 mg/tablet: Merck Whitehouse Station, New Jersey, USA), 0.2 mg/kg BW, PO, q14d for 3 treatments.
On day 11, results from a CBC revealed a mildly decreased hematocrit (0.31 L/L). The globulin levels remained elevated at 47 g/L. Urinalysis results showed no abnormalities. A 2nd standard gravitational fecal flotation was negative for parasite eggs.
On day 18, results from a CBC again revealed a mildly decreased hematocrit (0.30 L/L). The horse was still hyperglobulinemic (50 g/L) and had a low A/G ratio (0.6). In an attempt to identify evidence of dissemination of the infection, a transrectal ultrasonographic evaluation of the left kidney was performed (1), but it did not reveal any abnormalities.
On day 22, the horse was noted to be lethargic. Physical examination revealed a rectal temperature of 38.8°C. However, no other physical abnormalities were noted.
On day 23, the horse began to show neurological clinical signs. The animal was very depressed and began leaning to the right side. A physical examination revealed no other abnormal findings, and a cranial nerve examination was within normal limits. Results from a CBC were within normal limits. However, the globulin level (54 g/L) was increased compared with that on day 18. A catheter was placed in the left jugular vein and flunixin meglumine, 1.1 mg/kg BW, IV, omeprazole (GastroGard 2.28 g/tube; Merial Canada), 4 mg/kg BW, PO, and phenylbutazone (Phenylbutazone 200 mg/mL; Vétoquinol, Lavaltrie, Quebec), 2.2 mg/kg BW, IV, were administered. Flunixin and phenylbutazone were administered concurrently in an attempt to address the neurological signs. Although concurrent use of these 2 drugs may, in the long term, increase the likelihood of adverse reactions, it was decided that amelioration of the neurological signs was of greatest importance at this time. Omeprazole was administered to address potential negative effects of flunixin on the mucosal lining of the stomach.
On day 24, in light of the involvement of the central nervous system (CNS), the horse was administered thiabendazole (thiabendazole; The Veterinary Pharmacy, Guelph, Ontario), 44 mg/kg BW, PO, q24h, via a nasogastric tube. However, the animal became increasingly lethargic and dull throughout the day and, other than nibbling at hay, was relatively unresponsive to stimuli. Hydration status was adequate.
On day 25, the horse developed a head tilt to the right, became very dull, and collapsed once, but regained his feet, in the stall. In order to prevent dehydration, a fluid bolus of 10 L lactated Ringer’s solution (LACT-R, Vétoquinol) at 700 mL/h, supplemented with dextrose (Dextrose 50%, Vétoquinol), was administered, IV. At 10:00, while placing a nasogastric tube for thiabendazole administration, the horse collapsed, resulting in mild epistaxis. Twenty minutes later the animal regained his feet and seemed brighter than earlier in the day. Due to clinical constraints, fluid therapy was discontinued at 19:00. However, at 21:30, the horse was found in lateral recumbency and paddling. One liter of lactated Ringer’s solution containing 100 mL DMSO (dimethyl sulfoxide 99%; Bioniche Animal Health), flunixin meglumine, 1.1 mg/kg BW, and dexamethasone (dexamethasone 5, 5 mg/mL; Vétoquinol Canada), 0.1 mg/kg BW, were administered, IV, and the horse regained its feet by 02:00 on day 26.
On day 26, results from a CBC revealed an increased hematocrit (0.53 L/L), attributable to mild dehydration. Results from a biochemical panel revealed a marked hyperproteinemia due to hyperglobulinemia (65 g/L), an increase in aspartate aminotransferase (709 U/L; reference interval: 150 to 550 U/L), creatine kinase (4952 U/L; reference interval: 50 to 250 U/L), urea (13.0 mmol/L; reference interval: 3.5 to 8.5 mmol/L), and creatinine (299 μmol/L; reference interval: 70 to 170 μmol/L). Fluid therapy (LACT-R, Vétoquinol) was restarted at 700 mL/h to address concerns about both dehydration and renal failure. Throughout the day, the horse remained in lateral recumbency, was unresponsive to stimuli, and was paddling. By 18:30, the horse had a markedly elevated heart rate (60 beats/min), respiratory rate (40 breaths/min), capillary refill time (3 s; reference interval, < 2 s), and dark pink mucous membranes. Due to the animal’s poor condition and poor prognosis, the owners elected to have the horse euthanized, using pentobarbital sodium (Euthanyl Forte 540 mg/mL; Vétoquinol), 260 mg/kg BW, IV.
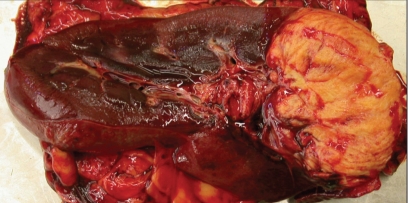
On day 27, the horse was transported to the Animal Health Laboratory, University of Guelph, for a postmortem examination. Because of human health concerns, all people involved in the postmortem wore a Powered Air Purifier Respirator (Air-Mate Hepa filter, 3M Occupational Health and Environmental Safety Division, St. Paul, Minnesota, USA) and double gloves. Gross findings included a 5.0-cm diameter hard grey swelling at the midpoint of the left mandible. The center of the mass was necrotic and extended to the root of the 3rd premolar tooth. There was a large, firm, yellow-brown area of necrosis involving the cranial 1/3rd of the left kidney (Figure 2). The affected area extended from the renal capsule to the pelvis. A firm yellow subendocardial cushion was located on the left ventricular papillary muscle, which measured approximately 3 × 2 × 0.5 cm and was edematous.
Figure 2.
Localized area of granulomatous inflammation affecting the cranial 30% of the left kidney.
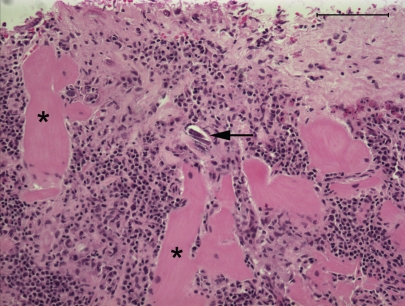
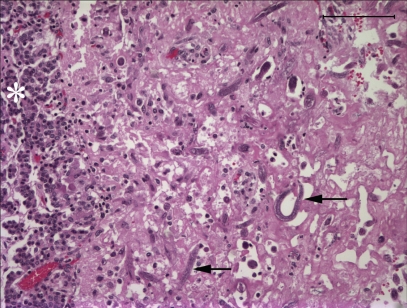
Microscopic lesions consisted of marked lysis, granulomatous inflammation, and fibrosis of the mandibular bone. Multinucleated giant cells formed part of the inflammatory reaction. Numerous H. gingivalis larvae were present within the lesion, which extended to the root of the tooth. There was a localized area of a mainly plasmacytic reaction of the endocardium and underlying myocardium (Figure 3). A few nematode larvae were evident within the inflamed area. The renal lesions consisted of numerous granulomas of both cortex and medulla, with lymphocytes, macrophages, and multinucleate giant cells, and large numbers of larvae in the centers of the granulomas. In the brain, lesions of a nonsuppurative meningoencephalitis were present in grey and white matter of all areas that were sectioned. Lesions were most prominent in the cerebral cortex and periventricular areas of the 4th ventricle. Larvae were present in most, but not all, affected areas. The meninges contained perivascular infiltrates of lymphocytes and macrophages, and the brain parenchyma contained similar perivascular infiltrates with focal areas of spongiosis, gliosis, and swollen axons. The pituitary gland contained a localized 3-mm diameter mass near the base of the pituitary, involving the pars nervosa and the pars intermedia, that contained extensive necrosis and large numbers of the nematode larvae (Figure 4). The morphology of nematode larvae (Figure 5) was identical with that seen in the original mandibular biopsy.
Figure 3.
Subendocardial myocarditis with presence of a cross section of a nematode larva (arrow) in the wall of the left ventricle, with plasmacytic infiltrates between cardiac myofibers (asterisk). Stain = hematoxylin and eosin, Bar = 100 μm.
Figure 4.
Area of necrosis with presence of nematode larvae (arrows) at the margin of the pituitary gland. Asterisk denotes the pars intermedia. Stain = hematoxylin and eosin, Bar = 100 μm.
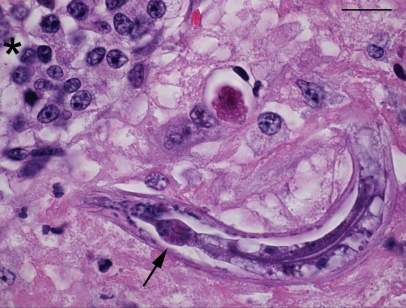
Figure 5.
Section of pituitary showing nematode larva with rhabditiform esophagus with isthmus and bulb (arrow), adjacent to pituitary epithelium (asterisk). Stain = hematoxylin and eosin, Bar = 15 μm.
Discussion
Halicephalobus gingivalis is a saprophytic free-living nematode that is infrequently identified in horses and humans (2). There have been approximately 30 reported infections in horses and 3 fatal infections in humans (3). Two of the human cases appeared to have become infected by deep penetrating wounds or contact with decubital ulcers (4,5). The route of infection in the 3rd case was not clear (6).
Halicephalobus gingivalis is usually diagnosed at postmortem examination in horses that have exhibited neurological signs. Although antemortem diagnoses are uncommon, clinical signs associated with H. gingivalis infections include facial swelling, uveitis, lameness, orchitis, renal disease, posthitis, and various CNS disorders (1,7–12). It remains speculative as to how H. gingivalis gains entry into the host; however, it appears likely that it enters through mucosal breaks in the oral cavity (2). This route of infection appears likely in the present case, since buccal erosions were observed on the day of initial presentation.
Halicephalobus gingivalis infections can be readily diagnosed by histological evaluation of biopsy tissue. In the present case, in which a submandibular lesion was the presenting sign, other more common differential diagnoses needed to be considered initially. On physical examination, the lesion found on the left mandible was consistent with a periapical infection of a premolar tooth, sialolithiasis of the parotid salivary duct, or osteomyelitis of the mandible (13–15). Although the use of radiography did not change the diagnostic plan in this case, in previous cases, infection with H. gingivalis in cortical bone appeared distinct from that of a typical periapical tooth root abscess (16). Radiographic signs of a tooth-root abscess include sclerosis of the periodontal bone, lysis of the alveolar bone, and decreased definition of the periodontal membrane surrounding the tooth. In this case, the radiographic signs included multifocal areas of bone lysis throughout the mandible at the level of the 2nd and 3rd premolar teeth, without a distinct connection between the apex of the tooth roots and the alveolar bone.
Management of horses with H. gingivalis infection must be considered individually, since it depends on the location of the infection. As described with the present case, surgical options were explored. However, the horse was treated medically due to the extent of the infection in the mandible, concern about hematogenous spread as a result of surgery, and lack of evidence for neurologic or systemic involvement at the time the infection was initially diagnosed.
Successful treatment of H. gingivalis infections has been reported in 3 horses, none of which had involvement of the CNS. One horse had an infection limited to the prepuce and was treated with ivermectin and diethylcarbamazine (10). The 2nd was a donkey with a renal infection; after nephrectomy the animal received a single dose of ivermectin and, reportedly, was healthy 18 mo later (17). Unfortunately, for both of these cases, the drug dosages and follow-up details were not stated. The 3rd horse was presented with a large granuloma on the head; after surgical debulking of the granuloma, the horse was treated with ivermectin at 1.2 mg/kg BW, PO, q2wk for 6 wk (3). This is considered the maximum safe dose in horses for ivermectin when administered on multiple occasions at 2-week intervals (18). In light of the efficacy of this treatment regimen, the same regimen was implemented in the case described here, as there was no evidence of systemic infection. In particular, there was no evidence of involvement of the CNS; ivermectin has a poor ability to cross the blood-brain barrier (19). When the horse developed neurological signs, thiabendazole was administered, since this drug has been used successfully to treat a human with disseminated Strongyloides stercoralis involving the CNS (20); S. stercoralis is closely related to H. gingivalis. Unfortunately, the horse was euthanized only 2 d after the thiabendazole treatment was initiated; likely too soon for the drug to have exerted significant activity. The rapid clinical deterioration that occurred after the appearance of the neurological clinical signs is consistent with that in other cases (21,22). Thus, in future, if the authors have to manage other cases of H. gingivalis infection without clinical evidence of CNS involvement, ivermectin and thiabendazole will be administered concurrently as soon as the diagnosis is made in order to determine if this increases the efficacy of treatment.
Since 3 human cases of H. gingivalis infection have been described, all of whom died with CNS involvement (4–6), the 14 individuals potentially exposed in this case were offered preventive treatment. In light of the known efficacy of ivermectin for treating H. gingivalis in a horse (3), and because ivermectin is considered the drug of choice for disseminated strongyloidiasis in humans without CNS involvement (23), all 14 were treated with ivermectin, 0.2 mg/kg BW, PO, with the treatment repeated 2 and 4 wk later. The treatment was repeated at 2-week intervals because this was consistent with the successful equine treatment protocol (3) and because 2 treatments with ivermectin (0.2 mg/kg BW), 14 d apart, rather than a single treatment, has been shown to increase the efficacy of the drug against disseminated strongyloidiasis in humans (24). Furthermore, the same protocol has previously been used to successfully treat an individual who inoculated large numbers of H. gingivalis deep into a finger with a contaminated scalpel blade (unpublished observation). At the time of writing, all 14 exposed individuals have remained well for the 18-month period since the time of exposure and treatment.
Acknowledgment
Dr. D.R. Trout is thanked for surgical advice at the time that radiographs were taken of the submandibular lesion. CVJ
Footnotes
Reprints will not be available from the authors.
A brief abstract with information contained in this case report has been published in the Proceedings of the Annual Conference of the Australian Society for Veterinary Pathology & C.L. Davis Foundation Australia, November 9–11, 2007, Attwood, Victoria.
Authors’ contributions Dr. Ferguson was the co-primary clinician for the case, wrote the majority of the first draft of the manuscript, and edited revised versions of the text. Dr. van Dreumel was the primary pathologist for the case, wrote the first draft of the pathology text, provided advice on appropriate images to include in the case report, and edited revised versions of the text. Dr. Keystone was the physician responsible for all the human cases described in the case report. He also provided assistance with revising all text referring to human cases. Dr. Manning was the co-primary clinician for the case. Dr. Malatestinic was a clinician who assisted with the case. Dr. Caswell was the pathologist who saw the original mandibular biopsy and made the original diagnosis. He also provided advice on revising the pathology text, assisted with revisions of the entire text, and provided advice on appropriate images/figure legends to include in the case report. Dr. Peregrine was the clinical parasitologist that assisted with the management of the case from the time it was originally diagnosed. He also managed the contributions of all the authors to the manuscript, assisted with writing the paper, and edited all versions of the manuscript.
References
- 1.Kinde H, Mathews M, Ash L, St Leger J. Halicephalobus gingivalis (H. deletrix) infection in two horses in southern California. J Vet Diagn Invest. 2000;12:162–165. doi: 10.1177/104063870001200213. [DOI] [PubMed] [Google Scholar]
- 2.Anderson RC, Linder KE, Peregrine AS. Halicephalobus gingivalis (Stefanski, 1954) from a fatal infection in a horse in Ontario, Canada with comments on the validity of H. deletrix and a review of the genus. Parasite. 1998;5:255–261. doi: 10.1051/parasite/1998053255. [DOI] [PubMed] [Google Scholar]
- 3.Pearce SG, Bouré LP, Taylor JA, Peregrine AS. Treatment of a granuloma caused by Halicephalobus gingivalis in a horse. J Am Vet Med Assoc. 2001;219:1735–1738. doi: 10.2460/javma.2001.219.1735. [DOI] [PubMed] [Google Scholar]
- 4.Hoogstraten J, Young WG. Meningoencephalomyelitis due to the saprophagous nematode, Micronema deletrix. Can J Neurol Sci. 1975;2:121–126. doi: 10.1017/s0317167100020102. [DOI] [PubMed] [Google Scholar]
- 5.Gardiner CH, Koh DS, Cardella TA. Micronema in man: Third fatal infection. Am J Trop Med Hyg. 1981;30:586–589. doi: 10.4269/ajtmh.1981.30.586. [DOI] [PubMed] [Google Scholar]
- 6.Shadduck JA, Ubelaker J, Telford VQ. Micronema deletrix meningoencephalitis in an adult man. Am J Clin Pathol. 1979;72:640–643. doi: 10.1093/ajcp/72.4.640. [DOI] [PubMed] [Google Scholar]
- 7.Powers RD, Benz GW. Micronema deletrix in the central nervous system of a horse. J Am Vet Med Assoc. 1977;170:175–177. [PubMed] [Google Scholar]
- 8.Keg PR, Mirck MH, Dik KJ, Vos JH. Micronema deletrix infection of a Shetland pony stallion. Equine Vet J. 1984;16:471–475. doi: 10.1111/j.2042-3306.1984.tb01987.x. [DOI] [PubMed] [Google Scholar]
- 9.Darien BJ, Belknap J, Nietfeld J. Cerebrospinal fluid changes in two horses with central nervous system nematodiasis (Micronema deletrix) J Vet Intern Med. 1988;2:201–205. doi: 10.1111/j.1939-1676.1988.tb00317.x. [DOI] [PubMed] [Google Scholar]
- 10.Dunn DG, Gardiner CH, Dralle KR, Thilsted JP. Nodular granulomatous posthitis caused by Halicephalobus (syn. Micronema) sp. in a horse. Vet Pathol. 1993;30:207–208. doi: 10.1177/030098589303000215. [DOI] [PubMed] [Google Scholar]
- 11.Rames DS, Miller RB, Craig TM. Ocular Halicephalobus (syn. Micronema) deletrix in a horse. Vet Pathol. 1995;32:540–542. doi: 10.1177/030098589503200514. [DOI] [PubMed] [Google Scholar]
- 12.Sturgeon BPR, Bassett H. Polydipsia in a foal with renal helminthiasis. Vet Rec. 2000;147:23–24. doi: 10.1136/vr.147.1.23. [DOI] [PubMed] [Google Scholar]
- 13.Freestone JF, Seahorn TL. Miscellaneous conditions of the equine head. Vet Clin North Am Equine Pract. 1993;9:235–242. doi: 10.1016/s0749-0739(17)30426-1. [DOI] [PubMed] [Google Scholar]
- 14.Baerg SD, Russel DA, Le Van LM, Kirker-Head CA. Endodontic therapy and surgical excision of a chronic suppurative osteomyelitic lesion in a horse: A case report. J Vet Dent. 1996;13:145–148. [PubMed] [Google Scholar]
- 15.Gayle JM, Redding WR, Vacek JR, Bowman KF. Diagnosis and surgical treatment of periapical infection of the third mandibular molar in five horses. J Am Vet Med Assoc. 1999;215:829–832. [PubMed] [Google Scholar]
- 16.Thrall DE. Textbook of Veterinary Diagnostic Radiology. 4. Toronto: WB Saunders; 2002. p. 97. [Google Scholar]
- 17.Schmitz DG, Chaffin MK. What is your diagnosis? J Am Vet Med Assoc. 2004;225:1667–1668. doi: 10.2460/javma.2004.225.1667. [DOI] [PubMed] [Google Scholar]
- 18.Pulliam JD, Preston JM. Safety of ivermectin in target animals. In: Campbell WC, editor. Ivermectin and Abamectin. New York: Springer-Verlag; 1989. pp. 149–161. [Google Scholar]
- 19.Plumb DC. Veterinary Drug Handbook. 4. Iowa State Pr; 2002. Ivermectin; pp. 454–459. [Google Scholar]
- 20.Wachter RM, Burke AM, MacGregor RR. Strongyloides stercoralis hyperinfection masquerading as cerebral vasculitis. Arch Neurol. 1984;41:1213–1216. doi: 10.1001/archneur.1984.04050220115030. [DOI] [PubMed] [Google Scholar]
- 21.Bröjer JT, Parsons DA, Linder KE, Peregrine AS, Dobson H. Halicephalobus gingivalis encephalomyelitis in a horse. Can Vet J. 2000;41:559–561. [PMC free article] [PubMed] [Google Scholar]
- 22.Bryant UK, Lyons ET, Bain FT, Hong CB. Halicephalobus gingivalis-associated meningoencephalitis in a Thoroughbred foal. J Vet Diagn Invest. 2006;18:612–615. doi: 10.1177/104063870601800618. [DOI] [PubMed] [Google Scholar]
- 23.Siddiqui AA, Berk SL. Diagnosis of Strongyloides stercoralis infection. Clin Infect Dis. 2001;33:1040–1047. doi: 10.1086/322707. [DOI] [PubMed] [Google Scholar]
- 24.Torres JR, Isturiz R, Murillo J, Guzman M, Contreras R. Efficacy of ivermectin in the treatment of strongyloidiasis complicating AIDS. Clin Infect Dis. 1993;17:900–902. doi: 10.1093/clinids/17.5.900. [DOI] [PubMed] [Google Scholar]