Abstract
In the fission yeast Schizosaccharomyces pombe, p34cdc2 plays a central role controlling the cell cycle. We recently isolated a new gene named srw1+, capable of encoding a WD repeat protein, as a multicopy suppressor of hyperactivated p34cdc2. Cells lacking srw1+ are sterile and defective in cell cycle controls. When starved for nitrogen source, they fail to effectively arrest in G1 and die of accelerated mitotic catastrophe if regulation of p34cdc2/Cdc13 by inhibitory tyrosine phosphorylation is compromised by partial inactivation of Wee1 kinase. Fertility is restored to the disruptant by deletion of Cig2 B-type cyclin or slight inactivation of p34cdc2. srw1+ shares functional similarity with rum1+, having abilities to induce endoreplication and restore fertility to rum1 disruptants. In the srw1 disruptant, Cdc13 fails to be degraded when cells are starved for nitrogen. We conclude that Srw1 controls differentiation and cell cycling at least by negatively regulating Cig2- and Cdc13-associated p34cdc2 and that one of its roles is to down-regulate the level of the mitotic cyclin particularly in nitrogen-poor environments.
INTRODUCTION
In virtually all eukaryotes, cyclin-dependent protein kinases (Cdk) play key roles controlling cell cycling (reviewed by Nurse, 1990; Nigg, 1995; Okayama et al., 1996; Murakami and Okayama, 1997). In the fission yeast Schizosaccharomyces pombe, the single Cdk encoded by cdc2+ (p34cdc2) controls the onsets of both S phase and mitosis (Nurse and Bissett, 1981). During cell cycle progression the activity of p34cdc2 is regulated positively and negatively at least by three kinds of biochemical events. One is association with a cyclin molecule, which is essential for kinase activity and strictly regulated in amount during cell cycling. The second is inhibitory phosphorylation at Tyr15 of p34cdc2. The third is negative regulation by Cdk inhibitors.
Three kinds of B-type cyclins are known to associate with p34cdc2. Cdc13 is a key cyclin essential for p34cdc2 to perform mitosis (Booher et al., 1989; Moreno et al., 1989) and is also involved in the cell cycle “start” (Fisher and Nurse 1996; Mondesert et al., 1996). Cig2 promotes the cell cycle start and negatively regulates differentiation (Obara-Ishihara and Okayama, 1994; Mondesert et al., 1996). Cig1 is the third cyclin that is thought to act in G2 or mitosis (Basi and Draetta, 1995). The protein levels of these cyclins are strictly regulated by transcription and ubiquitin-dependent proteolysis catalyzed by the 26S proteasome (Glotzer et al., 1991; Gorden et al., 1993; Funabiki et al., 1996; Gorden et al., 1996).
After passing through start in G1, p34cdc2/Cdc13 undergoes inhibitory phosphorylation at Tyr15, which is catalyzed by Wee1 and Mik1 kinases (Russell and Nurse, 1987; Gould and Nurse, 1989; Featherstone and Russell, 1991; Lundgren et al., 1991; Hayles and Nurse, 1995). At the G2–M boundary, the complex gets activated by Cdc25 and Pyp3 phosphatases-catalyzed dephosphorylation, which leads to the onset of mitosis (Russell and Nurse, 1986; Millar et al., 1991, 1992).
One major Cdk inhibitor known in fission yeast is rum1+, which was initially isolated as an inducer of multiple rounds of DNA replication without intervention by mitosis and subsequently shown to inhibit p34cdc2/Cdc13 (Moreno and Nurse, 1994; Correa-Bordes and Nurse, 1995). Cells deleted for rum1+ are sterile and defective in cell cycle control (Moreno and Nurse, 1994). The sterility but not the cell cycle defect is partially suppressed by deletion of Cig2 cyclin (Martin-Castellanos et al., 1996), suggesting a link between this inhibitor and B-type cyclins. However, understanding the regulation of p34cdc2 kinase as a key controller of cell cycling and differentiation is far from complete.
We recently launched an extensive search for factors regulating p34cdc2 and screened a S. pombe cDNA library for multicopy suppressors of the rad1–1 wee1–50 double mutant, a checkpoint mutant that dies of premature activation of p34cdc2 at the restrictive temperature (Al-Khodairy and Carr, 1992; Rowley et al., 1992). Here we report the identification of srw1+, a gene encoding a WD repeat protein that controls differentiation and cell cycling by negatively regulating p34cdc2/B-type cyclin complexes.
MATERIALS AND METHODS
Strains, Media, Libraries, and Vectors
The strains of S. pombe used in this study are listed in Table 3. Media were prepared as described previously (Egel and Egel-Mitani, 1974; Gutz et al., 1974; Moreno et al., 1990; Okazaki et al., 1990). The S. pombe cDNA library was constructed with mRNA from exponentially growing S. pombe cells by H. Tanaka. The vectors used have been described previously (Okazaki et al., 1990; Igarashi et al., 1991).
Table 3.
Strain list
| Strain | Genotype |
|---|---|
| DP2 | h−/h+ ade6-M210/ade6-M216 leu1-32/leu1-32 ura4-D18/ura4-D18 |
| ATCC38399 | h− leu1-32 |
| K150-A13 | h+S leu1-32 |
| K153-B25 | h90 leu1-32 |
| HM332 | h− ura4-294 leu1-32 |
| HM43 | h− mik1::ura4+ wee1-50 ura4-294 leu1-32 |
| HM65 | h− wee1-50 leu1-32 (from P. Nurse) |
| HM58 | h+N rad1-1 wee1-50 leu1-32 |
| HM60 | h+N rad3-136 wee1-50 leu1-32 |
| HM61 | h+N rad9-192 wee1-50 leu1-32 |
| HM64 | h−S chk1::ura4+ wee1-50 ura4-D18 leu1-32 |
| TI101 | h− cig2::ura4+ ura4-D18 ade6-M210 leu1-32 |
| HM270 | h− cdc2-M35 ura4-D18 leu1-32 (from P. Nurse) |
| HM1000 | cdc13::ura4+ int pREP41::cdc13+ ura4-D18 leu1-32 ade6-M216 (from P. Nurse, described in Fisher and Nurse, 1996) |
| SY1 | h− srw1::ura4+ ura4-D18 leu1-32 |
| SY2 | h+S srw1::ura4+ ura4-D18 leu1-32 |
| SY3 | h90 srw1::ura4+ ura4-D18 leu1-32 |
| SY4 | h+S srw1::ura4+ wee1-50 ura4-D18 leu1-32 |
| SY5 | h− srw1::ura4+ cig2/cyc17::ura4+ ura4-D18 leu1-32 |
| SY6 | h+S cdc2-M35 srw1::ura4+ ura4-D18 leu1-32 |
| SY7 | h− int pREP2::srw1+ ura4-294 leu1-32 |
| SY100 | h− rum1::ura4+ ura4-D18 leu1-32 (from S. Moreno) |
Isolation and Structural Analysis of the srw1+ Gene
The rad1–1 wee1–50 leu1–32 cells were cultured at 23°C to midlog phase in MB medium containing 0.15% leucine and transfected with the S. pombe cDNA library as described (Okazaki et al., 1990). The cells were incubated at 23°C for 24 h on minimum medium agar (MMA) plates and then selected at 32.2°C for 4 d. Plasmid DNA was recovered from authentic transformants and cloned in Escherichia coli. DNA sequences were determined by the dideoxy method (Sanger et al., 1977).
Gene Disruption
The srw1+ gene was disrupted as follows. A genomic DNA fragment containing the srw1+ gene was isolated from a S. pombe HindIII-genomic library by colony hybridization. The 2.2-kilobase (kb) EcoRI fragment containing 98% of the srw1+-coding region was replaced with the 1.8-kb HindIII-excised ura4+ gene. The linear fragment carrying the replaced gene was transfected into the h-/h+ ade6-M210/ade6-M216 leu1–32/leu1–32 ura4-D18/ura4-D18 diploid strain, and stable ura4+ cells were isolated. Disruptants were identified by Southern blot using the 0.7-kb HindIII–EcoRI fragment as a probe.
Assay for Conjugation
Mating frequencies were assayed as follows. The h- leu1–32, h− Δsrw1 leu1–32, h− Δcig2 Δsrw1 leu1–32, h+s cdc2-M35 Δsrw1 leu1–32 cells were grown in yeast extract liquid at 25°C, rinsed with water, and mixed with equivalent cultures of h+s leu1–32 or h- leu1–32 cells and incubated on malt extract agar plates for 2 d. Mating frequencies were calculated by dividing the number of zygotes by the number of total cells.
Flow Cytometry
Flow cytometry was performed as described previously (Tanaka et al., 1992), by using the FACScan system and the CellFIT cell cycle analysis program with the software LYSIS (Becton Dickinson, San Jose, CA).
Assay for Loss of Cell Viability Induced by the Expression of cdc2+F15 and cdc13+
The coding regions of cdc2+F15 and cdc13+ were inserted into the pcL expression vector, which contains a LEU2 selection marker, a replication origin, and the SV40 promoter to drive the expression of the insert. The vector carrying the insert was then transfected into Δsrw1 leu1–32, leu1–32, Δsrw1 wee1–50 leu1–32, and wee1–50 leu1–32 cells, and leu+-transformed cells were selected. The ratios of leu+ colonies formed with cdc2+F15 and cdc13+ to those formed with the empty vector were calculated and expressed as percent colony formation.
Northern Blot Analysis
Total RNA was prepared and Northern blot analysis was performed with the 32P-labeled 1.3-kb fragment of ste11+ as a probe as described previously (Nagata et al., 1991; Kato et al., 1996).
Western Blot Analysis
Cells (2–5 × 108) were washed once with water, resuspended in 200 μl of 10% trichloroacetic acid, and disrupted by vortexing with glass beads. After washing with acetone five times, proteins were solubilized by boiling for 5 min in the extraction buffer (50 mM Tris-HCl, pH 7.4, 1 mM EDTA, 1 mM dithiothreitol, 0.5 mM phenylmethylsulfonyl fluoride, and 1% SDS) (Watanabe et al., 1997). Cell extracts (20 μg per lane) were separated by 10% SDS-PAGE (Laemmli, 1970), transferred to Immobilon TM-P membrane (Millipore, Bedford, MA), and the desired protein was detected using ECL (Amersham). Immunoblot was carried out with 1:2000-diluted anti-Cdc13p rabbit antibodies (SP4), 1:2000-diluted anti-Cig2p affinity-purified antibody and 1:50,000-diluted anti-α-tubulin monoclonal antibody (Sigma T5168, Sigma Chemical, St. Louis, MO).
RESULTS
Isolation of the srw1+ Gene
To isolate new elements negatively regulating p34cdc2, we screened a S. pombe cDNA library for those that suppressed the lethality of the rad1–1 wee1–50 double mutant as described previously (Okazaki et al., 1990) and isolated several clones having such an activity. The rad1–1 wee1–50 double mutant is defective in a S-G2 checkpoint control and dies of mitotic catastrophe due to premature activation of p34cdc2 upon shift to the nonpermissive temperature (Al-Khodairy and Carr, 1992; Rowley et al., 1992). Consequently, any one of wee1+, mik1+, and rad1+ suppresses this mutant. One clone did not hybridize with any of them and was therefore characterized further.
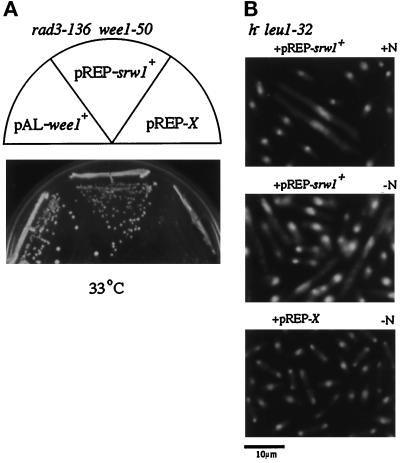
The clone suppressed the rad1–1 wee1–50 double mutant up to 34.5°C, 2°C above the restriction temperature. It also suppressed not only the rad3–136 wee1–50 (Figure 1A), rad9–192 wee1–50, Δchk1 wee1–50 but also Δmik1 wee1–50 double mutants, all of which die of mitotic catastrophe at the nonpermissive temperature, suggesting that this new gene inhibits the activity of p34cdc2/Cdc13 despite low levels of Tyr15 kinase activities. Consistently, overexpression of the clone in wild-type cells resulted in cell elongation, a typical phenotype of cell cycle retardation or arrest, although in a small population. Cell elongation became more evident in nitrogen-poor medium (Figure 1B), suggesting that the activity of this clone might be enhanced in nitrogen-starved environments. This gene was named srw1+ (suppressor of rad wee1) and characterized more extensively.
Figure 1.
Overexpression of srw1+ inhibits onset of mitosis. (A) Suppression of the rad3–136 wee1–50 double mutant by srw1+. The rad3–136 wee1–50 leu1–32 strain (HM60) was transformed to leu+ with pREP1-srw1+, pAL-wee1+, and empty pREP1-X (empty vector), and each transformant was spread on a minimum medium agar (MMA) plate and incubated at 33°C. (B) Morphology of wild-type cells overexpressing srw1+. The h- leu1–32 cells (ATCC38399) were transformed with pREP1-srw1+, grown to midlog in PM+N medium with thiamin, then incubated in thiamin-free PM+N or PM-N medium for 15 h, fixed with 70% ethanol, and stained with DAPI. +N and −N denote nitrogen-rich and free. Control is the cells similarly transformed with pREP1-X and incubated in thiamin-free PM-N medium.
srw1+ Encodes a WD Repeat Protein
srw1+ contains a contiguous open reading frame capable of encoding a 556-amino acid protein with seven WD repeats commonly present in the β-transducin family (Figure 2). It is significantly homologous (38% amino acid identity) with the hypothetical protein of S. cerevisiae Yg1003c identified by the Genome Sequence Project. In addition, Srw1 is also homologous with four distinct proteins, especially in the WD repeat domain. They are Fizzy (Drosophila melanogaster) (Dawson et al., 1995), p55CDC (Homo Sapiens)(Weinstein et al., 1994), CDC20 (S. cerevisiae)(Sethi et al., 1991), and Slp1 (S. pombe)(Matsumoto, 1997)(Figure 2). Fizzy is reportedly required for the degradation of cyclins A and B during mitosis. Accordingly, fizzy mutations cause metaphase arrest accompanied by failure to degrade mitotic cyclins. p55CDC is expressed in dividing cells. CDC20 is required for microtubule-dependent processes, such as nuclear movements before anaphase, chromosome separation, and nuclear fusion during mating of G1 cells, whereas Slp1 genetically interacts with Wee1 kinase or Cdc25 phosphatase, thereby promoting cells to restart the cell cycle after DNA repair is completed.
Figure 2.
Sequence and structure of srw1+. The predicted amino acid sequence of Srw1 is shown in single-letter code and compared with those of Yg1003c, Fizzy, p55CDC, Slp1, and CDC20. The amino acids identical to Srw1 are shaded. The positions of seven putative WD repeat motifs are indicated by bars with numbers, and WD by asterisks. The GenBank/EMBL/DDBJ accession number for srw1+ is AB005589.
Cells Lacking srw1+ Are Sterile
To investigate the biological role of srw1+, a null allele of srw1+ was constructed by one-step gene replacement. A genomic fragment containing srw1+ was isolated by colony hybridization, and the almost entire open reading frame was replaced with the ura4+ gene, followed by transfection into a diploid strain and by selection for stable ura+ diploid cells. Successful disruptants were identified by Southern blot analysis. The diploid cells, in which one allele of srw1+ was disrupted, were germinated to obtain haploid disruptants, which were then extensively backcrossed with wild-type cells to eliminate second mutations.
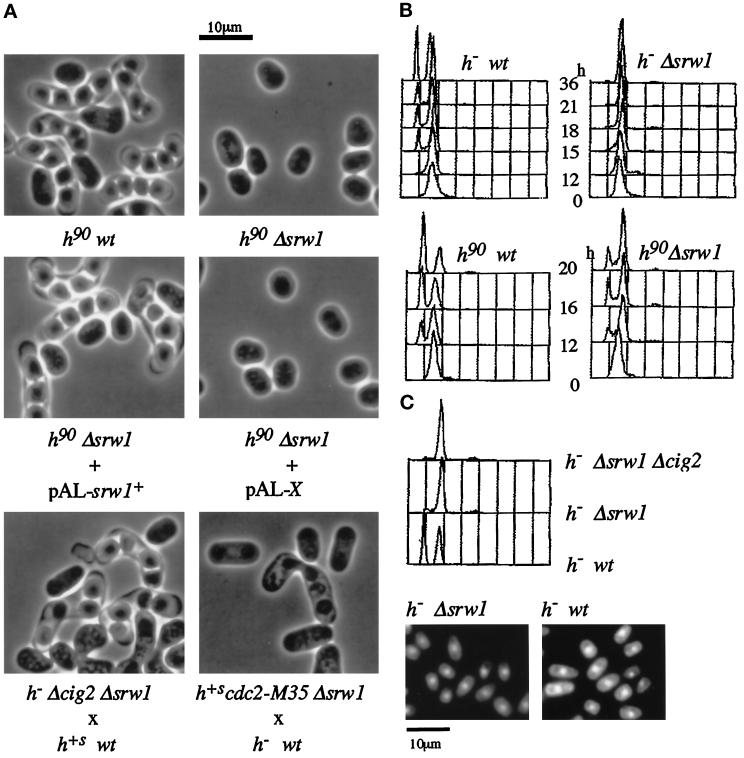
The srw1 disruptants (Δsrw1) grew in the regular medium with no apparent growth defects. However, they showed severe sterility even if wild- type cells were used as a mating partner (Figure 3A and Table 1). We failed to find even a single conjugated cell or spore ascus after extensive search. Sterility was indeed caused by the inactivation of srw1+ because it was effectively suppressed by the srw1+ cDNA as well as genomic DNA.
Figure 3.
Phenotypes of srw1 disruptant. (A) Δsrw1 cells are sterile, but sterility is suppressed by deletion of Cig2 or partial inactivation of Cdc2. The h90 leu1–32 (K153-B25) and h90 srw1::ura4+ ura4-D18 leu1–32 (SY3) cells were grown to midlog phase in YEL and plated on MEA plates for conjugation at 27°C for 2 days. The h90 srw1::ura4+ ura4-D18 leu1–32 (SY3) cells were transformed with pAL-srw1+ or pAL-X (empty pAL vector) and plated for conjugation as above. The h+s leu1–32 (K150-A13) and h- srw1::ura4+ cig2/cyc17::ura4+ ura4-D18 leu1–32 (SY5) cells, or h- leu1–32 (ATCC38399) and h+s cdc2-M35 srw1::ura4+ ura4-D18 leu1–32 (SY6) cells, were mixed and plated for conjugation. (B) Δsrw1 cells are unable to arrest or arrest weakly in G1. The h- leu1–32 (ATCC38399), h- srw1::ura4+ ura4-D18 leu1–32 (SY1), h90 leu1–32 (K153-B25), and h90 srw1::ura4+ ura4-D18 leu1–32 (SY3) cells were grown in YEL to midlog phase, washed, and then incubated at 25°C in malt extract liquid (MEL) for the indicated times and analyzed by flow cytometry. (C) Deletion of Cig2 is unable to restore the G1 arrest ability to Δsrw1 cells. The h- leu1–32 (ATCC38399), h- srw1::ura4+ ura4-D18 leu1–32 (SY1), and h- srw1::ura4+ cig2/cyc17::ura4+ ura4-D18 leu1–32 (SY5) were grown in YEL to midlog phase, incubated at 25°C for 36 h in malt extract liquid (MEL) and analyzed by flow cytometry. The h- srw1::ura4+ ura4-D18 leu1–32 (SY1) and h- leu1–32 (ATCC38399) cells were then stained with DAPI.
Table 1.
Cells lacking srw1+ are sterile but sterility is suppressed by deletion of cig2+ or inactivation of cdc2+
| Strain | % Mating
frequencies
|
|
|---|---|---|
| 27°C | 25°C | |
| h− leu1 × h+S leu1 | 57.0 | 55.2 |
| h−Δsrw1 leu1 × h+S leu1 | <0.01 | <0.01 |
| h−Δcig2 Δsrw1 leu1 × h+S leu1 | 35.6 | 34.7 |
| h+S cdc2-M35 Δsrw1 leu1 × h− leu1 | 0.44 | 0.02 |
Cells were grown to log phase in YEL, mixed and spotted on MEA plates followed by a 2-d incubation at the indicated temperature. Mating efficiencies were calculated as described in MATERIALS AND METHODS.
Deletion of cig2+ or Inactivation of Cdc2 Partially Suppresses Sterility
Cig2/Cyc17 cyclin negatively regulates sexual development as well as promotes the cell cycle start (Obara-Ishihara and Okayama, 1994), specifying p34cdc2 as a kinase partner (Martin-Castellanos et al., 1996). Because srw1+ was able to inhibit p34cdc2/Cdc13 as described above, we speculated that the sterility of Δsrw1 cells might have resulted, at least partly, from the hyperactivation of p34cdc2/Cig2. This proved true. In a conjugation assay using wild-type cells as a mating partner, cig2+ deletion restored fertility to the disruptant to more than one-half the ability of wild-type cells (Figure 3A and Table 1). The kinase partner of Cig2 for this mating inhibition is likely to be p34cdc2 because partial inactivation of p34cdc2 also restored mating ability to the disruptant, although only marginally. The temperature-sensitive cdc2-M35 mutant allele is partially inactivated at 25°C (Nurse and Thuriaux, 1980). At this temperature, the cdc2-M35 Δsrw1 double mutant was still sterile. However, when the temperature was raised to 27°C, a small fraction of the cells came to perform mating and sporulation (Figure 3A and Table 1). Further elevation in the temperature did not increase the mating frequencies perhaps because the cells tended to arrest in G2 due to more inactivation of p34cdc2. These results suggest that the srw1+ gene product promotes sexual development at least by inactivating p34cdc2/Cig2.
The transcriptional factor Ste11 is essential for the initiation of sexual development (Sugimoto et al., 1991), and various differentiation signals including nitrogen starvation signal regulate the expression of ste11+. Therefore, it was important to know whether srw1+ influenced ste11+ mRNA induction. Our unpublished observations show that in Δsrw1 cells ste11+ was expressed and induced to the same extent as wild-type cells upon nitrogen starvation. In addition, ectopic expression of ste11+ driven by the SV40 promoter failed to restore fertility to Δsrw1 cells, indicating that srw1+ promotes sexual development but not via transcriptional regulation of ste11+.
Δsrw1 Cells Are Partially Defective in Nitrogen Starvation-Induced G1 Arrest
The ability to arrest in G1 in response to both nitrogen starvation and mating pheromones is considered to be critical for cells to perform conjugation. We therefore examined the ability of the disruptant to arrest in G1 in response to nitrogen starvation. In conjugation-inducing malt extract medium, which contains a limited amount of nitrogen, Δsrw1 cells proliferated as rapidly as wild-type cells, and both ceased proliferation as nitrogen source was exhausted. At this point, approximately 50% of heterothallic wild-type cells arrested in G1 whereas virtually none of the Δsrw1 cells arrested in G1 (Figure 3B). In homothallic cells, in which mating pheromone signaling is activated, srw1+ was not essential but still required for full G1 arrest. Contrary to our expectation, unlike fertility, G1 arrest ability failed to be restored by the deletion of cig2+ (Figure 3C). Needless to say, lack of G1-arrested cells in the disruptant was not caused by a failure of cell separation before arrest. The growth-arrested Δsrw1 cells became small, and each cell had a single nucleus just like wild-type cells (Figure 3C). These results indicate that there are other target molecule(s) for srw1+ that are specifically required for G1 arrest.
Nevertheless, heterothallic Δsrw1 cells were not totally defective in G1 arrest ability. Abrupt removal of nitrogen source, such as shift to nitrogen-free minimum medium, induced G1 arrest to the disruptant although the G1 arrest was partial and significantly delayed (see below).
Δsrw1 wee1–50ts Double Mutant Dies of Mitotic Catastrophe
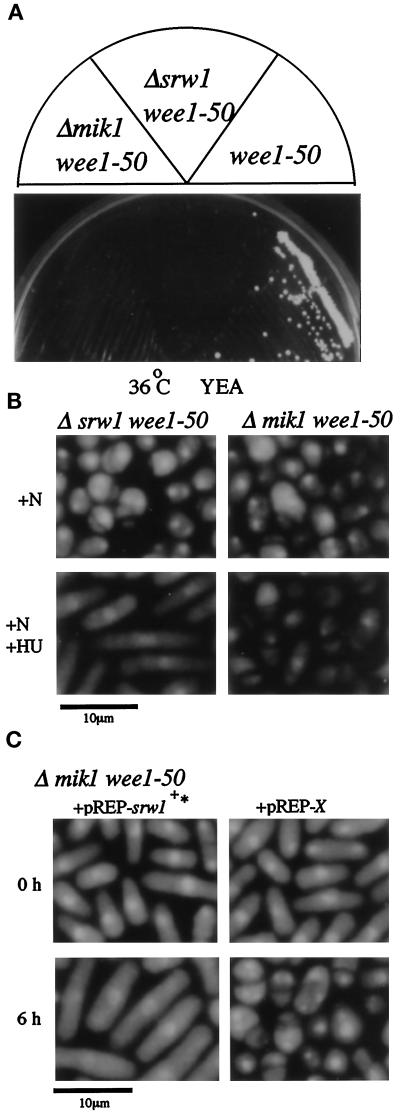
As shown already, overexpression of srw1+ inhibited the activity of p34cdc2/Cdc13. This does not necessarily mean that endogenous Srw1 is involved in mitotic control. We therefore investigated this point. As aforementioned, Δsrw1 cells showed no apparent defects in mitotic control in the regular growth conditions. However, srw1+ was absolutely required for proper mitotic control when the negative regulation of Cdc2/Cdc13 by tyrosine phosphorylation was compromised by inactivation of Wee1 kinase. Despite inactivation of Wee1, the temperature-sensitive wee1–50 mutant grows at 36°C because of the presence of the functionally redundant Mik1 kinase (Nurse, 1975; Thuriaux et al., 1978; Lundgren et al., 1991). However, the Δsrw1 wee1–50 double mutant was unable to grow at this temperature even on nutritionally rich YEA plates (Figure 4A). This resulted from massive entry into mitotic catastrophe, which was characterized by anucleated cells and cells with the nucleus divided by septum (“cut” phenotype) (Figure 4B). These results indicate that srw1+ plays a role negatively controlling the activity of p34cdc2/Cdc13 in cell cycling.
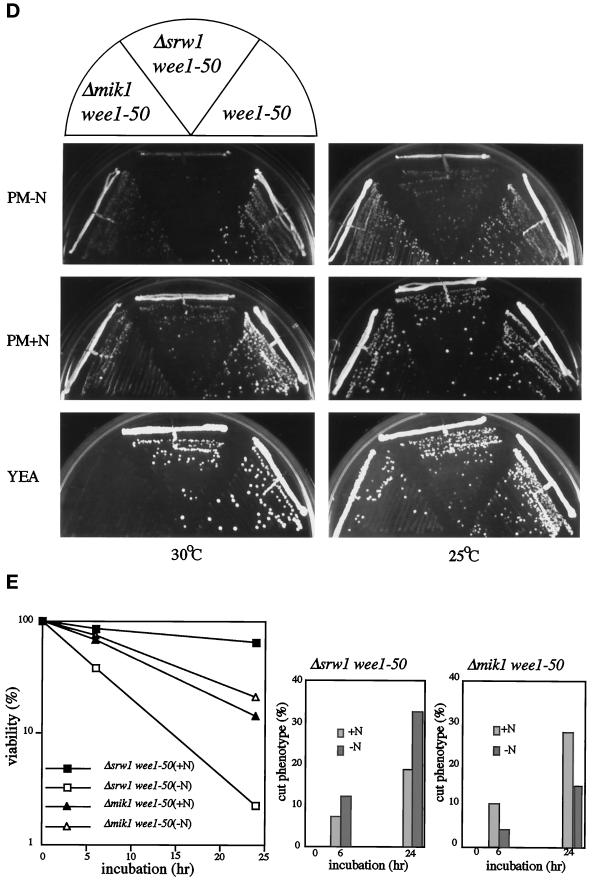
Figure 4.
Δsrw1 wee1–50 cells die of mitotic catastrophe. (A) Δsrw1 wee1–50 cells are unable to grow on YEA plate at 36°C. (B) Δsrw1 wee1–50 cells die of mitotic catastrophe, which is blocked by hydroxyurea. The h+s srw1::ura4+ wee1–50 ura4-D18 leu1–32 (SY4) cells were grown to midlog phase in PM+N medium at 25°C and incubated at 36°C for 6 h in PM+N medium with or without 12 mM hydroxyurea. Cells were fixed with 70% ethanol and stained with DAPI. (C) Overexpression of srw1+ rescues Δmik1 wee1–50 cells from mitotic catastrophe. The h- mik1::ura4+ wee1–50 ura4–294 leu1–32 (HM43) cells were transformed with pREP1-srw1+* (deleted for G-tail) or pREP1-X(empty vector), grown to midlog phase at 25°C in PM+N medium, and then shifted to 36°C. Cells were fixed with 70% ethanol and stained with DAPI. (D)Δsrw1 wee1–50 cells are unable to grow even at 30°C on PM-N plate. The h+s srw1::ura4+ wee1–50 ura4-D18 leu1–32 (SY4), wee1–50 leu1–32 (HM65), and h- mik1::ura4+ wee1–50 ura4–294 leu1–32 (HM43) cells were streaked on PM-N, PM+N, and YEA plates and incubated at 30°C and 25°C. (E) Δsrw1 wee1–50 cells lose viability in nitrogen-poor medium due to mitotic catastrophe. The h+s srw1::ura4+ wee1–50 ura4-D18 leu1–32 (SY4) and h- mik1::ura4+ wee1–50 ura4–294 leu1–32 (HM43) cells were grown to midlog phase in PM+N medium, incubated in PM+N or PM-N at 25°C for 6 h, and transferred to fresh PM+N or PM-N medium at 32°C followed by further incubation for the indicated time. The percent cell viability was calculated by dividing the number of colonies formed on YEA plates at 25°C by that of 0 h after appropriate dilution. The number of cells in cut phenotype was counted under the microscope after staining with DAPI.
The next question we addressed is how srw1+ regulates the activity of p34cdc2/Cdc13. Inhibition of DNA synthesis by hydroxyurea activates a S-G2 checkpoint control, which prevents the activation of p34cdc2/Cdc13 predominantly via inhibiting Tyr15 dephosphorylation (Enoch et al., 1992). Hydroxyurea treatment effectively blocked entry of Δsrw1 wee1–50 cells into mitotic catastrophe that occurred upon shift to the nonpermissive temperature, and induced cell elongation (Figure 4B). This is in sharp contrast to Δmik1 wee1–50 cells, which failed to arrest by hydroxyurea and prematurely entered mitosis with massive catastrophic cell death. This result suggests that srw1+ is likely to negatively regulate the p34cdc2/Cdc13 activity mainly by a mechanism independent of tyrosine 15 phosphorylation.
To further investigate the relationship between srw1+ and Tyr15 regulation, we used Δmik1 wee1–50 strain and Cdc2F15. As noted earlier, overexpressed srw1+ rescued not only the rad1 wee1–50 but also Δmik1 wee1–50 strain. As shown in Figure 4C, 4,6-diamidino-2-phenylindole (DAPI) staining revealed that overexpressed srw1+ effectively suppressed the mitotic catastrophe of the Δmik1 wee1–50 strain. This result supports our initial observation that the action of srw1+ is mostly, if not entirely, independent of tyrosine 15 phosphorylation. To further confirm and extend this observation, we tested the effect on cell viability of the expression of constitutively active Cdc2F15, in which tyrosine 15 was replaced with unphosphorylatable phenylalanine to completely eliminate the regulation by Tyr15 phosphorylation. The coding region of cdc2+F15 was inserted into the pcL expression vector driven by the SV40 promoter and containing the leu+ marker gene. If the cells transfected with the vector would lose viability by the expression of the insert, less leu+ colonies would be formed. Expression of pcL-cdc2+F15 was not highly toxic to wild type cells, and leu+ colonies were formed at 42% the efficiency of the empty vector (Table 2). On the contrary, pcL-cdc2+F15 was extremely toxic to Δsrw1 cells, and its colony-forming efficiency was reduced more than 200-fold. Thus, the combination of Cdc2F15 expression and inactivation of srw1+ seemed lethal to cells, reinforcing our initial observation.
Table 2.
The viability of cells expressing CdcF15 and Cdc13
| Strain | %
Leu+
|
||
|---|---|---|---|
| pcL-X | pcL-cdc2+F15 | pcL-cdc13+ | |
| h− leu1 | 100 | 41.7 ± 7.9 | 38.4 ± 7.6 |
| h− Δsrw1 leu1 | 100 | 0.157 ± 0.076 | 20.3 ± 5.66 |
| h− wee1-50 leu1 | 100 | ND | 48.7 ± 20.6 |
| h+S Δsrw1 wee1-50 leu1 | 100 | ND | 1.35 ± 0.11 |
Cells were transfected with the indicated plasmids and selected for leu+ phenotype at 30°C for the h− leu1-32 and h− Δsrw1 leu1-32 cells and at 25°C for the h− wee1-50 leu1-32 and h+S Δsrw1 wee1-50 leu1-32 cells, both temperatures being permissive for these strains. The ratios of leu+ colonies formed with the indicated plamids to those formed with the empty vector are expressed as % leu+. Each experiment was performed three times and values of mean ± SD are shown.
Our observation suggested that the function of srw1+ seemed to be enhanced during nitrogen starvation. To investigate this possibility, the effect of nitrogen starvation on the growth ability of Δsrw1 wee1–50 cells was studied. Both wee1–50 and Δmik1 wee1–50 cells were used as controls. At 30°C on PM+N plates, all these mutants grew without noticeable difficulties. But on nitrogen-poor PM-N plates or in such medium, unlike wee1–50 single and Δmik1 wee1–50 double mutants, Δsrw1 wee1–50 cells failed to grow (Figure 4D) and died of increased mitotic catastrophe (Figure 4E). By contrast, Δmik1 wee1–50 cells were unable to grow on nutritionally rich YEA plates or in nitrogen-rich PM+N medium and died of increased mitotic catastrophe. These results confirm the initial observation and strongly indicate that the biological role of Srw1 is signified in nitrogen-poor environments.
Overexpression of srw1+ Induces Endoreplication
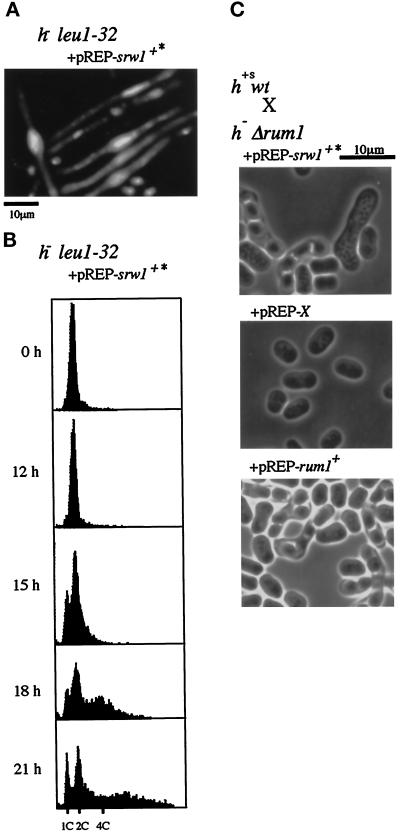
As one might have noticed previously, there is a striking functional similarity between srw1+ and rum1+. Both genes inhibit the onset of mitosis, both gene disruptants are phenotypically similar, being sterile and defective in nitrogen starvation-induced G1 arrest and in mitotic control, and their sterility is suppressed by deletion of Cig2 (Moreno and Nurse, 1994; Martin-Castellanos et al., 1996). To further investigate the similarity, we examined the ability of srw1+ to induce endoreplication. To obtain high expression of srw1+, the G-tail of the srw1+ cDNA was deleted, reinserted into the thiamin-repressible pREP1 vector (pREP-srw1+*), and expressed in wild-type cells. Upon removal of thiamin, many cells showed enlarged nuclei brightly stained with DAPI (Figure 5A). FACS analysis revealed that some cell population contained 4C (4 DNA content) or more DNA highly indicative of endoreplication (Figure 5B). Interestingly, a considerable fraction of cells arrested in 1C (1 DNA content), suggesting that Srw1 has the ability to block the onsets of both S phase and mitosis. Partial block of S phase onset was also observed with wild-type cells harboring an integrated copy of pREP2-srw1+*. In addition, overexpression of srw1+ restored fertility to Δrum1 cells (Figure 5C). With wild-type cells as a mating partner, srw1+-overexpressing Δrum1 cells conjugated at about 20% the frequency of control wild-type cells. By contrast, overexpression of rum1+ failed to restore fertility to Δsrw1 cells. These results establish functional similarities between Srw1 and Rum1 although the molecular mechanisms by which they inhibit p34cdc2/B-type cyclin are unlikely to be the same.
Figure 5.
Overexpression of srw1+ can induce endoreplication and restores fertility to Δrum1 cells. (A) DAPI staining and (B) Flow cytometry (FACS) analysis of wild-type cells expressing srw1+. The srw1+ cDNA was deleted for G-tail, reinserted into the pREP1 vector (pREP1-srw1+*) and transformed into wild-type cells. Cells were grown first in PM+N medium with thiamin and then washed and transferred to PM+N medium without thiamin (0 h) and incubated for the indicated times. (C) Overexpression of srw1+ restores fertility to Δrum1 cells. The h- rum1::ura4+ ura4-D18 leu1–32 (SY100) cells transformed with pREP1-srw1+*, pREP1-rum1+ or pREP1-X were mixed with h+s leu1–32 (K150-A13) cells and spotted on MEA plates and incubated at 27°C for 2 d.
Srw1 Is Involved in Regulation of the Amount of Cdc13
To gain further insights into the function of srw1+, we examined the effect of Cdc13 overproduction on the viability of Δsrw1 cells. Overexpression of cdc13+ in Δsrw1 cells had little effect but decreased colony formation slightly. However, when the disruptant was slightly inactivated for Wee1 kinase, Cdc13 overproduction had a dramatic effect. Even at a highly permissive temperature of 25°C for wee1–50, overexpressed cdc13+ was highly toxic to Δsrw1 wee1–50 cells (Table 2), and they died of mitotic catastrophe. These results, combined with the independence of Srw1 function from Tyr15 phosphorylation and its structural similarity to Fizzy reportedly required for cyclin degradation in Drosophila (Dawson et al., 1995), suggest that srw1+ inhibits the p34cdc2/Cdc13 activity possibly by modulating the amount of Cdc13.
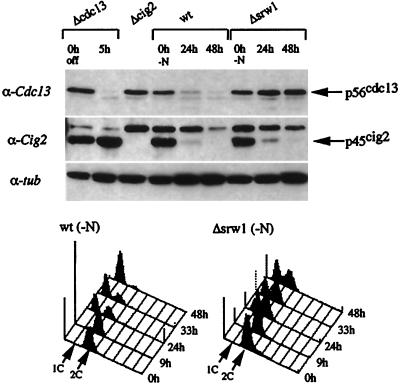
We therefore investigated the protein level of Cdc13 and also Cig2 B-type cyclins in Δsrw1 cells by Western blot analysis. As already noted, when transferred to nitrogen-free minimum medium, Δsrw1 cells could arrest in G1 despite a significant delay. At 24 h posttransfer, more than one-half of wild-type cells arrested in G1 with marked reduction in the level of Cdc13 (Figure 6). At 48 h, more than 90% of the cells were in G1 with a nearly undetectable level of Cdc13. By contrast, in Δsrw1 cells, the amount of Cdc13 did not decrease, but tended to rather slightly increase, at 48 h despite that more than one-half the cells arrested in G1. This result shows that Srw1 is essential for decrease of Cdc13 upon nitrogen starvation. By contrast, Cig2 cyclin behaved differently. In both wild-type and Δsrw1 cells, Cig2 disappeared upon nitrogen starvation, suggesting that Srw1 inhibits p34cdc2/Cig2 by a different mechanism and that the action of Srw1 to p34cdc2/Cig2 might be early in nitrogen starvation.
Figure 6.
Srw1 is required for nitrogen starvation-induced decrease of the amount of Cdc13 but not Cig2. The h- leu1–32 (wt, ATCC38399) and h- srw1::ura4+ ura4-D18 leu1–32 (Δsrw1, SY1) cells were grown in minimal medium to log-phase at 25°C and then incubated in nitrogen-free minimum medium for the indicated times. The h- cig2::ura4+ ura4-D18 ade6-M210 leu1–32 (Δcig2, TI101) and cdc13::ura4+ int pREP41::cdc13+ ura4-D18 leu1–32 ade6-M216 (Δcdc13, HM1000) strains were used as controls. For Δcdc13 cells, 5 μg/ml thiamin were added to the culture to shut off cdc13 expression. Extracts were prepared and separated by SDS-PAGE, and immunoblotted with anti-Cdc13, anti-Cig2, and anti-α-tubulin antibodies as probes. Flow cytometric analysis was performed. The 1 N and 2 N DNA contents are indicated by arrows.
DISCUSSION
How cell differentiation and the cell cycle are coordinately regulated is one critical question that remains to be addressed. Our data show that the newly identified srw1+ gene is involved in this regulation. Cells lacking this gene are defective in both cell differentiation and cell cycle controls. Their most apparent phenotypes are sterility and defects in G1 and mitotic controls. Sterility was so severe that no conjugated cells were detected throughout this series of experiments (Table 1). The defect in G1 control mainly involves the cell’s inability to arrest or slow down before the onset of S phase. However, the disruptant is not completely defective in G1 arrest ability. When mating pheromone signaling was activated or cells were rapidly starved for nitrogen, they could arrest in G1 at least partly. By contrast, their defect in mitotic control is dormant until the regulation of Cdc2 by tyrosine phosphorylation is slightly compromised by partial inactivation of Wee1 kinase. In such a situation, Srw1 is absolutely required for blocking premature mitosis and such a role of Srw1 is particularly evident in nitrogen-poor environments.
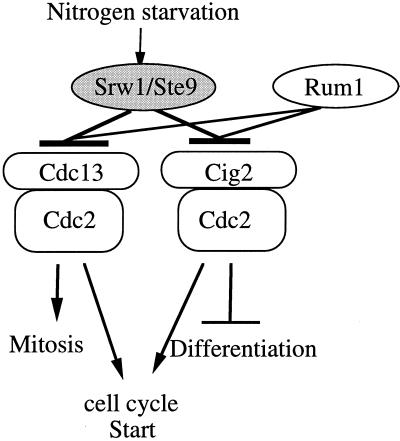
All the genetic and functional analysis data led us to conclude that, particularly responding to nitrogen starvation, Srw1 promotes differentiation and inhibits the onset of mitosis via inhibiting at least p34cdc2/Cig2 and p34cdc2/Cdc13, respectively (Figure 7). Cig2 cyclin plays a dual role inhibiting cell differentiation and promoting the cell cycle start (Obara-Ishihara and Okayama, 1994; Mondesert et al., 1996). Interestingly, the sterility but not the G1 arrest inability of Δsrw1 cells was effectively suppressed by the deletion of Cig2. The lack of the restoration of the G1 arrest ability was unexpected but may be explained by the presence of Cdc13 mitotic cyclin, which shares the S phase start function with Cig2 (Fisher and Nurse 1996; Mondesert et al., 1996). Thus, Srw1 plays a key role in the coordinated regulation of cell differentiation and proliferation by nutrient starvation.
Figure 7.
Proposed model for Srw1 function. Srw1 controls differentiation and the onsets of S phase and mitosis by negatively regulating Cdc2/B-type cyclin complexes.
Our genetic data suggested that the primary target(s) for the Srw1 action for mitotic control were not Tyr15 of Cdc2 but Cdc13 B-type cyclin. This was confirmed by the biochemical data demonstrating that Srw1 negatively regulates the level of Cdc13 upon nitrogen starvation (Figure 6). Taking the structural similarity with fizzy into consideration, it is most probable that Srw1 might directly promote degradation of Cdc13. However, contrary to our expectation, Srw1 is unlikely to play a role regulating the level of Cig2. Regardless of the presence or absence of Srw1, Cig2 was degraded upon nitrogen starvation, suggesting that Srw1 might inhibit p34cdc2/Cig2 by a different mechanism.
Interestingly, Srw1 shares striking functional similarity with Rum1 despite their structural dissimilarity. Both deletion mutants are sterile, and their sterility is suppressed at least in part by the inactivation of Cig2. In addition, both mutants are defective in G1 and mitotic controls, and their defects in mitotic control are displayed when the mitotic start regulation by tyrosine phosphorylation is compromised. Both overexpressed srw1+ and rum1+ block the onset of mitosis and induce endoreplication. The major target for both factors is Cdc2 kinase associated with B-type cyclins. But, the molecular mechanisms by which they inhibit p34cdc2/B-type cyclins appear to differ at least partly. Rum1 directly binds p34cdc2/Cdc13 and inhibits its activity (Correa-Bordes and Nurse, 1995), whereas the primary action of Srw1 to the mitotic kinase seems to be to regulate the level of Cdc13.
In addition to rum1+ deletion mutants, cells deficient in nuc2+ are phenotypically similar to Δsrw1 cells. They are sterile and defective in nitrogen starvation-induced G1 arrest (Kumada et al., 1995). Despite such similarity, srw1+ and nuc2+ seem to differ fundamentally at least in some functional aspects and therefore in their targets. srw1+ inhibits the onset of mitosis whereas nuc2+ promotes the metaphase–anaphase transition while inhibiting formation of septum.
Recently we learned that srw1+ is identical to ste9+ (Kitamura et al., personal communication). The ste9 mutant was initially isolated a long time ago but has not well been characterized until recently (Sipiczki, 1988).
ACKNOWLEDGMENTS
We thank P. Nurse and his laboratory members for supplying strains, antibodies, and helpful support; K. Kitamura for comparing the amino acid sequence of ste9+ with that of srw1+; and S. Moreno for supplying a strain. We also thank K. Okazaki for the genomic DNA library and helpful discussion. This work was supported by grants from the Ministry of Science, Education and Culture, Japan, and from Human Frontier Science Program.
REFERENCES
- Al-Khodairy F, Carr AM. DNA repair mutants defining G2 checkpoint pathways in Schizosaccharomyces pombe. EMBO J. 1992;11:1343–1350. doi: 10.1002/j.1460-2075.1992.tb05179.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basi G, Draetta G. p13suc1 of Schizosaccharomyces pombe regulates two distinct forms of the mitotic cdc2 kinase. Mol Cell Biol. 1995;15:2028–2036. doi: 10.1128/mcb.15.4.2028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booher RN, Alfa CE, Hyams JS, Beach DH. The fission yeast cdc2/cdc13/suc1 protein kinase: regulation of catalytic activity and nuclear localization. Cell. 1989;58:485–497. doi: 10.1016/0092-8674(89)90429-7. [DOI] [PubMed] [Google Scholar]
- Correa-Bordes J, Nurse P. p25rum1 orders S phase and mitosis by acting as an inhibitor of the p34cdc2 mitotic kinase. Cell. 1995;83:1001–1009. doi: 10.1016/0092-8674(95)90215-5. [DOI] [PubMed] [Google Scholar]
- Dawson IA, Roth S, Artavanis-Tsakonas S. The Drosophila cell cycle gene fizzy is required for normal degradation of cyclins A and B during mitosis and has homology to the CDC20 gene of Saccharomyces cerevisiae. J Cell Biol. 1995;129:725–737. doi: 10.1083/jcb.129.3.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egel R, Egel-Mitani M. Premeiotic DNA synthesis in fission yeast. Exp Cell Res. 1974;88:127–134. doi: 10.1016/0014-4827(74)90626-0. [DOI] [PubMed] [Google Scholar]
- Enoch T, Carr AM, Nurse P. Fission yeast genes involved in coupling mitosis to completion of DNA replication. Genes & Dev. 1992;6:2035–2046. doi: 10.1101/gad.6.11.2035. [DOI] [PubMed] [Google Scholar]
- Featherstone C, Russell P. Fission yeast p107wee1 mitotic inhibitor is a tyrosine/serine kinase. Nature. 1991;349:808–811. doi: 10.1038/349808a0. [DOI] [PubMed] [Google Scholar]
- Fisher DL, Nurse P. A single fission yeast mitotic cyclin B p34cdc2 kinase promotes both S-phase and mitosis in the absence of G1 cyclins. EMBO J. 1996;15:850–860. [PMC free article] [PubMed] [Google Scholar]
- Funabiki H, Yamano H, Kumada K, Nagao K, Hunt T, Yanagida M. Cut2 proteolysis required for sister-chromatid separation in fission yeast. Nature. 1996;381:438–441. doi: 10.1038/381438a0. [DOI] [PubMed] [Google Scholar]
- Glotzer M, Murry AW, Kirschner MW. Cyclin is degraded by the ubiquitin pathway. Nature. 1991;349:132–138. doi: 10.1038/349132a0. [DOI] [PubMed] [Google Scholar]
- Gorden C, McGurk G, Dillon P, Rosen C, Hastie ND. Defective mitosis due to a mutation in the gene for a fission yeast 26S protease subunit. Nature. 1993;366:355–357. doi: 10.1038/366355a0. [DOI] [PubMed] [Google Scholar]
- Gorden C, McGurk G, Wallace M, Hastie ND. A conditional lethal mutant in the fission yeast 26S protease subunit mts3+ is defective in metaphase to anaphase transition. J Biol Chem. 1996;271:5704–5711. doi: 10.1074/jbc.271.10.5704. [DOI] [PubMed] [Google Scholar]
- Gould KL, Nurse P. Tyrosine phosphorylation of the fission yeast cdc2+ protein kinase regulates entry into mitosis. Nature. 1989;342:39–45. doi: 10.1038/342039a0. [DOI] [PubMed] [Google Scholar]
- Gutz H, Heslot H, Leupold U, Loprieno N. Handbook of Genetics. R.C. King, New York: Plenum; 1974. Schizosaccharomyces pombe; pp. 395–446. [Google Scholar]
- Hayles J, Nurse P. A pre-start checkpoint preventing mitosis in fission yeast acts independently of p34cdc2 tyrosine phosphorylation. EMBO J. 1995;14:2760–2771. doi: 10.1002/j.1460-2075.1995.tb07276.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igarashi M, Nagata A, Jinno S, Suto K, Okayama H. Wee1+-like gene in human cells. Nature. 1991;353:80–83. doi: 10.1038/353080a0. [DOI] [PubMed] [Google Scholar]
- Kato T, Jr, Okazaki K, Murakami H, Stettler S, Fantes AP, Okayama H. Stress signal, mediated by a Hog1-like MAP kinase, controls sexual development in fission yeast. FEBS Lett. 1996;378:207–212. doi: 10.1016/0014-5793(95)01442-x. [DOI] [PubMed] [Google Scholar]
- Kumada K, Su S, Yanagida M, Toda T. Fission yeast TPR-family protein nuc2 is required for G1-arrest upon nitrogen starvation and is an inhibitor of septum formation. J Cell Sci. 1995;108:895–905. doi: 10.1242/jcs.108.3.895. [DOI] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lundgren K, Walworth N, Booher R, Dembski M, Kirschner M, Beach D. mik1 and wee1 cooperate in the inhibitory tyrosine phosphorylation of cdc2. Cell. 1991;64:1111–1122. doi: 10.1016/0092-8674(91)90266-2. [DOI] [PubMed] [Google Scholar]
- Martin-Castellanos C, Labib K, Moreno S. B-type cyclins regulate G1 progression in fission yeast in opposition to the p25rum1 cdk inhibitor. EMBO J. 1996;15:839–849. [PMC free article] [PubMed] [Google Scholar]
- Matsumoto T. A fission yeast homolog of CDC20/p55CDC/Fizzy is required for recovery from DNA damage and genetically interacts with p34cdc2. Mol Cell Biol. 1997;17:742–750. doi: 10.1128/mcb.17.2.742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millar JBA, Lenaers G, Russell P. Pyp3 PTPase acts as a mitotic inducer in fission yeast. EMBO J. 1992;11:4933–4941. doi: 10.1002/j.1460-2075.1992.tb05600.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millar JBA, McGowan CH, Lenaers G, Jones R, Russell P. p80cdc25 mitotic inducer is the tyrosine phosphatase that activates p34cdc2 kinase in fission yeast. EMBO J. 1991;10:4301–4309. doi: 10.1002/j.1460-2075.1991.tb05008.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mondesert O, McGowan CH, Russell P. Cig2, a B-type cyclin, promotes the onset of S in Schizosaccharomyces pombe. Mol Cell Biol. 1996;16:1527–1533. doi: 10.1128/mcb.16.4.1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno S, Hayles J, Nurse P. Regulation of p34cdc2 protein kinase during mitosis. Cell. 1989;58:361–372. doi: 10.1016/0092-8674(89)90850-7. [DOI] [PubMed] [Google Scholar]
- Moreno S, Klar A, Nurse P. Molecular genetic analysis of fission yeast Schizosaccharomyces pombe. Methods Enzymol. 1990;194:795–823. doi: 10.1016/0076-6879(91)94059-l. [DOI] [PubMed] [Google Scholar]
- Moreno S, Nurse P. Regulation of progression through the G1 phase of the cell cycle by the rum1+ gene. Nature. 1994;367:236–242. doi: 10.1038/367236a0. [DOI] [PubMed] [Google Scholar]
- Murakami H, Okayama H. Cell cycle checkpoint control. Mol Exp Med. 1997;29:1–11. [Google Scholar]
- Nagata A, Igarashi M, Jinno S, Suto K, Okayama H. An additional homolog of the fission yeast cdc25+ gene occurs in human and is highly expressed in some cancer cells. New Biol. 1991;3:959–968. [PubMed] [Google Scholar]
- Nigg EA. Cyclin-dependent protein kinases: key regulators of the eukaryotic cell cycle. Bioessays. 1995;17:471–480. doi: 10.1002/bies.950170603. [DOI] [PubMed] [Google Scholar]
- Nurse P. Genetic control of cell size at cell division in yeast. Nature. 1975;256:547–551. doi: 10.1038/256547a0. [DOI] [PubMed] [Google Scholar]
- Nurse P. Universal control mechanism regulating onset of M phase. Nature. 1990;344:503–508. doi: 10.1038/344503a0. [DOI] [PubMed] [Google Scholar]
- Nurse P, Bissett Y. Gene required in G1 for commitment to cell cycle and in G2 for control of mitosis in fission yeast. Nature. 1981;292:558–560. doi: 10.1038/292558a0. [DOI] [PubMed] [Google Scholar]
- Nurse P, Thuriaux P. Regulatory genes controlling mitosis in the fission yeast Schizosaccharomyces pombe. Genetics. 1980;96:627–637. doi: 10.1093/genetics/96.3.627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obara-Ishihara T, Okayama H. A B-type cyclin negatively regulates conjugation via interacting with cell cycle “start” genes in fission yeast. EMBO J. 1994;13:1863–1872. doi: 10.1002/j.1460-2075.1994.tb06455.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okayama H, Nagata A, Jinno S, Murakami H, Tanaka K, Nakashima N. Cell cycle control in fission yeast and mammals identification of new regulatory mechanisms. Adv Cancer Res. 1996;69:17–62. doi: 10.1016/s0065-230x(08)60859-3. [DOI] [PubMed] [Google Scholar]
- Okazaki K, Okazaki N, Kume K, Jinno S, Tanaka K, Okayama H. High frequency transformation method and library transducing vectors for cloning mammalian cDNAs by transcomplementation of Schizosaccharomyces pombe. Nucleic Acids Res. 1990;18:6485–6489. doi: 10.1093/nar/18.22.6485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowley R, Subramani S, Young PG. Checkpoint controls in Schizosaccharomyces pombe: rad1. EMBO J. 1992;11:1335–1342. doi: 10.1002/j.1460-2075.1992.tb05178.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell P, Nurse P. cdc25+ functions as an inducer in the mitotic control of fission yeast. Cell. 1986;45:145–153. doi: 10.1016/0092-8674(86)90546-5. [DOI] [PubMed] [Google Scholar]
- Russell P, Nurse P. Negative regulation of mitosis by wee1+, a gene encoding a protein kinase homolog. Cell. 1987;49:559–567. doi: 10.1016/0092-8674(87)90458-2. [DOI] [PubMed] [Google Scholar]
- Sanger F, Nicklen S, Goulson A. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA. 1977;74:5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sethi N, Monteagudo MC, Koshland D, Hogan E, Burke DJ. The CDC20 gene product of Saccharomyces cerevisiae, a β-transducin homolog, is required for a subset of microtubule-dependent cellular processes. Mol Cell Biol. 1991;11:5592–5602. doi: 10.1128/mcb.11.11.5592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sipiczki M. The role of sterility genes (ste and aff) in the initiation of sexual development in Schizosaccharomyces pombe. Mol & Gen Genet. 1988;213:529–534. doi: 10.1007/BF00339626. [DOI] [PubMed] [Google Scholar]
- Sugimoto A, Iino Y, Maeda T, Watanabe Y, Yamamoto M. Schizosaccharomyces pombe ste11+ encodes a transcription factor with an HMG motif that is a critical regulator of sexual development. Genes & Dev. 1991;5:1990–1999. doi: 10.1101/gad.5.11.1990. [DOI] [PubMed] [Google Scholar]
- Tanaka K, Okazaki K, Okazaki N, Ueda T, Sugiyama A, Nojima H, Okayama H. A new cdc gene required for S phase entry of Schizosaccharomyces pombe encodes a protein similar to the cdc10+ and SWI4 gene products. EMBO J. 1992;11:4923–4932. doi: 10.1002/j.1460-2075.1992.tb05599.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thuriaux P, Nurse P, Carter B. Mutants altered in the control coordinating cell division with cell growth in the fission yeast Schizosaccharomyces pombe. Mol & Gen Genet. 1978;161:215–220. doi: 10.1007/BF00274190. [DOI] [PubMed] [Google Scholar]
- Watanabe Y, Shinozaki-Yabana S, Chikashige Y, Hiraoka Y, Yamamoto M. Phosphorylation of RNA-binding protein controls cell cycle switch from mitotic to meiotic in fission yeast. Nature. 1997;386:187–190. doi: 10.1038/386187a0. [DOI] [PubMed] [Google Scholar]
- Weinstein J, Jacobsen FW, Hsu-Chen J, Wu T, Baum LG. A novel mammalian protein, p55CDC, present in dividing cells is associated with protein kinase activity and has homology to the Saccharomyces cerevisiae cell division cycle proteins Cdc20 and Cdc4. Mol Cell Biol. 1994;14:3350–3363. doi: 10.1128/mcb.14.5.3350. [DOI] [PMC free article] [PubMed] [Google Scholar]