Abstract
The somatic gonad of the nematode Caenorhabditis elegans has the myoepithelial sheath that surrounds oocytes and provides contractile forces during ovulation. Contractile apparatuses of the myoepithelial sheath cells are non-striated and similar to those of smooth muscle. We report identification of a specific isoform of actin depolymerizing factor (ADF)/cofilin as an essential factor for assembly of contractile actin networks in the gonadal myoepithelial sheath. Two ADF/cofilin isoforms, UNC-60A and UNC-60B, are expressed from the unc-60 gene by alternative splicing. RNA interference of UNC-60A caused disorganization of the actin networks in the myoepithelial sheath. UNC-60B, which is known to function in the body wall muscle, was not necessary or sufficient for actin organization in the myoepithelial sheath. However, mutant forms of UNC-60B with reduced actin filament severing activity rescued the UNC-60A-depletion phenotype. UNC-60A has much weaker filament severing activity than UNC-60B, suggesting that an ADF/cofilin with weak severing activity is optimal for assembly of actin networks in the myoepithelial sheath. In contrast, strong actin filament severing activity of UNC-60B was required for assembly of striated myofibrils in the body wall muscle. Our results suggest that an optimal level of actin filament severing activity of ADF/cofilin is required for assembly of actin networks in the somatic gonad.
Keywords: Actin dynamics, severing, contraction, ovulation, myoepithelial cells
Introduction
Striated and non-striated muscles have different organization of actin and myosin filaments and play distinct physiological roles. Striated muscle can repeat rapid cycles of contraction and relaxation and has uniformly oriented sarcomeric myofibrils so that the cell can contract in one direction (Squire, 1997). Non-striated (or smooth) muscle generally contracts and relaxes slowly and has a lattice-like network of contractile actomyosin filaments so that contraction changes the cell shape in a more flexible manner (Gerthoffer, 2005; Somlyo and Somlyo, 1994). Thus, the difference in the organization of actomyosin filaments is important for their different functions. However, it is currently unknown as to how actin and myosin are assembled into such different structures. In vertebrates, different sets of structural proteins are expressed in striated and smooth muscles, and perhaps different biochemical properties of the structural proteins may contribute to differentiation of the contractile apparatuses.
The nematode Caenorhabditis elegans is a relatively simple model to study the mechanism of cytoskeletal differentiation. The body wall muscle is obliquely striated muscle and has been extensively used as a model to study structure and function of striated muscle (Moerman and Fire, 1997; Waterston, 1988). C. elegans also has several different non-striated muscles such as the pharyngeal muscle and the myoepithelial cells of the somatic gonad, but cell biology of these muscles has not been studied as extensively as that of the body wall muscle. The myoepithelial sheath of the somatic gonad is particularly an interesting system, in which many of the structural components of the contractile apparatuses are expressed from genes that are also expressed in the striated body wall muscle (Ardizzi and Epstein, 1987; Ono and Ono, 2004; Ono et al., 2007; Rose et al., 1997). The myoepithelial sheath cells are smooth muscle-like cells that surround oocytes at the proximal gonad (Hall et al., 1999; Strome, 1986). Contraction of the myoepithelial sheath is coupled with oocyte maturation and is essential for ovulation (McCarter et al., 1997; McCarter et al., 1999). Thus, the gonadal myoepithelial sheath is substantially different from the body wall muscle, yet the same structural components are used to assemble striated and non-striated contractile apparatuses. Therefore, different regulatory mechanisms for cytoskeletal assembly are expected to operate in these muscles.
To explore the mechanism of assembly of non-striated contractile apparatuses in the myoepithelial sheath cells, we hypothesized that actin depolymerizing factor (ADF)/cofilin is a critical regulator of actin organization. ADF/cofilin enhances actin dynamics by severing and depolymerizing actin filaments and is involved in a wide range of actin cytoskeletal remodeling processes (Ono, 2007). In C. elegans, two ADF/cofilin isoforms, UNC-60A and UNC-60B, are expressed from the unc-60 gene by alternative splicing (Anyanful et al., 2004; McKim et al., 1994). In vitro, UNC-60A and UNC-60B exhibit different actin-regulatory activities (Ono and Benian, 1998; Yamashiro et al., 2005). UNC-60A only weakly severs actin filaments and strongly prevents polymerization, while UNC-60B strongly severs actin filaments and accelerates polymerization. UNC-60A is widely expressed in non-muscle cells including early embryos and required for embryonic cytokinesis (Ono et al., 2003). UNC-60B is expressed in the body wall muscle and required for organized assembly of myofibrils (Ono et al., 1999) and muscle arm development (Dixon and Roy, 2005). In this study, we identified UNC-60A as an essential ADF/cofilin isoform for actin organization in the somatic gonad. Although wild-type UNC-60B did not substitute for the function of UNC-60A, mutant forms of UNC-60B with defective actin severing activity rescued the UNC-60A-depletion phenotype. These results demonstrate non-redundant functions of the two ADF/cofilin isoforms and suggest that the difference in their actin filament severing activity determines the isoform-specific functions.
Results
UNC-60A is required for assembly of contractile apparatuses in the myoepithelial sheath cells
The myoepithelial sheath cells express a number of contractile proteins that are also expressed in the striated body wall muscle (Ardizzi and Epstein, 1987; Ono et al., 2007). Therefore, we predicted that UNC-60B, one of the two splice variants of ADF/cofilin, might have an important function, since it is expressed in the body wall muscle and required for organization of striated myofibrils (Ono et al., 2003; Ono et al., 1999). unc-60B(su158) is an unc-60B(null) allele that has a deletion in the unc-60B coding region without affecting the function of unc-60A (Ono et al., 2003). However, in unc-60B(su158) homozygous animals, actin filaments were nearly normally organized into non-striated meshwork in the myoepithelial sheath cells (Fig. 1D), and there was no major difference in actin organization from wild-type (Fig. 1A). In addition, unc-60B(su158) homozygotes could ovulate, and no endomitotic oocytes were detected (n=50) in the proximal gonad (Fig. 1D-F), indicating that the myoepithelial sheath cells function properly during ovulation, and that UNC-60B is not required in the myoepithelial sheath.
Figure 1.
Requirement of UNC-60A but not UNC-60B for assembly of organized actin networks in the myoepithelial sheath of the somatic gonad. Dissected hermaphroditic gonads from wild-type (A-C), unc-60B(su158) (D-F), control RNAi (G-I), or unc-60A(RNAi) worms (J-L) were stained for filamentous actin (F-actin) by tetramethylrhodamine-phalloidin (A, D, G, and J) and DNA by DAPI (B, E, H, and K). Merged images are shown in C, F, I, and L (F-actin in red and DNA in blue). Bar, 20 μm.
Instead, we found that UNC-60A was critical for assembly of the contractile apparatuses in the myoepithelial sheath cells. Previously, we showed that UNC-60A is widely expressed in non-muscle cells and essential for early embryogenesis (Ono et al., 2003), but function of UNC-60A in adult tissues have not been characterized. To bypass the effect of UNC-60A on embryogenesis, we started RNA interference treatment of unc-60A at the L1 larval stage and observed phenotypes in the adult. In unc-60A(RNAi) animals, actin filaments in the myoepithelial sheath cells were severely disorganized (Fig. 1J). Most of actin filaments were found in abnormal aggregates, and meshwork of thin actin filaments was nearly absent (Fig. 1J). unc-60A(RNAi) animals showed 100 % sterility with either endomitotic oocytes or abnormal clumps of premature oocytes in the proximal ovary (n=50) (Fig. 1J-L), indicating that ovulation was defective. unc-60A(RNAi) also caused severe defects in development of the distal gonad and oocytes, but these results will be reported elsewhere.
UNC-60A is expressed in the myoepithelial sheath cells
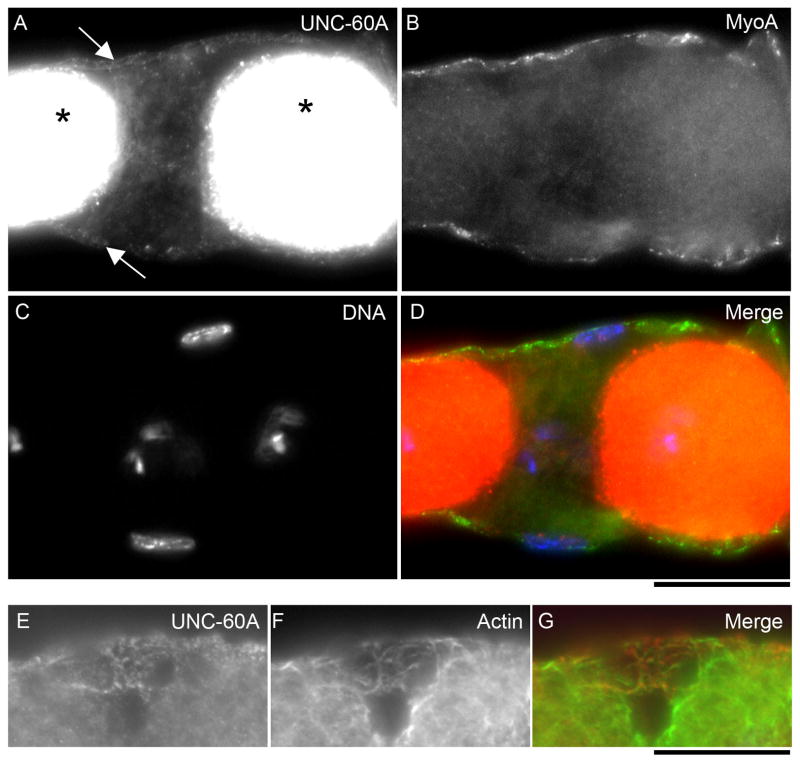
Previously, we reported that UNC-60A is highly expressed in the germline and oocytes in the hermaphroditic gonad (Ono et al., 2003). However, we overlooked the presence of UNC-60A in the myoepithelial sheath cells due to strong expression of UNC-60A in the oocytes. By focusing on the myoepithelial sheath, we detected immunofluorescent signals of UNC-60A (Fig. 2A) in the myoepithelial sheath cells that are positive for MyoA myosin heavy chain (Fig. 2B), although the signals were much weaker than those in the oocytes (Fig. 2A) (note that signals in the oocytes are saturated). Occasionally, we found areas where anti-UNC-60A antibody penetrated only into the myoepithelial sheath but not into the oocytes and were able to observe punctate localization of UNC-60A (Fig. 2E). These puncta localized along with filamentous actin (Fig. 2F and G), suggesting a role of UNC-60A in actin filament remodeling.
Figure 2.
Expression and localization of UNC-60A in the myoepithelial sheath cells. (A-D) A wild-type somatic gonad was stained for UNC-60A (A), MyoA myosin heavy chain (B), and DNA (C). A merged image is shown in D (UNC-60A in red, MyoA in green, and DNA in blue). UNC-60A was strongly expressed in oocytes (asterisks) and weakly in the myoepithelial sheath (arrows). Brightness and contrast in panel A are adjusted for weak UNC-60A staining in the myoepithelial sheath, so the strong signals in oocytes are saturated. (E-G) Co-localization of UNC-60A (E) and actin (F) in the myoepithelial sheath were detected where oocytes were not permeabilized for UNC-60A antibody. A merged image (G: UNC-60A in red and actin in green) shows that puncta of UNC-60A localize to actin filaments. Bars, 20 μm.
Somatic expression of UNC-60A is required for assembly of actin network in the myoepithelial sheath
UNC-60A is also highly expressed in the germline in the distal gonad (Ono et al., 2003), and unc-60A(RNAi) causes severe defects in the germline development and subsequent oocyte production (our unpublished results). Since the myoepithelial sheath and the germiline functionally interact during gonadal development (Killian and Hubbard, 2005), abnormal germline development could affect morphogenesis of the myoepithelial sheath non-cell autonomously. Therefore, to distinguish the effects of RNAi in the somatic gonads from those in the germ cells, we compared the RNAi phenotypes in wild-type and rrf-1 backgrounds. The rrf-1 mutants are defective with RNAi in the somatic cells but not in the germ cells (Sijen et al., 2001). Thus, if function of an RNAi target is important in the somatic gonad, the myoepithelial sheath will be disorganized in wild-type but normal in the rrf-1 background.
In rrf-1, unc-60A(RNAi) did not cause disorganization of the actin filaments in the myoepithelial sheath but caused abnormal oocyte formation (Fig. 3). rrf-1 worms with control RNAi treatment did not exhibit defects in either actin organization in the myoepithelial sheath (0 % defective, n=100) (Fig. 3A) or oocyte development (0 % defective, n=100) (Fig. 3A-C). unc-60A(RNAi);rrf-1 worms had clumps of germ cells or premature oocytes in the proximal gonad as shown by intense accumulation of DNA (99 % defective, n=100) (Fig. 3E), and they often showed abnormal accumulation of actin filaments (Fig. 3D; these actin accumulations are out of focus in the figure). In contrast, the myoepithelial sheath in 76 % of these animals (n=100) had nearly normal organization of actin filaments and do not have aggregates of actin filaments (24 % defective, n=100) (Fig. 3D). These results indicate that somatic expression of UNC-60A is required for organized assembly of actin filaments in the myoepithelial sheath independent of the germline and oocytes.
Figure 3.
Somatic requirement of UNC-60A for organized assembly of actin networks in the myoepithelial sheath of the somatic gonad. rrf-1(pk1417) (RNAi-defective in soma) worms were treated with control RNAi (A-C) or unc-60A(RNAi) (D-F), and the dissected gonads stained for F-actin (A and D) and DNA (B and E). Merged images are shown in C and F (F-actin in red and DNA in blue). unc-60A(RNAi) resulted in formation of abnormal clumps of oocytes (asterisks in D and E) but did not affect actin filament organization in the myoepithelial sheath. Note that the micrographs in A-C are composites of multiple micrographs of the same gonad. Bar, 20 μm.
The unc-60A(RNAi) phenotype is rescued by mutant UNC-60B with impaired actin filament severing activity
Our immunofluorescent localization indicates that UNC-60A is expressed in the myoepithelial sheath, but that UNC-60B is not. Therefore, requirement of UNC-60A may simply mean that UNC-60A is the only ADF/cofilin in the myoepithelial sheath, and the data do not demonstrate that the two ADF/cofilin isoforms are functionally different. We tested whether forced expression of UNC-60B can substitute for the function of UNC-60A in the myoepithelial sheath. GFP-tagged UNC-60B was expressed in the myoepithelial sheath from a transgene under the control of the let-502 promoter (Yin et al., 2004) and examined for its activity to rescue the unc-60A(RNAi) phenotype.
Because there was phenotypic variability in the actin organization, the actin phenotypes were classified into four classes (Table 1). Class 1 is the most severe phenotype in which large aggregates of actin were formed without thin filaments (e. g. Fig. 4D). Class 2 is “severely disorganized” in which large actin aggregates and some faint thin filament networks were formed (e. g. Fig. 4J). Class 3 is “moderately disorganized” in which small actin aggregates and fairly organized thin filament networks were formed (e. g. Fig. 4P). Class 4 is indistinguishable from wild-type.
Table 1.
Effects of unc-60A(RNAi) and expression of UNC-60B variants on actin filament organization in the myoepithelial sheath
| Actin phenotype (%)* | ||||||
|---|---|---|---|---|---|---|
| More severe | Less severe | |||||
| Transgene | GFP | RNAi | 1 | 2 | 3 | 4 |
| None | NA | Control | 0 | 0 | 0 | 100 |
| NA | unc-60A | 77 | 23 | 0 | 0 | |
| GFP-UNC-60B(WT) | + | Control | 0 | 0 | 0 | 100 |
| + | unc-60A | 37 | 37 | 20 | 6.7 | |
| − | Control | 0 | 0 | 0 | 100 | |
| − | unc-60A | 53 | 30 | 17 | 0 | |
| GFP-UNC-60B(Δ150) | + | Control | 0 | 0 | 0 | 100 |
| + | unc-60A | 0 | 40 | 43 | 17 | |
| − | Control | 0 | 0 | 0 | 100 | |
| − | unc-60A | 70 | 20 | 10 | 0 | |
| GFP-UNC-60B(Δ152) | + | Control | 0 | 0 | 0 | 100 |
| + | unc-60A | 10 | 60 | 27 | 6.7 | |
| − | Control | 0 | 0 | 0 | 100 | |
| − | unc-60A | 63 | 23 | 10 | 3.3 | |
Phenotypes of the actin filaments in the myoepithelial sheath were categorized into 4 classes (n=30). 1: large actin aggregates and no thin filaments (e. g. Fig. 4D); 2: large actin aggregates and weak thin filament networks (e. g. Fig. 4J); 3: small actin aggregates and strong thin filament networks (e. g. Fig. 4P); 4: nearly wild-type (e. g. Fig. 4A).
Figure 4.
Rescue of the unc-60A(RNAi) phenotype in the myoepithelial sheath by transgenic expression of UNC-60B mutants with defective severing activity. Wild-type worms with no transgene (A-F), Plet-502::GFP-UNC-60B(WT) (G-L), Plet-502::GFP-UNC-60B(Δ150) (M-R), or Plet-502::GFP-UNC-60B(Δ152) (S-X) were treated with control RNAi (A-C, G-I, M-O, and S-U) or unc-60A(RNAi) (D-F, J-L, P-R, and V-X), and the dissected gonads stained for F-actin (A, D, G, J, M, P, S, and V) and DNA (only shown in blue in merged images). The GFP localization is shown in H, K, N, Q, T, and W. Merged images are shown in C, F, I, L, O, R, U, and X (F-actin in red, GFP in green, and DNA in blue). Bar, 20 μm.
In gonads with control RNAi, GFP-UNC-60B(WT) diffusely localized in the cytoplasm (Fig. 4H) and did not cause any detectable alterations in the actin organization (Fig. 4G-I). When UNC-60A was depleted from the transgenic worms, the actin meshwork was disrupted, and large actin aggregates were formed (Fig. 4J). A majority of the worms exhibited class 1 (37 %) or class 2 (37 %) phenotype (Table 1), whereas some worms showed significantly less severe phenotypes (class 3: 20 %, class 4: 6.7 %). We also examined unc-60A(RNAi) phenotypes of the offspring of GFP-UNC-60B(WT) that did not inherit the extrachromosomal arrays (GFP-negative) and found that the phenotype was only slightly more severe than GFP-positive gonads (class 1: 53 %, class 2: 30 %) (Table 1). It should be noted that 17 % of these GFP-negative gonads exhibited class 3 phenotype, while gonads with no transgene (Fig. 4D) showed no class 3 or 4 phenotype (Table 1), suggesting that expression of GFP-UNC-60B(WT) below detection levels made the phenotype less severe. GFP-UNC-60B(WT) was functional in the body wall muscle (see below), indicating that the GFP tag did not impair the activity of UNC-60B. These results strongly suggest that wild-type UNC-60B can weakly but not efficiently substitute for the function of UNC-60A and that UNC-60B is not functionally equivalent to UNC-60A in vivo.
The major biochemical difference between UNC-60A and UNC-60B is in their F-actin severing activity. Our previous work demonstrated that UNC-60A has much weaker F-actin severing activity than UNC-60B (Yamashiro et al., 2005). Therefore, we tested if the difference in the severing activity confers their in vivo functional difference. We have shown that small truncations of 1–3 amino acids at the C-terminal tail of UNC-60B impair F-actin severing activity without affecting G-actin binding activity (Ono et al., 2001). Here, we examined effects of UNC-60B(Δ150) (lacking three C-terminal amino acids) and UNC-60B(Δ152) (lacking one C-terminal amino acid), both of which have impaired severing activity in vitro (Ono et al., 2001).
We previously used a bulk assay using pyrene-labeled actin to detect severing events (Ono et al., 2001). This assay quantitatively measures the relative number of exposed filament ends and is adequate to detect severing activity of wild-type UNC-60B. However, it was not sensitive enough to detect severing activity of UNC-60A and the C-terminal variants of UNC-60B (Ono, et al., 2001; Yamashiro et al., 2005). In this study, we re-evaluated actin filament severing activity of UNC-60B variants using a more sensitive microscopic assay (Ono et al., 2004; Yamashiro et al., 2005) and found that the UNC-60B variants have defects in severing activity to different extents. Severing activity of UNC-60A was very weak and almost non-detectable at 2 min of incubation in the range from 10 nM to 10 μM (Fig. 5A and B). In contrast, wild-type UNC-60B significantly severed actin filaments at 1μM and nearly completely disassembled filaments at >2 μM (Fig. 5C and D). UNC-60B(Δ150) had no detectable severing activity in the range from 10 nM to 10 μM under these conditions (Fig. 5E and F). However, UNC-60B(Δ152) severed filaments at 2 μM and nearly completely disassembled filaments at 10 μM (Fig. 5G and H). Thus, the microscopic assay revealed that actin filament severing activity was abolished in UNC-60B(Δ150) but only weakened in UNC-60B(Δ152).
Figure 5.
Different actin filament severing activity of UNC-60A and UNC-60B variants.Fluorescently labeled actin filaments were tethered to a glass coverslip and treated with 10 nM –10 μM of UNC-60A (A and B), UNC-60B(WT) (C and D), UNC-60B(Δ150) (E and F), or UNC-60B(Δ152) (G and H). The filaments were observed before (A, C, E, and G) or 2 minutes after the incubation (B, D, F, and H), and the same fields are shown for each treatment. Bar, 10 μm.
Interestingly, we found that the severing-defective UNC-60B variant showed much stronger ability to rescue the unc-60A(RNAi) phenotype than wild-type UNC-60B (Fig. 4M-X, Table 1). Expression of GFP-UNC-60B(Δ150) or GFP-UNC-60B(Δ152) in control gonads did not alter the actin organization (Fig. 4M-O and S-U). When unc-60A was depleted by RNAi, expression of GFP-UNC-60B(Δ150) (severing-defective) increased formation of fine actin networks in the myoepithelial sheath (Fig. 4P). Some actin aggregates were formed, but they were smaller (Fig. 4P showing class 3 phenotype) than those in the gonads without transgenic expression (Fig. 4D) or with expression of GFP-UNC-60B(WT) (Fig. 4J). Expression of GFP-UNC-60B(Δ150) in unc-60A(RNAi) worms significantly increased class 3 (43 %) and class 4 (17 %) phenotypes (Table 1). In contrast, GFP-UNC-60B(Δ152) (weak severing) only weakly rescued the unc-60A(RNAi) phenotype (Fig. 4V showing class 2 phenotype and Table 1). A majority of the unc-60A(RNAi) worms expressing GFP-UNC-60B(Δ152) exhibited class 2 (60 %) or class 3 (27 %) phenotype, which is slightly less severe than GFP-UNC-60B(WT)-expressing worms (Table 1). We still observed ~100 % sterility in unc-60A(RNAi) worms expressing GFP-UNC-60B(Δ150) or GFP-UNC-60B(Δ152). This is most likely because unc-60A(RNAi) also affected development of germline and oocytes (our unpublished data) and the transgene was expressed only in the somatic cells. The fluorescence levels suggest that wild-type and variants of GFP-UNC-60B were expressed at similar levels. However, an accurate comparison by image analysis or western blot was technically difficult because of occasional formation of aggregates of GFP-UNC-60B (Fig. 4N, Q, and W) and difficulty in dissecting and isolating the myoepithelial sheath. We concluded that a severing-defective form of UNC-60B, which is biochemically similar to UNC-60A, is more efficient in assembly of organized actin filaments than wild-type UNC-60B in the myoepithelial sheath.
Strong actin filament severing activity of UNC-60B is required for actin filament organization in the striated body wall muscle
Activities of GFP-UNC-60B (wild-type, Δ150, and Δ152) were also tested in the striated body wall muscle. These GFP-UNC-60B variants were expressed in the body wall muscle of the unc-60B(null) mutant by the myo-3 promoter and examined for their abilities to rescue disorganized actin filaments. Unlike in the myoepithelial sheath, wild-type UNC-60B efficiently rescued the phenotype, but weak-severing and severing-defective UNC-60B mutants showed only partial and no activity to rescue the phenotype, respectively. unc-60B(null) worms have very disorganized actin filaments with large aggregates in the body wall muscle (Fig. 6D-F) and are nearly paralyzed (Table 2). Expression of GFP-UNC-60B(WT) restored striated organization of actin filaments nearly as well as wild-type (100 % rescue, n = 50) (Fig. 6G-I). Worm motility was also improved by GFP-UNC-60B(WT) but not as fast as wild-type worms (Table 2). In this study, the transgenes were maintained as extrachromosomal arrays that are inherited independently from the chromosomes (Mello et al., 1991). Many of the transgenic worms had a few cells with no expression of GFP-UNC-60B, probably because the extrachromosomal arrays were lost, and these cells had disorganized actin filaments (Fig. 6G, arrow). Therefore, incomplete rescue of worm motility is likely due to mosaic expression of the transgene.
Figure 6.
Rescue of the unc-60B(null) phenotype in the body wall muscle by transgenic expression of UNC-60B. Actin filament organization in the body wall muscle of wild-type (A), unc-60B(su158) (D), unc-60B(su158); Pmyo-3::GFP-UNC-60B(WT) (G), unc-60B(su158); Pmyo-3::GFP-UNC-60B(Δ150) (J), and unc-60B(su158); Pmyo-3::GFP-UNC-60B(Δ152) (M) was visualized by staining with tetramethylrhodamine-phalloidin. The GFP fluorescence is shown in B, E, H, K, and N (wild-type and unc-60B(su158) do not have a transgene expressing GFP). Merged images are shown in C, F, I, L, and O (F-actin in red and GFP in green).sAn arrow in G indicates an actin aggregate in a cell that did not express GFP-UNC-60B(WT). Bar, 20 μm.
Table 2.
Effects of unc-60B(su158) and expression of UNC-60B variants on contractility and actin organization in the body wall muscle
| Genotype | Worm motility (beating/30 sec ± s.d., n=20) | Rescue of actin organization* (%, n=50) |
|---|---|---|
| Wild-type | 128 ± 4.33 | NA |
| unc-60B(su158) | 6.90 ± 4.00 | NA |
| unc-60B(su158); GFP-UNC-60B(WT) | 99.8 ± 15.8 | 100 |
| unc-60B(su158); GFP-UNC-60B(Δ150) | 1.85 ± 1.60 | 0 |
| unc-60B(su158); GFP-UNC-60B(Δ152) | 72.4 ± 14.2 | 72 |
When a majority of GFP-expressing muscle cells in a worm restored striated actin organization without actin aggregates, the worm was counted as “rescued”. Wild-type and unc-60B(su158) worms with no transgene had 100 % penetrant organized and disorganized actin filaments, respectively (n>200).
In contrast, GFP-UNC-60B(Δ150) showed no rescue, and GFP-UNC-60B(Δ152) only partially rescued the unc-60B-null phenotype. Expression of GFP-UNC-60B(Δ150) did not alter the disorganized actin filaments (0 % rescue, n = 50) (Fig. 6J-L, Table 2), and the transgenic worms remained paralyzed (Table 2). GFP-UNC-60B(Δ152) rescued the actin organization in 72 % of the worms investigated (n = 50) (Fig. 6M-O, Table 2), although the actin striation was not as sharp as wild-type (Fig. 6M). Worm motility was significantly improved by expression of GFP-UNC-60B(Δ150) but not as well as GFP-UNC-60B(WT) (Table 2). Wild-type and mutant forms of GFP-UNC-60B were expressed at comparable levels as determined by Western blot, and the extent of mosaic expression of these transgenes were similar (data not shown). Therefore, we concluded that the severing-defective UNC-60B variants are functionally inefficient for organized assembly of actin filaments in the striated body wall muscle, and that non-striated gonadal myoepithelial sheath and striated body wall muscle have different requirements for optimal actin filament severing activity of ADF/cofilin.
Discussion
In this study, we identified UNC-60A as an ADF/cofilin isoform that is specifically required for organized assembly of contractile actin networks in the myoepithelial sheath of the C. elegans somatic gonad. UNC-60A, but not UNC-60B, was expressed in the myoepithelial sheath. Transgenic expression of wild-type UNC-60B did not effectively rescue the unc-60A(RNAi) phenotype, indicating that the two ADF/cofilin isoforms are not functionally equivalent. However, variants of UNC-60B with reduced actin filament severing activity rescued the unc-60A(RNAi) phenotype. Since the major biochemical difference between UNC-60A and UNC-60B was in their actin filament severing activity, these results suggest that an ADF/cofilin with weak severing activity is suitable for morphogenesis of contractile actin networks in the myoepithelial sheath. In contrast, strong severing activity was required for actin filament organization in the striated body wall muscle. Thus, these observations suggest that striated and non-striated muscles require different levels of actin filament severing activity of ADF/cofilin.
Isoform-specific role of ADF/cofilin in the C. elegans somatic gonad and the body wall muscle
This is the first report of an essential function of a regulator of actin filament dynamics in the C. elegans somatic gonad. Previously, RNAi of talin has been shown to cause similarly severe disorganization of actin filaments in the myoepithelial sheath (Cram et al., 2003). However, talin is rather a structural component of adhesion structures where actin filaments are anchored to the membrane (Moulder et al., 1996). Tropomyosin stabilizes actin filaments against ADF/cofilin-dependent actin dynamics (Ono and Ono, 2002). However, in the myoepithelial sheath, depletion of tropomyosin inhibits contractile activity of the sheath but does not disrupt actin organization (Ono and Ono, 2004). We observed UNC-87, a calponin-like protein, localizes to the thin filaments in the myoepithelial sheath (our unpublished data) and may contribute to stabilizing actin filaments as we demonstrated in the body wall muscle (Yamashiro et al., 2007).
ADF/cofilin isoforms, UNC-60A and UNC-60B, are the only known cytoskeleton-related proteins with differential functions in the myoepithelial sheath and the body wall muscle. These two morphologically and functionally different muscles share many of the same structural components that are expressed from the same genes (Ardizzi and Epstein, 1987; Ono et al., 2007). To assemble non-striated and striated contractile apparatuses from the same components, different regulatory mechanisms must be employed. Although the difference in actin-regulatory activities of UNC-60A and UNC-60B appear to be a part of such differentiation mechanisms, further investigations are needed to identify additional genes that contribute to assembly of contractile apparatuses in these cells. Genome-wide promoter-reporter analysis (Dupuy et al., 2007) and cell-specific transcriptome analysis (Fox et al., 2007) may identify specific genes for the myoepithelial sheath or the body wall muscle. Further functional analysis of these genes should provide information on the differentiation of these muscle cells.
We observed that weaker actin filament severing activity of ADF/cofilin is preferred for organized assembly of actin filaments in the myoepithelial sheath. In contrast, full severing activity of UNC-60B was required for actin filament organization in the body wall muscle. The difference in the optimal filament severing activity of ADF/cofilin could be related to different architecture of striated and non-striated contractile apparatuses. Non-striated contractile apparatuses in the myoepithelial sheath are loosely arranged throughout the cytoplasm (Hall et al., 1999). Therefore, regulators of actin dynamics could be easily diffused into the actin network and interact with actin efficiently, but too strong actin regulators may disrupt the filamentous organization. In contrast, striated myofibrils in the body wall muscle are densely packed within 1–1.5 μm from the plasma membrane (Francis and Waterston, 1985; Waterston et al., 1980). Thus, soluble proteins may not be readily accessible to the core of myofibrils, and regulators of actin dynamics would have to be very efficient in actin remodeling. Our previous biochemical observations (Yamashiro et al., 2005) that UNC-60B is kinetically more efficient in enhancing actin turnover than UNC-60A support this hypothesis.
Alternatively, the two ADF/cofilin isoforms may have different interacting proteins in regulation of actin dynamics. UNC-60A and UNC-60B(Δ150) were very similar in their biochemical activities (Fig. 5), yet UNC-60B(Δ150) did not fully rescue the unc-60A(RNAi) phenotype (Fig. 4). Actin-interacting protein 1 (AIP1) is a conserved actin-regulatory protein that cooperates with ADF/cofilin to promote actin filament disassembly (Ono, 2003). In the C. elegans body wall muscle, UNC-60B cooperates with AIP1 that is encoded by the unc-78 gene (Ono, 2001). Interestingly, we have shown that UNC-60B and UNC-78/AIP1 efficiently cooperate to disassemble actin filaments in vitro, but UNC-60A does not (Mohri and Ono, 2003). UNC-78/AIP1 is not expressed in the myoepithelial sheath, and unc-78 mutants do not exhibit defects in the somatic gonad. C. elegans has a second AIP1 gene, K08F9.2, which is a candidate that may preferentially interact with UNC-60A. However, function of this gene is currently unknown, as K08F9.2(RNAi) did not show any obvious phenotype in the genome-wide RNAi analysis (Kamath et al., 2003). In addition, C. elegans has multiple isoforms of profilin (Polet et al., 2006) and cyclase-associated protein (K. Ono and S. Ono, American Society for Cell Biology Poster Abstract, 2006), which functionally interact with ADF/cofilin to enhance actin filament dynamics (Ono, 2007). Thus, isoform-specific interactions among these actin regulators may be important for cell type-specific organization of the actin cytoskeleton.
Implications for isoform-specific functions of ADF/cofilin in other systems
Our observations also provide insights into isoform-specific functions of ADF/cofilin in other multicellular organisms. Mammals have three ADF/cofilin isoforms: ADF/destrin, cofilin-1/non-muscle-type cofilin, and cofilin-2/muscle-type cofilin, and they are expressed in distinct tissue-specific patterns with some overlap (Moriyama et al., 1990; Ono et al., 1994; Vartiainen et al., 2002). Redundant functions of ADF and cofilin-1 in regulation of actin turnover are demonstrated in cultured cells (Hotulainen et al., 2005). A mutation in human cofilin-2 causes nemaline myopathy in the skeletal muscle (Agrawal et al., 2007). However, cardiac muscle is not affected by the mutation most likely because cofilin-1 is expressed in the heart and compensate for the function of cofilin-2. Nonetheless, the ADF/cofilin isoforms display quantitatively different activities in actin filament severing and depolymerization in vitro (Chen et al., 2004; Nakashima et al., 2005; Yeoh et al., 2002). Recently, differential involvements of ADF and cofilin-1 in migration of human colon cancer cells (Estornes et al., 2007) and mouse brain development (Bellenchi et al., 2007) have been reported, which may be due to the difference in their actin-regulatory activities. Moreover, actin severing activity of ADF/cofilin is regulated by pH (Bernstein et al., 2000; Hawkins et al., 1993; Hayden et al., 1993; Yonezawa et al., 1985), and local modulation of ADF/cofilin by pH changes may be important for assembly of different types of actin filament networks, such as contractile rings, stress fibers and lamella meshwork. Thus, actin severing activity of ADF/cofilin might be optimized by isoforms or cellular conditions for regulation of specific types of actin cytoskeletal organization.
Materials and Methods
Nematode strains
Wild-type C. elegans strain N2 and an RNAi-defective strain NL2098 rrf-3(pk1417) (Sijen et al., 2001) were obtained from the Caenorhabditis Genetics Center (Mineapolis, MN). unc-60B(su158) (Zengel and Epstein, 1980) was provided by Henry Epstein (University of Texas Medical Branch, Galveston, TX) and outcrossed in our laboratory (Ono et al., 2003). Nematodes were grown under standard conditions at 20 ºC (Brenner, 1974).
RNA interference experiments
Nematodes were treated with RNAi by feeding E. coli expressing double-stranded RNA (Timmons et al., 2001; Timmons and Fire, 1998). A vector for unc-60A(RNAi) was previously described (Ono et al., 2003). To bypass embryonic effects, gravid hermaphrodites were allowed to lay eggs on agar plates with E. coli expressing dsRNA, hatched worms cultured by feeding dsRNA-expressing E. coli, and phenotypes characterized when they became adults. Control experiments were performed with E. coli HT115(DE3) that was transformed with L4440 [a vector for feeding RNAi (Timmons et al., 2001)] with no insert. RNAi experiments were performed at least three times and quantification of one representative set of experiments is shown in the result.
Construction of expression vectors
Transgenic expression in the body wall muscle was driven by the myo-3 promoter (Okkema et al., 1993). The full-length UNC-60B cDNA was PCR-amplified and cloned at the Eco RI - Nhe I sites of pPD118.20, an expression vector with the myo-3 promoter and the GFP coding sequence (provided by Andrew Fire, Stanford University, Stanford, CA). UNC-60B(Δ150) and UNC-60B(Δ152) were produced by introducing premature stop codons in the reverse primers for PCR and cloned into pPD118.20. The entire coding regions were sequenced to confirm the presence of introduced mutations and the absence of PCR-induced errors. Transgenic expression in the myoepithelial sheath was driven by the let-502 promoter (Wissmann et al., 1999; Yin et al., 2004). A 2.9-kb fragment of the 5′-upstream sequence of the let-502 gene was amplified by PCR and fused with the GFP-UNC-60B sequence plus the let-858 3′-UTR that had been amplified from the body wall muscle-expression vector by fusion PCR (Hobert, 2002).
Transgenic nematodes
Transgenic nematodes were generated as described previously (Mohri et al., 2006). The plasmid vectors for Pmyo-3::GFP-UNC-60B (20 μg/ml) were mixed with 80 μg/ml pET-32a (Novagen) as carrier DNA. The fusion-PCR products for Plet-502::GFP-UNC-60B were diluted to 1:10 in a buffer containing 80 μg/ml pRF6 as carrier DNA. The DNA mixtures were injected into the distal arm of the hermaphroditic gonad of wild-type N2 as described (Mello and Fire, 1995). Transformants were selected by expression of GFP as observed by epifluorescence, and the transgenes maintained as extrachromosomal arrays. Transgenic animals carrying Pmyo-3::GFP-UNC-60B were further crossed with unc-60B(su158), and unc-60B(su158) homozygotes carrying Pmyo-3::GFP-UNC-60B were established.
Fluorescence microscopy
The gonads were dissected by cutting adult hermaphrodites at the level of pharynx on poly-lysine-coated slides as described previously (Rose et al., 1997). For staining with tetramethylrhodamine-phalloidin (Sigma-Aldrich), the samples were fixed with 4 % paraformaldehyde in cytoskeleton buffer (138 mM KCl, 3 mM MgCl2, 2 mM EGTA, and 10 mM MES-KOH, pH 6.1) containing 0.32 M sucrose for 10 min at room temperature followed by treatment with phosphate-buffered saline (PBS) containing 0.5 % Triton X-100 and 30 mM glycine for 10 min. They were incubated with 0.2 μg/ml tetramethylrhodamine-phalloidin and 0.2 μg/ml 4′6-diamidino-2-phenylindole (DAPI) in PBS containing 0.5 % Triton X-100 and 30 mM glycine for 10 min for 1 hour and washed with PBS containing 0.5 % Triton X-100 and 30 mM glycine. For immunolocalization of UNC-60A, dissected gonads were freeze-cracked and fixed with methanol at −20 ºC for 5 min, washed with PBS, and incubated with rabbit anti-UNC-60A antibody (Ono et al., 1999) and mouse anti-MyoA monoclonal antibody 5–6 (Miller et al., 1983) or mouse anti-actin monoclonal antibody C4 (MP Biomedicals). They were visualized by Cy3-conjugated donkey anti-rabbit IgG (Jackson ImmunoResearch Laboratories) and Alexa488-conjugated goat anti-mouse IgG (Invitrogen).
Staining of whole worms with tetramethylrhodamine-phalloidin was performed as described previously (Ono, 2001).
Samples were mounted with ProLong Gold (Invitrogen) and viewed by epifluorescence using a Nikon Eclipse TE2000 inverted microscope with a 40x or 60x CFI Plan Fluor objective. Images were captured by a SPOT RT Monochrome CCD camera (Diagnostic Instruments) and processed by the IPLab imaging software (Scanalytics, Inc.) and Adobe Photoshop 6.0.
Worm motility assay
Worm motility was quantified as described (Epstein and Thomson, 1974). Briefly, adult worms were placed in M9 buffer. Then, one beat was counted when a worm swung its head to either right or left. The total number of beats in 30 seconds was recorded.
Microscopic assay for actin filament severing
Bacterially expressed UNC-60A, UNC-60B, UNC-60B(Δ150), and UNC-60B(Δ152) were purified as described previously (Ono and Benian, 1998; Ono et al., 2001). Observation of actin filament severing by fluorescence microscopy was performed as described previously (Ono et al., 2004; Yamashiro et al., 2005) with slight modifications. Previously, we used anti-biotin monoclonal antibody (Invitrogen) to immobilize biotin-labeled actin on the glass surface. However, this antibody has been discontinued by the company, and we found several other commercially available anti-biotin antibodies were not very efficient in tethering actin filaments. Therefore, unlabeled actin (1.4 μM) and Alexa488-labeled actin (0.4 μM) were co-polymerized and attached to a glass coverslip using heavy meromyosin (Cytoskeleton Inc.). Other procedures were the same as our previous reports.
Acknowledgments
Monoclonal antibody 5–6 was developed by Dr. Henry Epstein (University of Texas Medical Branch, Galveston, TX) and was obtained from the Developmental Studies Hybridoma Bank developed under the auspices of the NICHD and maintained by The University of Iowa, Department of Biological Sciences, Iowa City, IA 52242. Some C. elegans strains were provided by the Caenorhabditis Genetics Center, which is supported by the National Institute of Health National Center for Research Resources. This work was supported by grants from the National Institute of Health (R01 AR48615) and American Heart Association to S. O.
References
- Agrawal PB, Greenleaf RS, Tomczak KK, Lehtokari VL, Wallgren-Pettersson C, Wallefeld W, Laing NG, Darras BT, Maciver SK, Dormitzer PR, et al. Nemaline myopathy with minicores caused by mutation of the CFL2 gene encoding the skeletal muscle actin-binding protein, cofilin-2. Am J Hum Genet. 2007;80:162–167. doi: 10.1086/510402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anyanful A, Ono K, Johnsen RC, Ly H, Jensen V, Baillie DL, Ono S. The RNA-binding protein SUP-12 controls muscle-specific splicing of the ADF/cofilin pre-mRNA in C. elegans. J Cell Biol. 2004;167:639–647. doi: 10.1083/jcb.200407085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ardizzi JP, Epstein HF. Immunochemical localization of myosin heavy chain isoforms and paramyosin in developmentally and structurally diverse muscle cell types of the nematode Caenorhabditis elegans. J Cell Biol. 1987;105:2763–2770. doi: 10.1083/jcb.105.6.2763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellenchi GC, Gurniak CB, Perlas E, Middei S, Ammassari-Teule M, Witke W. N-cofilin is associated with neuronal migration disorders and cell cycle control in the cerebral cortex. Genes Dev. 2007;21:2347–2357. doi: 10.1101/gad.434307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein BW, Painter WB, Chen H, Minamide LS, Abe H, Bamburg JR. Intracellular pH modulation of ADF/cofilin proteins. Cell Motil Cytoskeleton. 2000;47:319–336. doi: 10.1002/1097-0169(200012)47:4<319::AID-CM6>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Brenner S. The genetics of Caenorhabditis elegans. Genetics. 1974;77:71–94. doi: 10.1093/genetics/77.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Bernstein BW, Sneider JM, Boyle JA, Minamide LS, Bamburg JR. In vitro activity differences between proteins of the ADF/cofilin family define two distinct subgroups. Biochemistry. 2004;43:7127–7142. doi: 10.1021/bi049797n. [DOI] [PubMed] [Google Scholar]
- Cram EJ, Clark SG, Schwarzbauer JE. Talin loss-of-function uncovers roles in cell contractility and migration in C. elegans. J Cell Sci. 2003;116:3871–3878. doi: 10.1242/jcs.00705. [DOI] [PubMed] [Google Scholar]
- Dixon SJ, Roy PJ. Muscle arm development in Caenorhabditis elegans. Development. 2005;132:3079–3092. doi: 10.1242/dev.01883. [DOI] [PubMed] [Google Scholar]
- Dupuy D, Bertin N, Hidalgo CA, Venkatesan K, Tu D, Lee D, Rosenberg J, Svrzikapa N, Blanc A, Carnec A, et al. Genome-scale analysis of in vivo spatiotemporal promoter activity in Caenorhabditis elegans. Nat Biotechnol. 2007;25:663–668. doi: 10.1038/nbt1305. [DOI] [PubMed] [Google Scholar]
- Epstein HF, Thomson JN. Temperature-sensitive mutation affecting myofilament assembly in Caenorhabditis elegans. Nature. 1974;250:579–580. doi: 10.1038/250579a0. [DOI] [PubMed] [Google Scholar]
- Estornes Y, Gay F, Gevrey JC, Navoizat S, Nejjari M, Scoazec JY, Chayvialle JA, Saurin JC, Abello J. Differential involvement of destrin and cofilin-1 in the control of invasive properties of Isreco1 human colon cancer cells. Int J Cancer. 2007;121:2162–2171. doi: 10.1002/ijc.22911. [DOI] [PubMed] [Google Scholar]
- Fox RM, Watson JD, Von Stetina SE, McDermott J, Brodigan TM, Fukushige T, Krause M, Miller DM., 3rd The embryonic muscle transcriptome of Caenorhabditis elegans. Genome Biol. 2007;8:R188. doi: 10.1186/gb-2007-8-9-r188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis GR, Waterston RH. Muscle organization in Caenorhabditis elegans: localization of proteins implicated in thin filament attachment and I-band organization. J Cell Biol. 1985;101:1532–1549. doi: 10.1083/jcb.101.4.1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerthoffer WT. Actin cytoskeletal dynamics in smooth muscle contraction. Can J Physiol Pharmacol. 2005;83:851–856. doi: 10.1139/y05-088. [DOI] [PubMed] [Google Scholar]
- Hall DH, Winfrey VP, Blaeuer G, Hoffman LH, Furuta T, Rose KL, Hobert O, Greenstein D. Ultrastructural features of the adult hermaphrodite gonad of Caenorhabditis elegans: relations between the germ line and soma. Dev Biol. 1999;212:101–123. doi: 10.1006/dbio.1999.9356. [DOI] [PubMed] [Google Scholar]
- Hawkins M, Pope B, Maciver SK, Weeds AG. Human actin depolymerizing factor mediates a pH-sensitive destruction of actin filaments. Biochemistry. 1993;32:9985–9993. doi: 10.1021/bi00089a014. [DOI] [PubMed] [Google Scholar]
- Hayden SM, Miller PS, Brauweiler A, Bamburg JR. Analysis of the interactions of actin depolymerizing factor with G- and F-actin. Biochemistry. 1993;32:9994–10004. doi: 10.1021/bi00089a015. [DOI] [PubMed] [Google Scholar]
- Hobert O. PCR fusion-based approach to create reporter gene constructs for expression analysis in transgenic C. elegans. Biotechniques. 2002;32:728–730. doi: 10.2144/02324bm01. [DOI] [PubMed] [Google Scholar]
- Hotulainen P, Paunola E, Vartiainen MK, Lappalainen P. Actin-depolymerizing factor and cofilin-1 play overlapping roles in promoting rapid F-actin depolymerization in mammalian nonmuscle cells. Mol Biol Cell. 2005;16:649–664. doi: 10.1091/mbc.E04-07-0555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamath RS, Fraser AG, Dong Y, Poulin G, Durbin R, Gotta M, Kanapin A, Le Bot N, Moreno S, Sohrmann M, et al. Systematic functional analysis of the Caenorhabditis elegans genome using RNAi. Nature. 2003;421:231–237. doi: 10.1038/nature01278. [DOI] [PubMed] [Google Scholar]
- Killian DJ, Hubbard EJ. Caenorhabditis elegans germline patterning requires coordinated development of the somatic gonadal sheath and the germ line. Dev Biol. 2005;279:322–335. doi: 10.1016/j.ydbio.2004.12.021. [DOI] [PubMed] [Google Scholar]
- McCarter J, Bartlett B, Dang T, Schedl T. Soma-germ cell interactions in Caenorhabditis elegans: multiple events of hermaphrodite germline development require the somatic sheath and spermathecal lineages. Dev Biol. 1997;181:121–143. doi: 10.1006/dbio.1996.8429. [DOI] [PubMed] [Google Scholar]
- McCarter J, Bartlett B, Dang T, Schedl T. On the control of oocyte meiotic maturation and ovulation in Caenorhabditis elegans. Dev Biol. 1999;205:111–128. doi: 10.1006/dbio.1998.9109. [DOI] [PubMed] [Google Scholar]
- McKim KS, Matheson C, Marra MA, Wakarchuk MF, Baillie DL. The Caenorhabditis elegans unc-60 gene encodes proteins homologous to a family of actin-binding proteins. Mol Gen Genet. 1994;242:346–357. doi: 10.1007/BF00280425. [DOI] [PubMed] [Google Scholar]
- Mello C, Fire A. DNA transformation. Methods Cell Biol. 1995;48:451–482. [PubMed] [Google Scholar]
- Mello CC, Kramer JM, Stinchcomb D, Ambros V. Efficient gene transfer in C.elegans: extrachromosomal maintenance and integration of transforming sequences. EMBO J. 1991;10:3959–3970. doi: 10.1002/j.1460-2075.1991.tb04966.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller DM, Ortiz I, Berliner GC, Epstein HF. Differential localization of two myosins within nematode thick filaments. Cell. 1983;34:477–490. doi: 10.1016/0092-8674(83)90381-1. [DOI] [PubMed] [Google Scholar]
- Moerman DG, Fire A. Muscle: Structure, Function, and Development. In: Riddle DL, Blumenthal T, Meyer BJ, Priess JR, editors. C elegans II. Plainview, NY: Cold Spring Harbor Laboratory Press; 1997. pp. 417–470. [PubMed] [Google Scholar]
- Mohri K, Ono K, Yu R, Yamashiro S, Ono S. Enhancement of actin-depolymerizing factor/cofilin-dependent actin disassembly by actin-interacting protein 1 is required for organized actin filament assembly in the Caenorhabditis elegans body wall muscle. Mol Biol Cell. 2006;17:2190–2199. doi: 10.1091/mbc.E05-11-1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohri K, Ono S. Actin filament disassembling activity of Caenorhabditis elegans actin-interacting protein 1 (UNC-78) is dependent on filament binding by a specific ADF/cofilin isoform. J Cell Sci. 2003;116:4107–4118. doi: 10.1242/jcs.00717. [DOI] [PubMed] [Google Scholar]
- Moriyama K, Nishida E, Yonezawa N, Sakai H, Matsumoto S, Iida K, Yahara I. Destrin, a mammalian actin-depolymerizing protein, is closely related to cofilin. Cloning and expression of porcine brain destrin cDNA. J Biol Chem. 1990;265:5768–5773. [PubMed] [Google Scholar]
- Moulder GL, Huang MM, Waterston RH, Barstead RJ. Talin requires beta-integrin, but not vinculin, for its assembly into focal adhesion-like structures in the nematode Caenorhabditis elegans. Mol Biol Cell. 1996;7:1181–1193. doi: 10.1091/mbc.7.8.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakashima K, Sato N, Nakagaki T, Abe H, Ono S, Obinata T. Two mouse cofilin isoforms, muscle-type (MCF) and non-muscle type (NMCF), interact with F-actin with different efficiencies. J Biochem. 2005;138:519–526. doi: 10.1093/jb/mvi152. [DOI] [PubMed] [Google Scholar]
- Okkema PG, Harrison SW, Plunger V, Aryana A, Fire A. Sequence requirements for myosin gene expression and regulation in Caenorhabditis elegans. Genetics. 1993;135:385–404. doi: 10.1093/genetics/135.2.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono K, Ono S. Tropomyosin and troponin are required for ovarian contraction in the Caenorhabditis elegans reproductive system. Mol Biol Cell. 2004;15:2782–2793. doi: 10.1091/mbc.E04-03-0179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono K, Parast M, Alberico C, Benian GM, Ono S. Specific requirement for two ADF/cofilin isoforms in distinct actin-dependent processes in Caenorhabditis elegans. J Cell Sci. 2003;116:2073–2085. doi: 10.1242/jcs.00421. [DOI] [PubMed] [Google Scholar]
- Ono K, Yu R, Ono S. Structural components of the nonstriated contractile apparatuses in the Caenorhabditis elegans gonadal myoepithelial sheath and their essential roles for ovulation. Dev Dyn. 2007;236:1093–1105. doi: 10.1002/dvdy.21091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono S. The Caenorhabditis elegans unc-78 gene encodes a homologue of actin-interacting protein 1 required for organized assembly of muscle actin filaments. J Cell Biol. 2001;152:1313–1319. doi: 10.1083/jcb.152.6.1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono S. Regulation of actin filament dynamics by actin depolymerizing factor/cofilin and actin-interacting protein 1: new blades for twisted filaments. Biochemistry. 2003;42:13363–13370. doi: 10.1021/bi034600x. [DOI] [PubMed] [Google Scholar]
- Ono S. Mechanism of depolymerization and severing of actin filaments and its significance in cytoskeletal dynamics. Int Rev Cytol. 2007;258:1–82. doi: 10.1016/S0074-7696(07)58001-0. [DOI] [PubMed] [Google Scholar]
- Ono S, Baillie DL, Benian GM. UNC-60B, an ADF/cofilin family protein, is required for proper assembly of actin into myofibrils in Caenorhabditis elegans body wall muscle. J Cell Biol. 1999;145:491–502. doi: 10.1083/jcb.145.3.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono S, Benian GM. Two Caenorhabditis elegans actin depolymerizing factor/cofilin proteins, encoded by the unc-60 gene, differentially regulate actin filament dynamics. J Biol Chem. 1998;273:3778–3783. doi: 10.1074/jbc.273.6.3778. [DOI] [PubMed] [Google Scholar]
- Ono S, McGough A, Pope BJ, Tolbert VT, Bui A, Pohl J, Benian GM, Gernert KM, Weeds AG. The C-terminal tail of UNC-60B (ADF/cofilin) is critical for maintaining its stable association with F-actin and is implicated in the second actin-binding site. J Biol Chem. 2001;276:5952–5958. doi: 10.1074/jbc.M007563200. [DOI] [PubMed] [Google Scholar]
- Ono S, Minami N, Abe H, Obinata T. Characterization of a novel cofilin isoform that is predominantly expressed in mammalian skeletal muscle. J Biol Chem. 1994;269:15280–15286. [PubMed] [Google Scholar]
- Ono S, Mohri K, Ono K. Microscopic evidence that actin-interacting protein 1 actively disassembles actin-depolymerizing factor/cofilin-bound actin filaments. J Biol Chem. 2004;279:14207–14212. doi: 10.1074/jbc.M313418200. [DOI] [PubMed] [Google Scholar]
- Ono S, Ono K. Tropomyosin inhibits ADF/cofilin-dependent actin filament dynamics. J Cell Biol. 2002;156:1065–1076. doi: 10.1083/jcb.200110013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polet D, Lambrechts A, Ono K, Mah A, Peelman F, Vandekerckhove J, Baillie DL, Ampe C, Ono S. Caenorhabditis elegans expresses three functional profilins in a tissue-specific manner. Cell Motil Cytoskeleton. 2006;63:14–28. doi: 10.1002/cm.20102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose KL, Winfrey VP, Hoffman LH, Hall DH, Furuta T, Greenstein D. The POU gene ceh-18 promotes gonadal sheath cell differentiation and function required for meiotic maturation and ovulation in Caenorhabditis elegans. Dev Biol. 1997;192:59–77. doi: 10.1006/dbio.1997.8728. [DOI] [PubMed] [Google Scholar]
- Sijen T, Fleenor J, Simmer F, Thijssen KL, Parrish S, Timmons L, Plasterk RH, Fire A. On the role of RNA amplification in dsRNA-triggered gene silencing. Cell. 2001;107:465–476. doi: 10.1016/s0092-8674(01)00576-1. [DOI] [PubMed] [Google Scholar]
- Somlyo AP, Somlyo AV. Signal transduction and regulation in smooth muscle. Nature. 1994;372:231–236. doi: 10.1038/372231a0. [DOI] [PubMed] [Google Scholar]
- Squire JM. Architecture and function in the muscle sarcomere. Curr Opin Struct Biol. 1997;7:247–257. doi: 10.1016/s0959-440x(97)80033-4. [DOI] [PubMed] [Google Scholar]
- Strome S. Fluorescence visualization of the distribution of microfilaments in gonads and early embryos of the nematode Caenorhabditis elegans. J Cell Biol. 1986;103:2241–2252. doi: 10.1083/jcb.103.6.2241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmons L, Court DL, Fire A. Ingestion of bacterially expressed dsRNAs can produce specific and potent genetic interference in Caenorhabditis elegans. Gene. 2001;263:103–112. doi: 10.1016/s0378-1119(00)00579-5. [DOI] [PubMed] [Google Scholar]
- Timmons L, Fire A. Specific interference by ingested dsRNA. Nature. 1998;395:854. doi: 10.1038/27579. [DOI] [PubMed] [Google Scholar]
- Vartiainen MK, Mustonen T, Mattila PK, Ojala PJ, Thesleff I, Partanen J, Lappalainen P. The three mouse actin-depolymerizing factor/cofilins evolved to fulfill cell-type-specific requirements for actin dynamics. Mol Biol Cell. 2002;13:183–194. doi: 10.1091/mbc.01-07-0331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterston RH. Muscle. In: Wood WB, editor. The Nematode C elegans. Cold Spring Harbor Laboratory; 1988. pp. 281–335. [Google Scholar]
- Waterston RH, Thomson JN, Brenner S. Mutants with altered muscle structure of Caenorhabditis elegans. Dev Biol. 1980;77:271–302. doi: 10.1016/0012-1606(80)90475-3. [DOI] [PubMed] [Google Scholar]
- Wissmann A, Ingles J, Mains PE. The Caenorhabditis elegans mel-11 myosin phosphatase regulatory subunit affects tissue contraction in the somatic gonad and the embryonic epidermis and genetically interacts with the Rac signaling pathway. Dev Biol. 1999;209:111–127. doi: 10.1006/dbio.1999.9242. [DOI] [PubMed] [Google Scholar]
- Yamashiro S, Gimona M, Ono S. UNC-87, a calponin-related protein in C. elegans, antagonizes ADF/cofilin-mediated actin filament dynamics. J Cell Sci. 2007;120:3022–3033. doi: 10.1242/jcs.013516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashiro S, Mohri K, Ono S. The two Caenorhabditis elegans actin depolymerizing factor/cofilin proteins differently enhance actin filament severing and depolymerization. Biochemistry. 2005;44:14238–14247. doi: 10.1021/bi050933d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeoh S, Pope B, Mannherz HG, Weeds A. Determining the differences in actin binding by human ADF and cofilin. J Mol Biol. 2002;315:911–925. doi: 10.1006/jmbi.2001.5280. [DOI] [PubMed] [Google Scholar]
- Yin X, Gower NJ, Baylis HA, Strange K. Inositol 1,4,5-trisphosphate signaling regulates rhythmic contractile activity of myoepithelial sheath cells in Caenorhabditis elegans. Mol Biol Cell. 2004;15:3938–3949. doi: 10.1091/mbc.E04-03-0198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonezawa N, Nishida E, Sakai H. pH control of actin polymerization by cofilin. J Biol Chem. 1985;260:14410–14412. [PubMed] [Google Scholar]
- Zengel JM, Epstein HF. Identification of genetic elements associated with muscle structure in the nematode Caenorhabditis elegans. Cell Motil. 1980;1:73–97. doi: 10.1002/cm.970010107. [DOI] [PubMed] [Google Scholar]