Abstract
Neuropilin 1 (NRP1), a non-tyrosine kinase receptor for vascular endothelial growth factor and class 3 Semaphorins, is highly expressed in many human tumour cell lines, but its function is poorly understood. Here, we describe the expression of a new chondroitin sulphate-modified NRP1 (NRP1-CS) in human tumour cell lines. Expression of a non-modifiable NRP1 mutant (S612A) in U87MG human glioma cells results in enhanced invasion in three dimensions (3D), whereas wild-type NRP1 has no effect. Furthermore, the S612A NRP1 cells show a significant increase in p130Cas tyrosine phosphorylation compared with control and wild-type NRP1 cells. Silencing of p130Cas in S612A NRP1 cells resulted in a loss of increased invasive phenotype. Interestingly, p130Cas silencing does not inhibit basal 3D invasion, but leads to a mesenchymal to amoeboid transition. Biopsies from both low- and high-grade human gliomas show strong expression of NRP1, and little expression of NRP1-CS. Our data establish distinct roles for NRP1 and NRP1-CS in modulating a new NRP1-p130Cas signalling pathway contributing to glioblastoma cell invasion in 3D.
Keywords: growth factors, VEGF, metastasis, proteoglycan
Introduction
Neuropilin 1 (NRP1) acts as a co-receptor for Semaphorin 3A (SEMA3A) and vascular endothelial growth factor (VEGF). In neuronal cells, NRP1 regulates axon guidance, in part, by acting as a co-receptor with Plexin-A1 and SEMA3A (He & Tessier-Lavigne, 1997). NRP1 is a receptor for VEGF in endothelial cells and is essential for embryonic angiogenesis and vascular development (Soker et al, 1998; Gu et al, 2003). NRP1 is overexpressed in several types of cancer cells and human tumours, and increased expression is associated with poor patient prognosis (Osada et al, 2004; Bielenberg et al, 2006). These results have led to several groups trying to identify new therapeutic approaches for the inhibition of NRP1 (Cheng et al, 2004; Jia et al, 2006; Pan et al, 2007).
Recent findings suggest that NRP1 in endothelial and vascular smooth muscle cells (SMCs) can be post-translationally modified by the addition of glycosaminoglycans (GAGs; Shintani et al, 2006). GAGs are principal structural components of proteoglycans and important components of the extracellular matrix, participating in the regulation of cell proliferation, differentiation, and cell–cell and cell–matrix interactions (Kjellen & Lindahl, 1991). Deregulated expression of GAGs occurs in several cancers and correlates with clinical prognosis in several malignant neoplasms (Theocharis et al, 2006), stimulating research in the development of new therapeutic drugs targeting GAGs (Yip et al, 2006).
Here, we report the existence of two populations of NRP1 in diverse human tumour cell lines. One comprises an N-linked glycosylated core protein, and the other is a high molecular weight species modified by the addition of chondroitin sulphate GAG (NRP1-CS) to a single conserved serine residue (Ser 612). Surprisingly, overexpression of the S612A NRP1 mutant but not wild-type NRP1 in the human malignant glioma cell line (U87MG) leads to enhanced cell invasion in a three-dimensional (3D) matrix. Furthermore, S612A NRP1 cells show markedly increased levels of tyrosine phosphorylated p130Cas, an adaptor molecule implicated in invasive signalling in several cell types (Defilippi et al, 2006). Finally, biopsies from both low- and high-grade human gliomas show strong expression of NRP1 and little expression of NRP1-CS, suggesting that the highly invasive nature of these tumours is established, in part, by reduction in the expression of NRP1-CS.
Results And Discussion
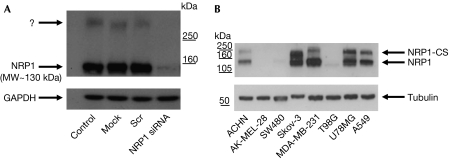
Silencing of NRP1 in A549 lung carcinoma cells by using short interfering RNA decreased expression of a band corresponding to the predicted molecular weight of NRP1 (130 kDa), but also inhibited expression of a high molecular weight band recognized by the NRP1 antibody and unaffected by treatment with a scrambled siRNA (Fig 1A). A similar co-migrating high molecular weight band was expressed in several other human tumour cells, including ACHN, MDA-MB-231, Skov-3, A549 and U87MG (Fig 1B).
Figure 1.
Identification of a high molecular weight NRP1 species in human tumour cell lines. (A) Identification of a high molecular weight band (>250 kDa) in Western blot analysis of NRP1, which is eliminated by NRP1 siRNA treatment. (B) The high molecular weight NRP1 species is expressed in several human tumour cell lines. Blots shown here and in all subsequent figures are representative of at least three separate experiments. NRP1, neuropilin 1; NRP1-CS, chondroitin sulphate-modified NRP1; siRNA, short interfering RNA.
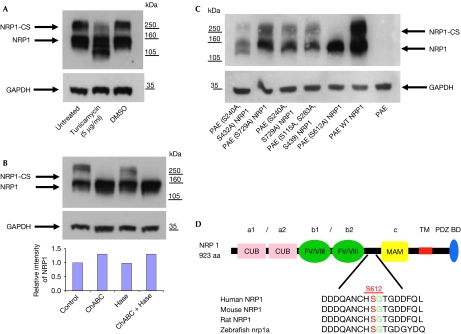
Treatment of A549 cells with tunicamycin, an inhibitor of N-linked glycosylation, caused a decrease in the apparent molecular weight of 130 kDa NRP1, but only modestly reduced the size of the high molecular weight species. These results indicated that although both NRP1 species contain N-linked oligosaccharide groups, modification of the high molecular weight NRP1 was not primarily due to N-linked glycosylation (Fig 2A). One type of post-translational modification known to produce high molecular weight molecules is the addition of GAGs, containing chondroitin sulphate, dermatan sulphate or heparin/heparan sulphate, to specific serine residues within the consensus motif, SG (Bourdon et al, 1987). We addressed whether NRP1 is modified by the addition of a GAG in human tumour cells by treating A549 cells in culture with chondroitinase ABC (ChABC) and heparinase/heparitinase, enzymes that cleave the respective GAGs from membrane surface proteoglycans. Treatment with ChABC alone completely removed the higher molecular weight band with a concomitant increase in the levels of 130 kDa NRP1, whereas heparinase/heparitinase treatment caused no reduction in the high molecular weight band (Fig 2B). Therefore, we conclude that NRP1 in A549 cells exists in two distinct populations: an N-glycosylated non-GAG-modified form (NRP1) and a chondroitin sulphate GAG-modified form (NRP1-CS).
Figure 2.
NRP1 is post-translationally modified by N-linked glycosylation and by the addition of O-linked chondroitin sulphate to Ser 612 (S612). (A) A549 cells were untreated or pretreated with 5 μg/ml tunicamycin or control vehicle (0.1% DMSO) for 16 h, lysed and blotted with NRP1 antibody. (B) A549 cells were untreated or treated with 1 U/ml ChABC, 1 U/ml heparitinase (Hase), or both for 2 h before lysis and blotting for NRP1. A graphical representation of the relative intensity of the NRP1 band measured by densitometry is shown below. (C) Transient transfection of PAE cells with WT and NRP1 mutants, which show that only S612A NRP1 transfection results in the expression of non-CS-modified NRP1. (D) S612 in the region between the b2 and MAM domains of NRP1 is conserved between several vertebrate species. ChABC, chondroitinase ABC; CS, chondroitin sulphate; NRP1, neuropilin 1; PAE, porcine aortic endothelium; WT, wild type.
To determine which serine residues were modified by the addition of chondroitin sulphate to NRP1, all seven extracellular serine residues in the species conserved SG consensus sequences were mutated to alanine. Transfection of these mutants into porcine aortic endothelial (PAE) cells, which do not express endogenous NRP1 (Jia et al, 2006), showed that only the S612A mutant was not CS-GAG modified. Interestingly, mutation of Ser 240 and Ser 432 caused a partial reduction in the level of NRP1-CS, suggesting that these sites might also contribute to CS-GAG modification (Fig 2C). Nevertheless, these results show that Ser 612 is the principal site of modification and that mutation of this site results in the complete loss of CS-GAG NRP1 modification. Furthermore, the SG motif corresponding to Ser 612 is highly conserved between vertebrate species (Fig 2D). Modification of NRP1 by both chondroitin sulphate and heparin sulphate GAGs on Ser 612 in SMCs and endothelial cells was recently reported (Shintani et al, 2006). However, our results indicate that NRP1 is modified by the addition of chondroitin sulphate alone, in both tumour cells (Fig 2B) and SMCs (C.P.-M., P.F. & I.C.M., unpublished data). This difference could be due to the extremely sensitive nature of ChABC activity to slight changes in pH and temperature (Tester et al, 2007). In addition, we are unaware of any reports of a specific serine that can be modified by the addition of both chondroitin sulphate and heparin sulphate in vivo. Indeed, other GAG-modified proteins (for example, syndecan 1) contain distinct sites of unambiguous attachment mediated by structural elements within the core protein (Kokenyesi & Bernfield, 1994).
High levels of expression of NRP1 have been correlated with increased invasiveness of several human cancers and tumour cell lines (Bachelder et al, 2002; Hansel et al, 2004), but the role of NRP1 in tumour cell invasion remains unclear. The role of NRP1 and NRP1-CS in invasion was investigated by using the highly invasive human malignant glioma cell line U87MG (Stan et al, 1999), which expresses relatively equal amounts of endogenous NRP1 and NRP1-CS (Fig 1B).
Initially, we examined the cellular distributions of NRP1 and chondroitin sulphate in U87MG cells by immunofluorescent staining for both NRP1 and chondroitin sulphate. NRP1 and chondroitin sulphate were localized in both overlapping and distinct areas on the cell membrane, and both were enriched in areas of cell–cell contact and on the leading edge of lamellipodia, suggesting that NRP1 and chondroitin sulphate partly colocalize (data not shown). We went on to stably overexpress wild-type NRP1 and S612A NRP1 in U87MG cells by retroviral infection, which resulted in a homogeneous pattern of expression (supplementary Fig 1A online). S612A NRP1 U87MG cells expressed almost entirely unmodified NRP1 relative to NRP1-CS, whereas wild-type NRP1 U87MG cells predominantly expressed the modified NRP1-CS (supplementary Fig 1B online).
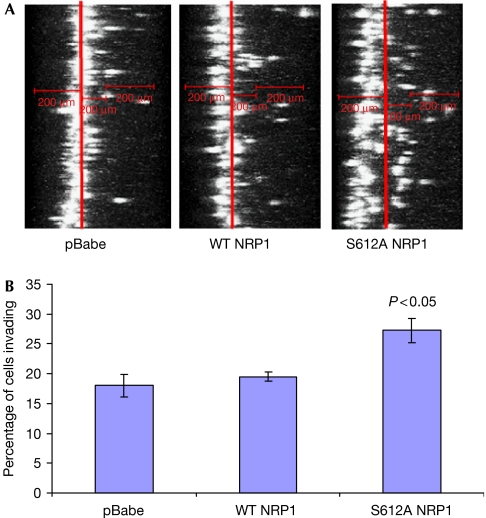
The invasiveness of NRP1-overexpressing U87MG cells was examined by allowing cells to invade through a 3D collagen matrix, representative of the in vivo environment experienced by malignant tumour cells (Sahai, 2005). Surprisingly, although wild-type NRP1 cells showed no significant effect on invasion compared with the control, S612A NRP1 cells showed a significant increase in invasion of about 50% (Fig 3A,B). By contrast, expression of S612A NRP1 caused a small but reproducible inhibition of cell proliferation, whereas wild-type NRP1 had no significant effect on proliferation (supplementary Fig 2 online). Interestingly, global removal of cell surface CS-GAGs in U87MG cells by incubation with ChABC markedly decreased invasion (data not shown), consistent with reports describing a role for CS-GAGs in tumour cell invasion (Denholm et al, 2001; Yip et al, 2006). These findings emphasize that CS-GAG modification of specific surface molecules can have distinct effects on tumour cell invasion, which might be masked by global changes in proteoglycan modifications. For example, heparan sulphate GAG modification of the proteoglycan syndecan 1 was found to have a negative effect on invasiveness (Liu et al, 1998).
Figure 3.
Expression of S612A NRP1 increases three-dimensional invasion. (A) xz reconstructions of U87MG pBabe, WT NRP1 and S612A NRP1 cells labelled with sytox green in collagen I invasion assays, performed as described in the Methods. (B) Quantification of invasion of U87MG pBabe, WT NRP1 and S612A NRP1 cells. Analysis was carried out as described in the supplementary information online. Data are expressed as the means±s.e.m. from three independent experiments. NRP1, neuropilin 1; WT, wild type.
Given that Ser 612 lies within the linker region between the MAM and b2 extracellular domains (Fig 2D), we considered whether GAG modification at this site might affect VEGF-A165 or SEMA3A binding to NRP1. Measurement of high-affinity 125I-VEGF-A165 binding revealed that only wild-type NRP1 U87MG cells showed increased (around 50%) VEGF-A165 binding. Although we detected an increase in binding for SEMA3A in both wild-type and S612A cell lines compared with the control, we could not detect any significant difference between wild-type and S612A NRP1-expressing cell lines as determined by FACS analysis (supplementary Fig 3A,B online). These data indicate that the increased invasiveness of the S612A cells does not result from increased VEGF-A165 of SEMA3A binding to NRP1. In agreement, Shintani et al (2006) showed that in both SMCs and endothelial cells expression of S612A NRP1 significantly reduced the level of VEGF-A165 binding compared with wild-type NRP1. Furthermore, a recent report shows that overexpression of wild-type NRP1 promotes glioma progression in vivo through a VEGF-A165-independent pathway that is dependent on HGF/c-Met signalling (HGF for hepatocyte growth factor; Hu et al, 2007).
Next, we investigated the effects of S612A NRP1 expression on signalling pathways known to mediate invasion (Sahai, 2005). Western blots showed that there were equal amounts of total and phosphorylated ERK (extracellular-signal-regulated kinase) 1/2 and AKT present in the control, wild-type NRP1 and S612A NRP1 cell lines (supplementary Fig 4A online). Similarly, we found no detectable differences in the expression of total protein and levels of phosphorylation of Src (Tyr 416) or FAK (Tyr 397) in the control, wild-type NRP1 and S612A NRP1 cell lines (supplementary Fig 4B online).
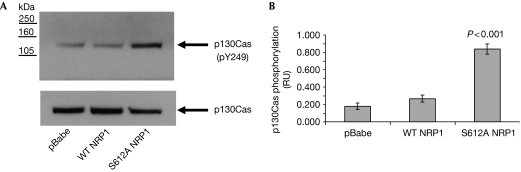
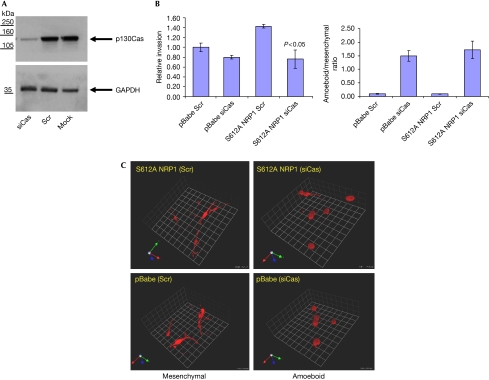
p130Cas is an adaptor protein involved in cytoskeleton reorganization, which can be both tyrosine and serine/tyrosine phosphorylated. Increased p130Cas tyrosine phosphorylation is associated with increased cell invasion (Defilippi et al, 2006) and results in the recruitment of effector proteins into multimolecular complexes necessary for intracellular signalling. Surprisingly, S612A NRP1 cells showed a fourfold increase in p130Cas Tyr 249 phosphorylation compared with control and wild-type NRP1 cells. This effect was not due to an increase in the total level of p130Cas; indeed, we consistently found some reduction in total p130Cas in the S612A NRP1 cell line (Fig 4A,B). To determine whether p130Cas was required for S612A NRP1-mediated increased invasion, we inhibited the expression of p130Cas by using siRNA (Fig 5A). Treatment with p130Cas siRNA reduced invasion of the S612A NRP1 cells to the level observed in control cells treated with either scrambled or p130Cas siRNA (Fig 5B). Consistent with our findings, two recent reports describe both a SRC-independent pathway leading to increased p130Cas phosphorylation (Ambrogio et al, 2005), and FAK-mediated cell invasion, which is not dependent on p130Cas (Natarajan et al, 2006). Interestingly, a recent report describes NRP1 association with β1 integrin modulating pancreatic cancer cell growth, survival and invasion (Fukasawa et al, 2007). Given the involvement of p130Cas in the integrin signalling machinery (Defilippi et al, 2006), it is plausible that NRP1 could signal to p130Cas through an integrin-mediated pathway. Therefore, we examined the effect of β1 integrin silencing in the control and S612A NRP1-expressing cell lines. Interestingly, although we achieved a strong knockdown of β1 integrin subunit expression, we could detect no significant effect on invasion in either the control or S612A-expressing cell lines (supplementary Fig 5A,B).
Figure 4.
Effect of expression of wild-type and S612A NRP1 on p130Cas phosphorylation in U87MG cells. (A) U87MG pBabe, WT NRP1 and S612A NRP1 cells grown on collagen I-coated plates and incubated in 1% FCS for 18 h were lysed and blotted for total and phosphor-Tyr 249 (pY239) p130Cas. (B) Quantification of p130Cas phosphorylation was performed by densitometry using Image J. Data from three independent experiments are presented as p130Cas phosphorylation relative units (RUs) normalized to total p130Cas and expressed as means±s.e.m. NRP1, neuropilin 1; WT, wild type.
Figure 5.
p130Cas is required for S612A NRP1-mediated elongated invasion in three-dimensions. (A) S612A NRP1 cell lysates were immunoblotted for p130Cas and GAPDH 72 h after transfection of cells with scrambled siRNA (Scr) or p130Cas siRNA. Similar levels of p130Cas knockdown were obtained in U87MG pBabe cells (data not shown). (B) Invasion of U87MG pBabe and S612A NRP1 cells, 24 h after transfection with the indicated siRNAs. Data are expressed as the means±s.e.m. from three experiments. Invasion index was normalized to pBabe Scr siRNA (set at 1). (C) Depletion of p130Cas leads to a transition from elongated (mesenchymal) to a rounded (amoeboid) mode of invasion. U87MG pBabe and S612A NRP1 cells invading through collagen plugs stained with AF546-Phalloidin (cytoskeleton). Confocal sections (1.75 μm intervals) were obtained using a × 40 LWD objective. Three-dimensional reconstructions (1 U=25 μm) were performed with Volocity imaging software (Improvision). The ratio of amoeboid cells to mesenchymal cells is shown on the right and was determined from two of the three independent experiments performed above. LWD, long working distance; NRP1, neuropilin 1; siRNA, short interfering RNA.
Control and S612A NRP1 cell lines show a classical elongated (mesenchymal) phenotype (Sahai & Marshall, 2003; Wolf et al, 2003) when invading through collagen I (Fig 5C). However, p130Cas knockdown caused distinct morphological changes in both cell lines, including a rounding of the cell body and a loss of filopodia-type extensions (Fig 5C), similar to the more rounded (amoeboid) morphology observed when mesenchymal invasion is blocked with protease inhibitors (Wolf et al, 2003). This transition from mesenchymal to amoeboid invasion had no significant effect on the amount of invasion observed in the control cells (Fig 5B), indicating that the S612A NRP1 invasive phenotype might be mediated through modification of signalling pathways regulating the mesenchymal mode of invasion.
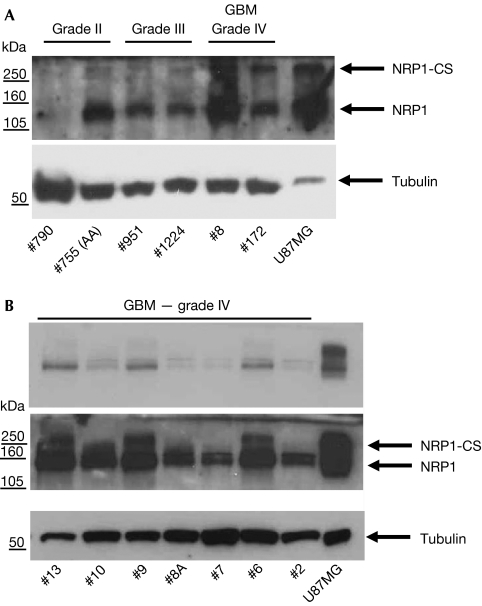
To determine whether there exists a pathophysiological relevance to increased invasion caused by the expression of S612A NRP1, we probed several biopsies of human gliomas for the expression of NRP1. Although several samples showed strong expression of NRP1 in both low- and high-grade gliomas, we detected little expression of NRP1-CS in all samples (Fig 6A,B). These data suggest that the highly invasive nature of these tumours is established, in part, by maintaining a low NRP1-CS to NRP1 expression ratio.
Figure 6.
Expression of NRP1 and NRP1-CS in low- and high-grade gliomas. Protein lysates of several biopsies of human glioma (all provided with separate code numbers) separated by gel electrophoresis, and probed using antibodies to NRP1 and tubulin as described in the supplementary information online. (A) Low- and high-grade glioma. (B) High-grade glioblastoma multiforme (GBM). Upper panel is a lower exposure of the middle NRP1 blot. CS, chondroitin sulphate; NRP1, neuropilin 1.
Our findings show that CS-GAG modification of NRP1 regulates cell signalling involved in invasion, and suggest that the balance between chondroitin sulphate-modified and unmodified NRP1 might be important for determining invasive potential. Recently, it has been suggested that inhibition of NRP1 does not reduce tumour cell proliferation or growth directly, but acts primarily through an anti-angiogenic mode of action, partly through inhibition of VEGF-mediated functions in endothelial cells (Jia et al, 2006; Pan et al, 2007). However, no studies have examined the role of NRP1 in tumour metastatic spread. The results presented here indicate that NRP1 regulates tumour cell invasion and might therefore be involved in the mechanisms underlying tumour metastasis.
Methods
Cell culture. A549 and U87MG cells were cultured in DMEM containing 10% FCS supplemented with Pen/Strep (1:100; P4333; Sigma). PAE cells and PAE cells expressing NRP1 (PAE/NRP1) were grown in Ham's F12 medium containing 10% FCS. PAE/NRP1 cells were routinely grown in 25 μg/ml hygromycin B to maintain the expression of the transgene. For cell signalling assays, cells were grown on collagen I-coated plates and shifted to DMEM medium containing 1% FCS for 18 h before collecting cells.
Transfections, plasmids and siRNA. A549, PAE and U87MG cells were transfected with Lipofectamine 2000 (Invitrogen). Briefly, the cells were plated at 60% confluence and transfected on the following day using 1 μg plasmid DNA or 25 nM final concentration of siRNA. pcDNA 3.1(+)wild-type NRP1 was generated by subcloning the full-length open reading frame of NRP1 (Origene, Cambridge Bioscience, Cambridge, UK) into pcDNA 3.1(+). pcDNA 3.1(+) S612A NRP1 was generated by using the Quickchange II site-directed mutagenesis kit (Stratagene, La Jolla, CA, USA) according to the manufacturer's instructions. Both wild-type NRP1 and S612A NRP1 coding sequences were subcloned into pBabe puro (a gift from E. Sahai, LRI, Cancer Research UK, UK) through the SnaBI site. p130Cas siRNA 5′-GGUCGACAGUGGUGUGUAU-3′ and β1 integrin siRNA 5′-GAACAGAUCUGAUGAAUGA-3′ were purchased from Dharmacon (Lafayette, CO, USA).
Supplementary information is available at EMBO reports online (http://www.emboreports.org).
Supplementary Material
supplementary Information
Acknowledgments
This study was supported by British Heart Foundation grants FS/06/019to C.P.-M. and RG/06/003 to I.C.Z., and by financial assistance from Ark Therapeutics Ltd. We thank David I.R. Holmes for generation of pcDNA 3.1(+) wild-type NRP1 and Ian Evans for helpful discussions.
Footnotes
This study was financially assisted in part by Ark Therapeutics Plc, which is developing therapies targeted at inhibition of neuropilin. I.C.Z. is a consultant to Ark Therapeutics Plc. P.F., L.C. and P.L. are employees of Ark Therapeutics Plc. M.L.T. was a past employee of Ark Therapeutics Plc.
References
- Ambrogio C et al. (2005) p130Cas mediates the transforming properties of the anaplastic lymphoma kinase. Blood 106: 3907–3916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachelder RE, Wendt MA, Mercurio AM (2002) Vascular endothelial growth factor promotes breast carcinoma invasion in an autocrine manner by regulating the chemokine receptor CXCR4. Cancer Res 62: 7203–7206 [PubMed] [Google Scholar]
- Bielenberg DR, Pettaway CA, Takashima S, Klagsbrun M (2006) Neuropilins in neoplasms: expression, regulation, and function. Exp Cell Res 312: 584–593 [DOI] [PubMed] [Google Scholar]
- Bourdon MA, Krusius T, Campbell S, Schwartz NB, Ruoslahti E (1987) Identification and synthesis of a recognition signal for the attachment of glycosaminoglycans to proteins. Proc Natl Acad Sci USA 84: 3194–3198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng L, Jia H, Lohr M, Bagherzadeh A, Holmes DI, Selwood D, Zachary I (2004) Anti-chemorepulsive effects of vascular endothelial growth factor and placental growth factor-2 in dorsal root ganglion neurons are mediated via neuropilin-1 and cyclooxygenase-derived prostanoid production. J Biol Chem 279: 30654–30661 [DOI] [PubMed] [Google Scholar]
- Defilippi P, Di Stefano P, Cabodi S (2006) p130Cas: a versatile scaffold in signaling networks. Trends Cell Biol 16: 257–263 [DOI] [PubMed] [Google Scholar]
- Denholm EM, Lin YQ, Silver PJ (2001) Anti-tumor activities of chondroitinase AC and chondroitinase B: inhibition of angiogenesis, proliferation and invasion. Eur J Pharmacol 416: 213–221 [DOI] [PubMed] [Google Scholar]
- Fukasawa M, Matsushita A, Korc M (2007) Neuropilin-1 interacts with integrin β1 and modulates pancreatic cancer cell growth, survival and invasion. Cancer Biol Ther 6: 1173–1180 [DOI] [PubMed] [Google Scholar]
- Gu C, Rodriguez ER, Reimert DV, Shu T, Fritzsch B, Richards LJ, Kolodkin AL, Ginty DD (2003) Neuropilin-1 conveys semaphorin and VEGF signaling during neural and cardiovascular development. Dev Cell 5: 45–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansel DE, Wilentz RE, Yeo CJ, Schulick RD, Montgomery E, Maitra A (2004) Expression of neuropilin-1 in high-grade dysplasia, invasive cancer, and metastases of the human gastrointestinal tract. Am J Surg Pathol 28: 347–356 [DOI] [PubMed] [Google Scholar]
- He Z, Tessier-Lavigne M (1997) Neuropilin is a receptor for the axonal chemorepellent Semaphorin III. Cell 90: 739–751 [DOI] [PubMed] [Google Scholar]
- Hu B, Guo P, Bar-Joseph I, Imanishi Y, Jarzynka MJ, Bogler O, Mikkelsen T, Hirose T, Nishikawa R, Cheng SY (2007) Neuropilin-1 promotes human glioma progression through potentiating the activity of the HGF/SF autocrine pathway. Oncogene 26: 5577–5586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia H et al. (2006) Characterization of a bicyclic peptide neuropilin-1 (NP-1) antagonist (EG3287) reveals importance of vascular endothelial growth factor exon 8 for NP-1 binding and role of NP-1 in KDR signaling. J Biol Chem 281: 13493–13502 [DOI] [PubMed] [Google Scholar]
- Kjellen L, Lindahl U (1991) Proteoglycans: structures and interactions. Annu Rev Biochem 60: 443–475 [DOI] [PubMed] [Google Scholar]
- Kokenyesi R, Bernfield M (1994) Core protein structure and sequence determine the site and presence of heparan sulfate and chondroitin sulfate on syndecan-1. J Biol Chem 269: 12304–12309 [PubMed] [Google Scholar]
- Liu W, Litwack ED, Stanley MJ, Langford JK, Lander AD, Sanderson RD (1998) Heparan sulfate proteoglycans as adhesive and anti-invasive molecules. Syndecans and glypican have distinct functions. J Biol Chem 273: 22825–22832 [DOI] [PubMed] [Google Scholar]
- Natarajan M, Stewart JE, Golemis EA, Pugacheva EN, Alexandropoulos K, Cox BD, Wang W, Grammer JR, Gladson CL (2006) HEF1 is a necessary and specific downstream effector of FAK that promotes the migration of glioblastoma cells. Oncogene 25: 1721–1732 [DOI] [PubMed] [Google Scholar]
- Osada H, Tokunaga T, Nishi M, Hatanaka H, Abe Y, Tsugu A, Kijima H, Yamazaki H, Ueyama Y, Nakamura M (2004) Overexpression of the neuropilin 1 (NRP1) gene correlated with poor prognosis in human glioma. Anticancer Res 24: 547–552 [PubMed] [Google Scholar]
- Pan Q et al. (2007) Blocking neuropilin-1 function has an additive effect with anti-VEGF to inhibit tumor growth. Cancer Cell 11: 53–67 [DOI] [PubMed] [Google Scholar]
- Sahai E (2005) Mechanisms of cancer cell invasion. Curr Opin Genet Dev 15: 87–96 [DOI] [PubMed] [Google Scholar]
- Sahai E, Marshall CJ (2003) Differing modes of tumour cell invasion have distinct requirements for Rho/ROCK signalling and extracellular proteolysis. Nat Cell Biol 5: 711–719 [DOI] [PubMed] [Google Scholar]
- Shintani Y et al. (2006) Glycosaminoglycan modification of neuropilin-1 modulates VEGFR2 signaling. EMBO J 25: 3045–3055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soker S, Takashima S, Miao HQ, Neufeld G, Klagsbrun M (1998) Neuropilin-1 is expressed by endothelial and tumor cells as an isoform-specific receptor for vascular endothelial growth factor. Cell 92: 735–745 [DOI] [PubMed] [Google Scholar]
- Stan AC, Casares S, Radu D, Walter GF, Brumeanu TD (1999) Doxorubicin-induced cell death in highly invasive human gliomas. Anticancer Res 19: 941–950 [PubMed] [Google Scholar]
- Tester NJ, Plaas AH, Howland DR (2007) Effect of body temperature on chondroitinase ABC's ability to cleave chondroitin sulfate glycosaminoglycans. J Neurosci Res 85: 1110–1118 [DOI] [PubMed] [Google Scholar]
- Theocharis AD, Tsolakis I, Tzanakakis GN, Karamanos NK (2006) Chondroitin sulfate as a key molecule in the development of atherosclerosis and cancer progression. Adv Pharmacol 53: 281–295 [DOI] [PubMed] [Google Scholar]
- Wolf K, Mazo I, Leung H, Engelke K, von Andrian UH, Deryugina EI, Strongin AY, Brocker EB, Friedl P (2003) Compensation mechanism in tumor cell migration: mesenchymal–amoeboid transition after blocking of pericellular proteolysis. J Cell Biol 160: 267–277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yip GW, Smollich M, Gotte M (2006) Therapeutic value of glycosaminoglycans in cancer. Mol Cancer Ther 5: 2139–2148 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
supplementary Information