Abstract
Mouse P19 embryonal carcinoma cells undergo cardiogenesis in response to high density and DMSO. We have derived a clonal subline which undergoes cardiogenesis in response to high density, but without requiring exposure to DMSO. The new subline retains the capacity to differentiate into skeletal muscle and neuronal cells in response to DMSO and retinoic acid. However, upon aggregation, these Oct 4-positive cells, termed P19-SI because they “self-induce” cardiac muscle, exhibit increased mRNAs encoding the mesodermal factor Brachyury, cardiac transcription factors Nkx 2.5 and GATA 4, the transcriptional repressor Msx-1, and cytokines Wnt 3a, Noggin and BMP 4. Exposure of aggregated P19-SI cells to BMP 4, a known inducer of cardiogenesis, accelerates cardiogenesis, as determined by rhythmic beating and myosin staining. However, cardiogenesis is severely inhibited when P19-SI cells are aggregated in the presence of BMP 4. These results demonstrate that cell-cell interaction is required before P19-SI cells can undergo a cardiogenic response to BMP 4. A concurrent increase in the expression of Msx-1 suggests one possible process underlying the inhibition of cardiogenesis. The phenotype of P19-SI cells offers an opportunity to explore new aspects of cardiac induction.
Keywords: P19, BMP, cardiogenesis, aggregation, self-induction
INTRODUCTION
Signaling between the anterior endoderm and the adjacent anterior mesoderm is pivotal for vertebrate cardiogenesis (Arai et al., 1997; Schultheiss et al., 1995; Nascone and Mercola, 1995). In vivo and explant culture experiments have strongly implicated endoderm-derived bone morphogenetic proteins (BMPs) as molecules which are crucial for normal cardiac induction and development (Schultheiss et al., 1997; Ladd et al., 1998; Ehrman and Yutzey, 1999; Sparrow et al., 1998; Zhang and Bradley, 1996).
Pluripotent mouse cell lines, such as the embryonal carcinoma (EC) cell line, P19, are useful model systems for investigating the roles of various factors in cardiac induction and differentiation (Grepin et al., 1997; Skerjanc et al., 1998; van der Heyden and Defize, 2003). Aggregated P19 cells can be induced to undergo cardiogenesis by co-culture with endodermal cell lines (Mummery et al., 1991), or by exposure to exogenously-added agents such as DMSO (McBurney et al., 1982; Edwards et al., 1983). In the latter experiments, peripheral cells in the aggregate display an endodermal phenotype (Smith et al., 1987), while the “core” cells express the mesodermal marker, Brachyury (Yamaguchi et al., 1999). Consistent with in vivo and explant results, BMP signaling appears to be required for cardiac induction of P19 cell aggregates. These conclusions have been drawn primarily from studies using the BMP inhibitor, noggin (Jamali et al., 2001a; Monzen et al., 1999). Complementrary data have been obtained from experiments in which exogenously-added BMP was shown to induce monolayer cultures of P19 cells which had been stably transfected with the cardiac transcription factor, Nkx 2.5 (Jamali et al., 2001a). Therefore, aggregated P19 cells appear to recapitulate at least some of the more fundamental events, including BMP signaling, known to be critical for cardiac induction.
In this study, we describe a unique variant, “P19-SI”, of the P19 embryonal carcinoma cell line. P19-SI cells are called “self inducing” because they exhibit significant cardiogenesis under conditions of high density and in the absence of any added inducing agent. P19-SI cells express BMP4, but not until after about 48 hrs of aggregate culture. Supplementing the endogenous BMP4 at this time accelerates cardiogenesis, as measured by the percentage of beating aggregates or the appearance of myosin-positive cells. However, exposure of cells to BMP4 prior to aggregate formation effectively inhibits any cardiogenesis, as measured by the failure to induce the cardiac transcription factors GATA 4 and Nkx 2.5, sarcomeric myosin or rhythmic beating. Hence, the timing of exposure to BMP4 is critical for cardiac induction. In contrast, expression of Wnt 3a, a global signaling molecule which is known to play one or more roles in cardiogenesis (Marvin et al., 2001; Naito et al., 2005; Nakamura et al., 2003), is increased by “early” exposure of P19 cells to exogenous BMP4. Furthermore, a rapid increase in Msx-1 expression also occurs in response to exogenously added BMP4. Msx-1 is a known transcriptional repressor (Zhang et al., 1996; Lee et al., 2004; Bendall and Abate-Shen, 2000) and inhibitor of cadherin-mediated cell adhesion (Lincecum et al., 1998), suggesting its expression may need to be tightly controlled in order to permit effective cardiac induction by BMP4.
RESULTS
P19-SI cells undergo cardiogenesis in the absence of DMSO
DMSO-treated aggregates of pluripotent P19-EC (“embryonal carcinoma”) cells can be induced to terminally differentiate into cell types from all three germ layers (Marcel et al., 2003). In the course of investigating the induction of cardiac muscle from P19 cells, we noted that some untreated aggregates contained beating cardiocytes. To determine whether the appearance of differentiated cardiac cells in untreated aggregates was due to cells that had undergone a stable genetic or epigenetic change, the parental population was subjected to clonal analysis. These assays showed that, in the absence of DMSO, 1–2% of cells in the parental P19 population produced clones which, based on their spontaneous rhythmic contractions, contained cardiac muscle. Analysis of clonal cultures prior to the onset of beating revealed a subpopulation of colonies whose cells appeared less cohesive and more migratory (Fig. 1). The subclonal progeny of this morphologically distinct population are Oct 3/4-positive (Fig. 2A–C), indicating that they are “undifferentiated” prior to aggregation (Pesce and Scholer, 2001). However, these cells are cardiogenic when aggregated in the absence of DMSO. Within a few days of aggregation, a population of Sox17 positive cells emerged. These endoderm-like cells (Kubo et al., 2003) were concentrated at the periphery of aggregates (Fig. 3). All aggregates contained myosin-positive cells by day 4, spontaneous contractions began in ~10% of the aggregates by day 10, and >70% of the aggregates were beating by day 19 (Fig. 4, open boxes). These data are comparable to those obtained with the donor “parental” P19 cells aggregated and induced with DMSO (Fig. 4, filled boxes), and are qualitatively similar to results from other laboratories studying P19 cell induction (see, for example, Edwards et al., 1983). Accordingly, these P19 cell variants were called “self-inducers” (SI).
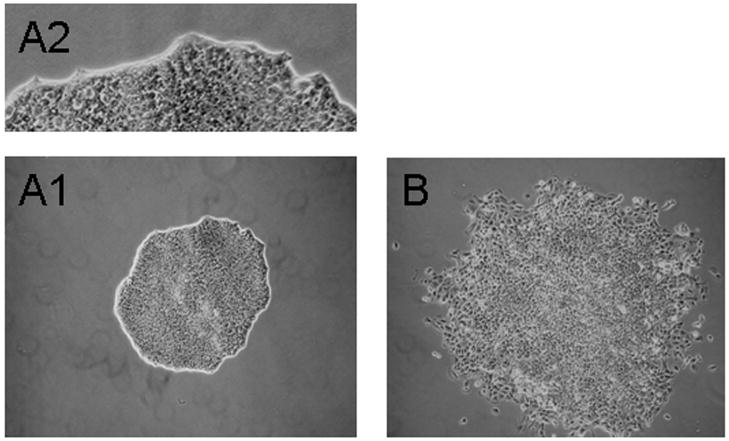
Fig. 1.
Clonal morphology of “parental” P19 and variant P19-SI cells. Parental P19 cells were plated at clonal density (see Experimental Procedures) and grown for 5 days. The clones were located, marked and observed using an inverted, phase contrast microscope. The majority of clones (>95%) displayed a “tight” morphology (A1 and A2), while the morphology of the minority clone population was less compact (B). When clones of the latter type were expanded, the cells were found to be “self-inducing” to a cardiac phenotype (see text). The images in A1 and B are the same magnification.
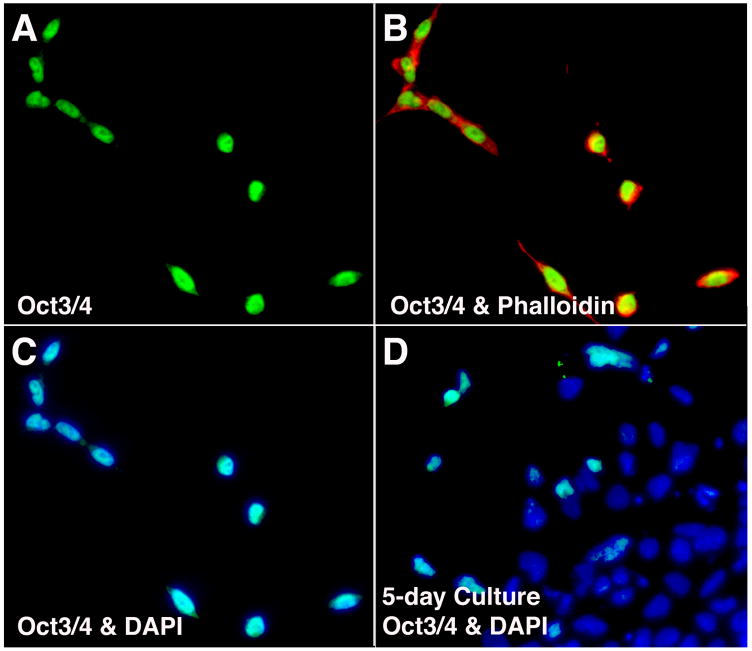
Fig. 2.
Assessment of the “undifferentiated state” of P19-SI cells. Mass cultures of P19-SI cells were prepared for antibody staining after 15 hrs (A–C) and after 5 days (D) of culture. (A) Oct 3/4. (B) Oct 3/4 and phalloidin. (C) and (D) Oct 3/4 and DAPI.
Fig. 3.
Assessment of endoderm formation in 5-day P19-SI aggregates. Cryosectioned P19-SI aggregates were stained for: (A) Sox 17 (green & arrow) and alpha myosin heavy chain (red & arrowhead); (B) composite of (A) and DAPI staining; (C) composite of (A) and phase contrast image.
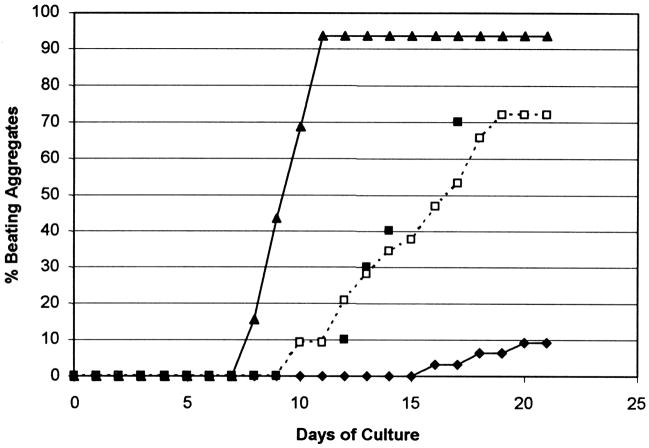
Fig. 4.
BMP 4 effects on P19-SI cell cardiogenesis. P19 parental and P19-SI cells were cultured as hanging drops (50–100 cells per 30ul) as described in Materials and Methods. (□) P19-SI cells cultured with no BMP 4 added; (◆) 10ng/ml BMP 4 added at the time the hanging drop cultures were established; (▲) 10 ng/ml BMP 4 added in 3ul on day 2; (■) “parental” P19 cells cultured with 0.5% DMSO. Hanging drop cultures (32 per experimental condition) were scored daily for beating activity. BMP 4 added prior to cell aggregation (◆) inhibits cardiogenesis; BMP 4 added after the cells have aggregated (▲) enhances cardiogenesis. Control P19-SI aggregates (□) appear to “self-induce” to cardiocytes as efficiently as do “parental” P19 cell aggregates induced with DMSO (■).
Aggregation (or high density) is required for P19-SI cell cardiogenesis
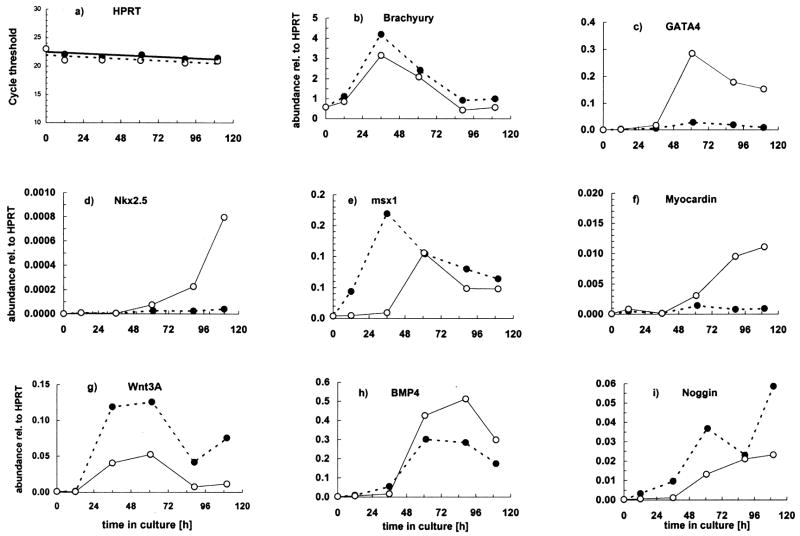
We examined the expression patterns of genes known to impact cardiogenesis in DMSO-induced P19 cell aggregates: GATA 4 (Grepin et al., 1995 & 1997), Nkx 2.5 (Jamali et al., 2001 a & b; Skerjanc et al., 1998), Myocardin (Ueyama et al., 2003), BMP 4 (Monzen et al., 1999 & 2001), Wnt 3a (Naito et al., 2005) and the BMP inhibitor, noggin (Monzen et al., 1999 & 2001); as well as the mesodermal transcription factor, Brachyury (Vidricaire et al., 1994) and the transcriptional repressor, Msx-1 (Lee et al., 2004). To determine how aggregation affects the temporal expression patterns of the genes listed above, we performed real time RT-PCR studies on aggregate and on monolayer cells during the first 5 days of culture in the absence of DMSO. These data were normalized to the transcript levels of a “housekeeping” gene, HPRT, the Ct values of which were nearly identical at each time point tested (Fig. 5a). Figure 5 shows results from 1 of 2 similar experiments.
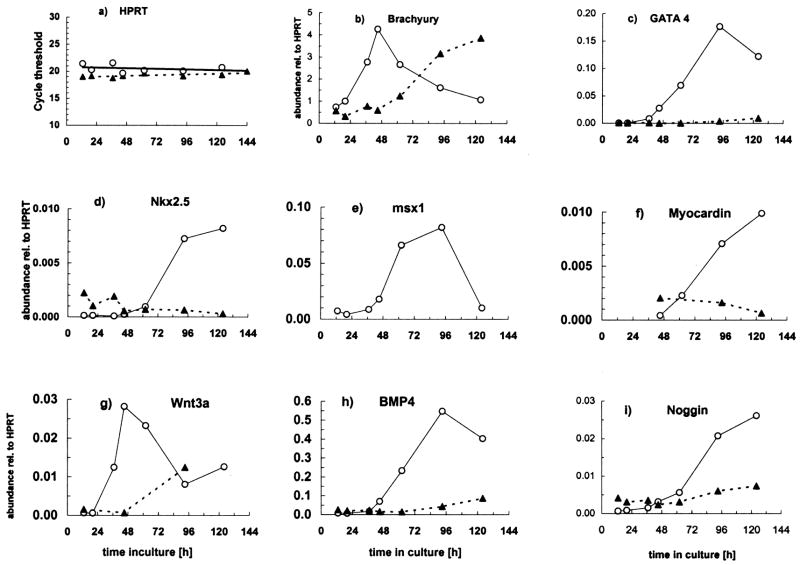
Fig. 5.
Effect of aggregation on P19-SI cell gene expression patterns. P19-SI cells were grown in monolayer (
 ) or mass aggregate (
) or mass aggregate (
 ) cultures. Samples were harvested from 13–124 hrs and assayed by quantitative RT-PCR to detect expression of the early mesodermal marker, Brachyury T; known cardiac transcription factors (Nkx 2.5, GATA 4, Myocardin); the cardiogenic signaling molecule, BMP 4; Noggin, Wnt 3a and Msx-1. Data in b) through i) are normalized to HPRT (a) expression. Msx-1 mRNA was not measured in monolayer cultures. See text for complete discussion.
) cultures. Samples were harvested from 13–124 hrs and assayed by quantitative RT-PCR to detect expression of the early mesodermal marker, Brachyury T; known cardiac transcription factors (Nkx 2.5, GATA 4, Myocardin); the cardiogenic signaling molecule, BMP 4; Noggin, Wnt 3a and Msx-1. Data in b) through i) are normalized to HPRT (a) expression. Msx-1 mRNA was not measured in monolayer cultures. See text for complete discussion.
Brachyury (Bra), an early marker of mesodermal induction (Wilson et al., 1995), dramatically increases shortly after P19-SI cells begin to aggregate, and then declines (Fig. 5b). It has been demonstrated with P19 cells that, even without exogenous inducing agents, Bra transcription transiently increases in response to cell aggregation (Vidricaire et al., 1994). Bra transcription also increases in monolayer cultures of P19-SI cells, but increased expression is delayed at least 36 hrs relative to that in aggregates (see Fig. 5b). The increased Bra transcription in monolayer culture occurs as the cells begin to reach confluency in local areas, some of which contain cells that are Oct 3/4 negative (Fig. 2D). These two events are indications that the process(es) underlying self-induction among P19-SI cells are particularly responsive to “density”, as opposed to requiring any 3rd dimensional parameters of aggregation, per se.
Also consistent with previous reports of DMSO-induced P19 cell induction (Jamali et al., 2001b; Nakamura et al., 2003), Wnt 3a gene expression transiently increases early in the process of P19-SI aggregation (Fig. 5g). In contrast to the kinetics of Wnt 3a espression in aggregates, and similarly to Bra, Wnt 3a expression in monolayer culture is delayed (Fig. 5g).
A causal relationship between BMP signaling and GATA 4 expression during DMSO-induced P19 cell cardiogenesis has been demonstrated by noggin inhibition studies (Jamali et al., 2001a; Monzen et al., 1999). The increased expression of GATA 4 in P19-SI aggregates is consistent with a rapid BMP-mediated response (Fig. 5c & h). Shortly thereafter, the cardiac marker, Nkx 2.5 (Komuro and Izumo, 1993) increases (Fig. 5d), as does one of its important targets, Myocardin (Fig. 5f) (Ueyama et al., 2003). Expression of noggin, an inhibitor of BMP signaling, occurs relatively late and well after the initial increases in BMP4 and GATA 4 (Fig. 5i).
The expression of Msx-1, a homeodomain transcription factor and a target of BMP 4 signaling (Tucker, 1998; Ishimura, 2000; Alvarez Martinez et al., 2001; Binato et al., 2005), increases in P19-SI aggregates at about the same time as do BMP 4 and GATA 4 (Fig. 5e). Msx-1 transcripts reach maximum levels at about day 3, and then decline. A specific role for Msx-1 in early cardiac development has yet to be demonstrated.
In contrast to the results with P19-SI aggregates, no significant increase was detected in GATA 4, Nkx 2.5, myocardin or BMP4 expression in monolayer cultures (Fig. 5c, d, f & h), even though Bra expression had increased significantly (see above and Fig. 5b). The marked differences between P19-SI expression patterns in aggregate vs monolayer cultures concur with previous studies which demonstrated a requirement for intimate cell-cell interaction(s) early in cardiac induction (Grover et al., 1987; Smith et al., 1987).
Histological examination of older P19-SI aggregates indicates that they contain small clusters of cardiocytes, identified by alpha-cardiac myosin heavy chain (α-cMyHC)-positive immunostaining, that are surrounded by (α-cMyHC)-negative cells (Fig. 6). This restricted pattern of cardiogenesis within developing aggregates could be a consequence of noggin accumulation, inhibiting the ability of endogenous BMP to induce cardiogenesis among the continuously increasing cell population within P19-SI aggregates.
Fig. 6.
P19-SI cardiocytes form clusters within aggregates. Six – day 27 beating P19-SI aggregates were pooled, fixed and sectioned as described in Experimental Procedures so as to illustrate clusters of cardiomyocytes within several individual aggregates. The sections were stained with DAPI (blue) to display the nuclei, and with alpha-myosin heavy chain mAb (green) to locate the cardiomyocytes.
Consistent with the formation of multiple clusters of cardiogenic cells within P19-SI cell aggregates, rhythmic contractions within individual aggregates are initially observed in multiple foci which then become synchronized by about 2 weeks (see Supplemental video data). Since the entire aggregate seems to eventually beat synchronously, this suggests that additional cardiogenesis must occur to form interconnecting bridges of cardiomyocytes or Purkinje fiber cells between contracting centers. The beating rate within these older aggregates was variable (28.0 +/− 10.2 bpm, n=7) and increased by 130% (+/− 69) in response to Isoproterenol (200 nM), indicating that these cells express β-adrenergic receptors and the components of their associated signaling pathways.
A cardiogenic response to BMP4 signals depends on the time of initial exposure
Based on their DMSO-independent cardiac induction, we hypothesized that P19-SI cells may represent cells at an early state in the cardiac lineage. The numerous reports of BMP 2/4 involvement in early events leading to cardiogenesis (Monzen et al., 1999 & 2001; Ladd et al., 1998; Schultheiss et al., 1997), led us to ask whether P19-SI cells were responsive to exogenously-added BMPs. As assessed by the appearance of beating aggregates, when BMP4 was added at the time that hanging drop cultures were established (i.e., prior to aggregate formation), cardiogenesis was severely repressed. No beating aggregates were observed until day 16, and after day 16, no more than 10% of the aggregates beat (Fig. 4, filled diamonds), and these beat much more weakly than did untreated aggregates. Similar data were obtained with BMP 2 (data not shown). Dose-response studies with up to 100 ng/ml BMP 4 demonstrated that 10 ng/ml BMP 4 was sufficient to achieve maximal inhibition of cardiogenesis, 1 ng/ml BMP 4 gave an intermediate response, and 0.1 ng/ml had no inhibitory effect (data not shown).
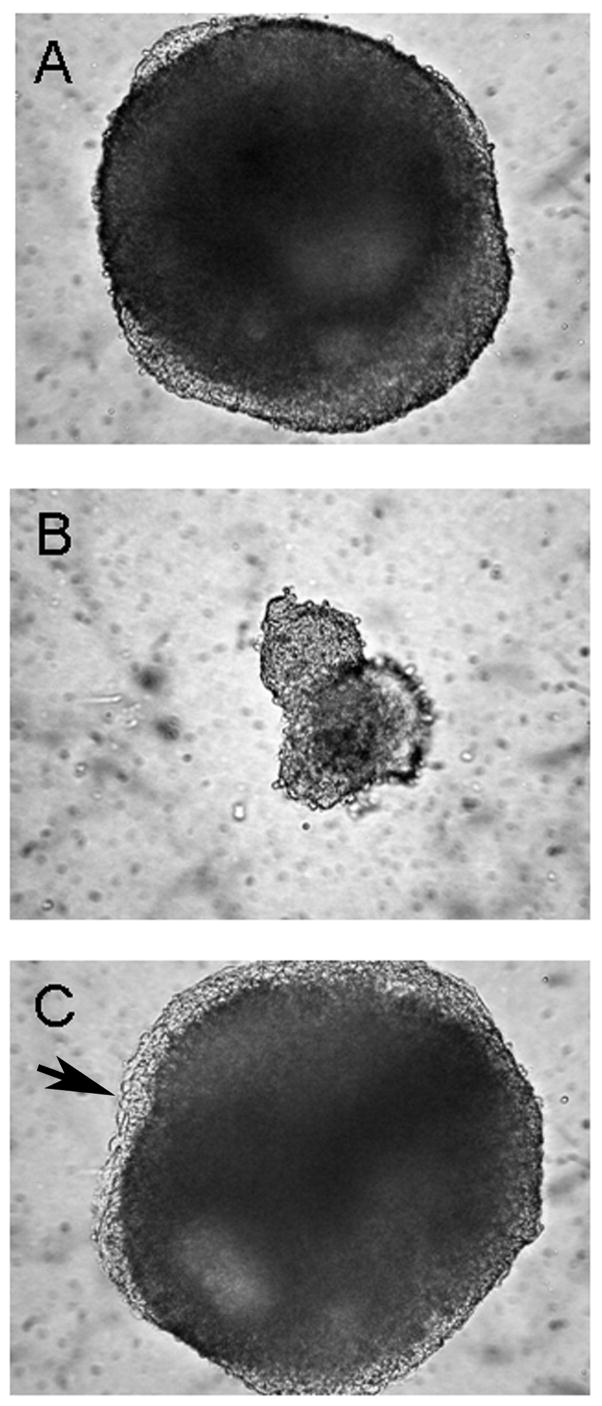
In addition to BMP 4-mediated repression of cardiac development, aggregates formed in the presence of BMP4 were much smaller than controls (Fig. 7B vs A). There was no evidence of cell death in the smaller aggregates; however, clonal growth rate and time-lapse video microscopy studies indicated that BMP4 added to the culture medium increased the cell cycle time by 20–25%, which may partially account for the difference in aggregate size. Importantly, and in stark contrast to the inhibitory effect just described, 10ng/ml BMP4 did potentiate cardiogenesis - but only when added after the cells had aggregated for several days (Fig. 4, filled triangles), suggesting that the qualitative BMP response of pre-cardiac cells is dependent on the history of cell-cell interactions. Interestingly, aggregates which were treated with BMP4 on day 2 formed a more extensive peripheral endoderm-like layer (Fig. 7 C vs A, arrow), an observation evocative of reports showing that endodermal formation in embryoid bodies is itself dependent on BMPs (Coucouvanis and Martin, 1999), perhaps through expression of GATA 4 (Soudais et al., 1995)
Fig. 7.
BMP 4 effects on P19-SI aggregate morphology. P19-SI aggregates were grown for 5 days and then photographed (see legend to Figure 2). When 10 ng/ml BMP 4 was added at the time hanging drop cultures were established (B), the resulting aggregates were much smaller than Controls (A). When BMP 4 was added after 2 days of aggregate formation (C), the aggregates developed a more pronounced “zone” of peripheral cells. Arrow shows peripheral endoderm-like layer (see Fig. 3).
BMP4 addition to P19-SI cells prior to aggregation inhibits expression of cardiogenic genes but stimulates precocious expression of Msx-1
The inhibition of cardiogenesis by BMP 4 addition to aggregating P19-SI cells was further investigated by comparing the transcriptional status of Brachyury, GATA 4, Nkx 2.5, Myocardin, Msx-1, BMP 4, Noggin and Wnt 3a in aggregates formed with and without added BMP4. When P19-SI cells were aggregated in the presence of (10 ng/ml) BMP 4 (Fig. 8; data represent 1 of 3 similar experiments), the timing of Bra expression is unaffected and levels are similar to aggregates without BMP 4 (Fig. 8b). However, increases in GATA 4, Nkx 2.5 and Myocardin expression are completely abolished (Fig. 8c, d & f), which is consistent with the lack of cardiac differentiation seen under these conditions (Fig. 4, filled diamonds). Interestingly, GATA 4 expression is inhibited in spite of the presence of both exogenous BMP 4 protein and endogenous BMP 4 gene expression (although the latter is slightly decreased relative to control; Fig. 8h). In contrast, BMP 4-treated aggregates exhibit increased expression of Wnt 3a and Noggin, with no detectable change in the kinetics of their appearance (Fig. 8g and i).
Fig. 8.
P19-SI cell cardiogenesis is inhibited by exposure to BMP 4 at the beginning of aggregation. P19-SI cultures were established in aggregation conditions either with (
 ) or without (
) or without (
 )10ng/ml BMP4. Samples were harvested up to 110 hrs after plating and assayed by quantitative RT-PCR to detect expression of the early mesodermal marker, Brachyury T; known cardiac transcription factors (Nkx 2.5, GATA 4, Myocardin); the cardiogenic signaling molecule, BMP 4; Noggin, Wnt 3a and Msx-1. Data in b) through i) are normalized to HPRT expression. Cardiac transcription factors do not increase if BMP 4 is added before the cells have begun to aggregate. Note also the earlier increase in Msx-1 expression when BMP 4 is added at the time the aggregate cultures are established. See text for complete discussion.
)10ng/ml BMP4. Samples were harvested up to 110 hrs after plating and assayed by quantitative RT-PCR to detect expression of the early mesodermal marker, Brachyury T; known cardiac transcription factors (Nkx 2.5, GATA 4, Myocardin); the cardiogenic signaling molecule, BMP 4; Noggin, Wnt 3a and Msx-1. Data in b) through i) are normalized to HPRT expression. Cardiac transcription factors do not increase if BMP 4 is added before the cells have begun to aggregate. Note also the earlier increase in Msx-1 expression when BMP 4 is added at the time the aggregate cultures are established. See text for complete discussion.
Among the transcripts measured, Msx-1 exhibits the most dramatic change when BMP4 is added prior to cell aggregation. Msx-1 expression increases much earlier (as early as Bra) and reaches higher levels than in untreated P19-SI aggregates (Fig. 8e).
P19-SI cells can express non-cardiac genes without DMSO treatment
Since cardiocytes develop within P19-SI aggregates in the absence of DMSO, it was of interest to determine whether other cell types were also induced in these aggregates. This was assessed by performing conventional RT-PCR analysis on RNA obtained from aggregates after 2–3 days and after 20 days of culture. As expected, substantial gene expression associated with cardiac induction (GATA 4) was detectable at the earlier time, as was a low level of the endothelial cell marker, von Willibrand’s factor. Markers of skeletal muscle (Myo D, myogenin and MRF4) and neuronal cells (Mash) were not evident (data not shown). However, by 20 days of aggregate culture, markers of all these cell types were readily detected (Fig. 9). Thus, the genetic or epigenetic difference between P19-SI cells and their parental P19 cell line does not completely restrict their differentiation to cardiac muscle. As for cardiogenic development, DMSO treatment is not required for P19-SI cell expression of endothelial, skeletal muscle or neuronal genes.
Fig. 9.
Analysis of markers for both cardiac and non-cardiac gene expression in P19-SI aggregates. Twenty (20) day-old beating aggregates of P19-SI cells were pooled and mRNA was prepared as described in Material and Methods. “Conventional” reverse transcription-polymerase chain reaction (RT-PCR) analysis showed that genes representative of all three germ layers are expressed in these older aggregates (lane 1). Lanes 2 & 3, showing results with mRNA isolated from control tissues, demonstrate the specificity of the primers.
Induction of non-cardiac phenotypes in P19-SI cells is enhanced by DMSO and Retinoic Acid
Although exogenously-added inducing agents are not required for the eventual expression of skeletal muscle and neuronal cell markers in P19-SI aggregates (see Fig. 9), these cell types constitute relatively small and variable cohorts whose size varies among different aggregates. However, more extensive populations emerge when either DMSO or retinoic acid (RA), respectively, is included during aggregate culture.
To test P19-SI cells for DMSO-stimulated skeletal muscle induction, cultures were aggregated in the presence of DMSO for 4 days and then replated onto plastic for 15 days. Skeletal myocytes exhibit bipolar morphology (Fig. 10A) and stain positively with anti-skeletal myosin antibody (Fig. 10B). The definitive skeletal myocytes seen at this time of culture may represent only a fraction of the entire DMSO-induced skeletal muscle precursor population, as inferred from Pax 7 (Halevy et al., 2004) and Desmin antibody staining of a similar area of the culture (Fig. 10D & E). Furthermore, continued culturing of DMSO-treated aggregates supports the emergence of elongated, multi-nucleated cells which exhibit the characteristic “twitch” behavior of skeletal myotubes (see: Supplemental material – videos of skeletal- vs cardiac-like beating behaviors).
Fig. 10.
Assessment of skeletal muscle induction of P19-SI cells by DMSO. P19-SI cells were aggregated in the presence of 0.5% DMSO. Day 4 aggregates were plated onto plastic tissue culture plates (an attachable surface), cultured for 15 days, then fixed for antibody staining (see Materials and Methods). (A) Phase contrast image of an area containing a cohort of bipolar cells. (B) The same area stained with antibody to skeletal myosin heavy chain. (C) Phase contrast image of another aggregate-derived “island”. (D) The same area stained with antibody to Pax 7 or (E) desmin. (F) Merged image of Pax 7 - and desmin-positive cells.
To test P19-SI cells for retinoic acid-stimulated neuronal induction, cultures were aggregated in the presence of RA for 6 days and then replated onto plastic and allowed to attach and spread. Within 2 days, a population of round, refractile cells, some with nascent projections, emerged from throughout the culture (Fig. 11A). Upon further incubation (8 additional days), this population increased and also formed “clusters” (Fig. 11B). Clusters such as these, together with their associated projections, are neuronal as determined by their reactivity with antibody to neurofilament protein (Fig. 11C & D).
Fig. 11.
Assessment of neuronal cell induction of P19-SI cells by retinoic acid. P19-SI cells were aggregated in the presence of 0.2 uM retinoic acid. After 6 days, aggregates were plated onto plastic tissue culture plates, onto which they attached and spread. (A) After 2 days of incubation (10x objective). (B) The same aggregate-derived “island” after 10 days of incubation (20x objective). Eleven (11)-day cultures were fixed for antibody staining. (C) Phase contrast image of an area containing a putative neuronal “cluster”. (D) The same area stained with antibody to neurofilament protein.
DISCUSSION
P19 embryonal carcinoma cells, and a number of P19 cell variants and stably transfected sublines, have been successfully employed to clarify the roles of various cardiogenic factors (van der Heyden and Defize, 2003; McBurney et al., 1982; Edwards et al., 1983; Habara-Ohkubo, A, 1996). These cell lines have been useful because the conditions for culturing, inducing and stably transfecting them are well developed (Grepin et al., 1997; Jamali et al., 2001a; Jamali et al., 2001b; Skerjanc et al., 1998). The “P19-SI” cells described in this study are unique because they undergo spontaneous cardiac induction in the absence of any exogenously added inducing agent or transfected “cardiac factors”. Furthermore, the phenotype of P19-SI cells reveals a previously unreported requirement for cell-cell interaction in aggregates before the cells are competent to receive cardiogenic signals from BMPs.
Although the molecular distinction between P19-SI and parental P19 cells is unknown, the capacity of P19-SI cells to undergo spontaneous cardiogenesis in response to aggregation has not altered their capacity to generate non-cardiogenic cell lineages. Cells representing skeletal muscle, neuronal and endothelial lineages appear both spontaneously (Fig. 9) and in response to DMSO- and retinoic acid-mediated induction (Figs. 10 and 11).
Similar to what has been previously reported for parental P19 cells induced with DMSO (Jamali et al., 2001b; Nakamura et al., 2003), aggregation of P19-SI cells is promptly followed by transient increases in mRNA encoding the mesodermal marker Brachyury (Bra) and the signaling factor Wnt 3a, then by increases in transcripts for genes known to play integral roles in cardiac induction: BMP 4, GATA 4, Nkx 2.5 and myocardin (Ueyama et al., 2003). This suggests that both the parental line and the derivative “SI” subline are following the same, or a very similar, pathway to cardiogenesis. Clearly, hundreds of additional temporal changes in gene expression patterns occur during establishment of the cardiac cell lineage, as illustrated by the recent microarray data of Afrakhte and Schultheiss (2004).
A central role for BMPs in cardiac induction has been demonstrated in vivo using the chick embryo system (Schultheiss et al., 1997; Lough et al., 1996; Ladd et al., 1998). In vitro studies of P19 cells (Jamali et al., 2001a; Monzen et al., 1999 and 2005) as well as ES cells (Hu et al., 2005; Yamashita et al., 2005) have also shown that BMP 2/4 is required for cardiogenesis, presumably because BMPs stimulate expression of the cardiac transcription factor, GATA 4 (Grepin et al., 1995; Grepin et al., 1997). In our study, endogenous BMP 4 and GATA 4 mRNAs begin to increase at about the same time (36–48 hrs. of aggregate culture; Fig. 5c & h), which may imply that GATA 4 transcription in P19-SI aggregates can also be regulated by a pathway other than BMP signaling. The report of Heicklen-Klein and Evans (2004) suggests T-box factors as alternative regulators. This would be consistent with the important roles of T-box factors in early cardiac cell lineage determination (Plageman and Yutzey, 2005).
The timing of BMP signaling for cardiac induction appears to be critical. Noggin, which inhibits BMP-mediated effects by binding directly to BMPs (Chen et al., 2004), markedly enhances cardiogenesis in “undifferentiated” ES cells only if it is present prior to and during the early stages of aggregate formation (Yuasa et al, 2005). This is consistent with the results of our study, in which we found that exogenously-added BMPs inhibit cardiogenesis when included in the culture medium of aggregating P19-SI cells (Fig. 4, filled diamonds). Under these conditions, there was no increase in expression of GATA 4, Nkx 2.5 or myocardin in the aggregates, although the putative endogenous signal for GATA 4 transcription, BMP 4, was still expressed. In contrast, when BMP 4 was added after the cells had aggregated, cardiogenesis was enhanced (Fig. 2, filled triangles). Our data suggest that, in order for BMPs to induce a cardiac response in P19-SI cells, the cells must first be aggregated. Furthermore, prior to cell aggregation, BMPs inhibit cardiogenesis. Our results are also consistent with in vivo chick embryo studies that found BMP 2/4 inhibition of cardiac myogenesis when BMPs were added prior to gastrulation (Ladd, 1998).
It is useful to consider how the in vitro model of cardiac induction within P19 aggregates might reflect events in vivo. In vivo, there is no counterpart to the cell dissociation and subsequent aggregation which are standard procedures for manipulating P19 cells in culture. However, these cell culture protocols may mimic the in vivo, cell-cell associations established among mesodermal cells as they migrate during gastrulation. As noted by Marvin et al., (2001), precardiac cells in the avian embryo migrate from the Wnt-rich primitive streak, where they presumably are “conditioned” for a later cardiogenic response. When these cells emerge as anterior mesoderm, Wnt activity would presumably be repressed by endogenous inhibitors in order for the mesoderm to “properly” respond to endoderm-derived BMP. The in vitro studies of Nakamura et al. (2003) with P19CL6 cells are consistent with canonical Wnts being one indispensable early signal in the process of cardiogenesis. Although a relationship between Wnt 3a signaling and BMP response in P19-SI cells has yet to be demonstrated, the early expression of Wnt 3a in P19-SI aggregates may “condition” subgroups of cells to respond to endogenous BMPs, whose transcription increases about a day after that of Wnt 3a (Fig. 5g and h).
The mechanism(s) underlying inhibition of cardiogenesis in P19-SI aggregates by early exposure to BMP have yet to be elucidated, but may include a precocious expression of Msx-1 (Fig. 8e), a known transcriptional repressor in many systems (Zhang et al., 1996; Bendall and Abate-Shen, 2000) including skeletal myogenesis (Lee et al., 2004). Of the transcripts whose expression was quantified in this report, only that of Msx-1 was both significantly increased and induced earlier when BMP 4 was present during the process of aggregate formation (Fig. 8). The upregulation of Msx-1 expression by BMPs has been documented by many investigators (Tucker et al., 1998; Ishimura et al., 2000; Alvarez Martinez et al., 2001; Binato et al., 2005), and Msx-1 has been shown to inhibit (Song et al., 1992; Lee et al., 2004; Hu et al., 2001) and in some cases reverse (Oldenberg et al., 2000) terminal differentiation. Interestingly, one reported activity of Msx-1 is to alter the quality of cell-cell interactions toward a “less cohesive” phenotype (Lincecum et al., 1998). Because cardiac induction is dependent on BMP signals, regulating the response to BMPs by controlling precardiac cell-cell associations might be critical for optimal cardiac differentiation.
Our P19-SI data are consistent with a model in which an endodermal-like subpopulation that produces BMPs emerges shortly after aggregate formation (Fig. 3). Subsequent signaling of adjacent cells within aggregates results in cardiac induction. In vitro – and especially with randomly aggregated, pluripotent cells such as P19 or ES cells - inappropriately-timed signaling could have unforeseen consequences for subsequent differentiation. In the case of P19-SI cells, the addition of BMPs before the cells even aggregate results in the functional loss of cells with a BMP-responsive pre-cardiac potential. At this time, it is unclear whether BMPs are inhibiting the emergence of an inducing cell type, e.g., endodermal-like cells, or whether BMPs are influencing the lineage decision options of pre-cardiac cells. Data from our studies support the hypothesis that BMP signaling must follow some, as yet undescribed, early cell-cell interactions. P19-SI cell aggregates offer an attractive model system for investigating these interactions.
EXPERIMENTAL PROCEDURES
Cells and culturing
Monolayer cultures of P19 embryonal carcinoma cells were established at 50K cells per 100 mm tissue culture dish in 10mls of DMEM containing 10 % selected fetal bovine serum (FBS) and the antibiotics penicillin (50 U/ml) and streptomycin (50 ug/ml). Cultures were passaged, using saline/trypsin/versene, every other day, before the cells reached confluency.
Hanging drop aggregate cultures were established as previously described (Angello et al., 1997). Briefly, dissociated cells were serially diluted to 50–100 cells per 30 ul of medium. Sixteen – 30 ul drops were placed 1–2 cm apart onto the lids of 100mm tissue culture plates and the cultures were incubated over a 8 ml reservoir of complete medium placed in the bottom of the plate. The cultures were monitored daily, using a phase contrast microscope, for aggregate formation, morphology and beating activity.
“Mass aggregates” were established at 200,000 cells per 10 mls complete medium in 100 mm tissue culture dishes which had been previously prepared with 15 mls of 2 % agar and equilibrated with complete medium. Under these conditions, the cells did not attach to the substratum. After the cells were plated, the plates were gently rocked for 60 min. to initiate aggregate formation. The nascent aggregates were dispersed by manual agitation every 12–15 hrs in order to minimize “consolidation” of aggregates.
Clones were established at 10 and 50 cells per 100 mm dish, and grown without disturbance until it was time to evaluate their morphology (see Results).
Selected FBS
FBS was tested extensively for its ability to support: a population doubling time of 9–12 hrs; substantial cardiac differentiation of “parental” P19 cell aggregates only when induced with 0.5 % DMSO; “self-induced” cardiac differentiation of P19-SI aggregates (without DMSO); clonal growth and self-induced differentiation of P19-SI cell clones.
BMP 4
BMP 4 (Sigma B 2680) was reconstituted, according to the manufacturer’s specifications, to 10 ug/ml in sterile phosphate-buffered saline containing 0.1 % bovine serum albumin.
Immunocytochemistry
P19-SI aggregates were pooled and rinsed with PBS, stained with 1% methylene blue for 10 min at room temp, rinsed twice more with PBS and either left unfixed or fixed in MeOH for 10 min at −20°C. The aggregates were then embedded in O.T.C. (Tissue Tek, Miles, INC) and frozen in a bath with ethanol and dry ice. Frozen sections (5 – 10 um) were mounted on Superfrost-Plus glass slides (VWR), treated with blocking solution (1 % BSA, 0.05 % tween-20 in PBS) for 20 min. at room temperature (RT), and incubated with primary antibody for 2 hr at RT. After 3 – 5 min. washes with blocking solution, the sections were incubated with secondary antibody for 90 min. at RT. The samples were then washed with blocking solution (3 times for 5 min. each) and then with PBS (twice), and mounted in gelvatol (Air Products) with 100 mg/ml DABCO (Sigma, St. Louis, MO).
The cardiac myosin primary antibody was derived from the hybridoma cell line Ba-G5 (ATCC Number HB-276), which is specific for alpha-cardiac myosin heavy chain (MyHC) (Rudnicki et al., 1990). Ba-G5 hybridoma monoclonal antibody (mAb) supernatant was used at a dilution of 1/25, and was followed with a secondary antibody (goat anti-mouse, conjugated with Alexa 488 or rabbit anti-mouse Alexa 594(Molecular Probes)). Cells expressing Sox-17 were identified using a goat anti Sox-17 polyclonal antibody (pAb) raised against the N-terminus of mouse Sox-17 (Santa Cruz Biotechnology, Inc; Santa Cruz, CA) followed by rabbit anti-goat Alexa 488 (Molecular Probes). The sections were counter-stained with DAPI (0.5 ug/ml) to visualize the nuclei.
Cultured cells and plated aggregates were rinsed twice with PBS, fixed with 2% PFA in PBS for 10 min and permeabilized with 1% Triton X-100 in PBS for 10 min. After washing in PBS, cultures were incubated in blocking solution for 20 min and incubated with primary antibodies for 1 to 2 h at RT. After three 5 min washes with blocking solution, the cultures were incubated with secondary antibodies and washed again with blocking solution for 0.5 to 1 h at RT. After a final wash with PBS, the specimens were rinsed in distilled water and mounted as above. Skeletal muscle cells were identified using anti Pax7 mAb supernatant (Hybridoma Bank at the University of Iowa) at a dilution of 1:10, anti-desmin pAb (Abcam Inc, Cambridge, MA) at 1:500 and anti-skeletal fast MyHC mAb ascites fluid (Sigma) at 1:100. Neuronal cells were identified using anti-neurofilament 200 pAb (Sigma) at a dilution of 1:100. Pluripotent cells were identified using a pAb against Oct 3/4 (Santa Cruz Biotechnology). Fifteen hr. mass cultures were then counter-stained with Alexa-594 phalloidin (red cytoplasmic staining).
Real-time qRT-PCR
Mass aggregates were pooled, centrifuged and resuspended in 0.5 ml chilled guanidinium thiocyanate (GTC) buffer. Monolayer cultures were solubilized directly with 0.5 ml buffer. Dissociated cells were centrifuged and then resuspended in buffer. In all cases, the DNA was sheared by passing the extract 20 times through a 16 gauge needle. The samples were then flash-frozen in liquid nitrogen and stored at −80 °C until use. Total RNA was extracted as described (Chomczynski and Sacchi, 1987).
Samples were treated with RNase-free DNase I, amplification grade (Invitrogen, Carlsbad, CA). 1.5 ug total RNA was reverse transcribed in a volume of 60 ul as previously described (Kastner et al., 2000). As a negative control, reverse transcriptase was omitted in duplicate reverse transcription reactions. For real-time PCR, 2 ul of cDNA (40 ng) was added to 18 ul of master mix containing 1x SYBR Green (Applied Biosystems, Foster City, CA) and 400 nM sense and anti-sense primers. Triplicates of the cDNA were amplified for 40 cycles either with the Applied Biosystems 7700 or with the Opticon I Real-Time thermal cycler (MJ Research, Inc., Waltham, MA). The PCR product level is calculated from the threshold cycle (Ct), the amplification cycle at which the emission intensity of the product rises above a set threshold level.
The mouse specific primers used in this study were:
| HPRT (fw): | 5′ TGG AAA GAA TGT CTT GAT TGT TGA A
(rv): 5′ AGC TTG CAA CCT TAA CCA TTT YG |
| GATA 4 (fw): | 5′ CAA GAT GAA CGG CAT CAA CC
(rv): 5′ CAC AGC GTG GTG GTG GTA GT |
| Nkx 2.5 (fw): | 5′ GAG CCT ACG GTG ACC CTG AC
(rv):5′ GTC CAG CTC CAC TGC CTT CT |
| Myocardin (fw): | 5′ CAA CAG GAG TGT GAA GGA CCA
(rv): 5′ GCC TTG GCC AAA CTG TAT GTT |
| Msx-1 (fw): | 5′ CCT CAA GCT GCC AGA AGA TG
(rv): 5′ GTT GGT CTT GTG CTT GCG TAG |
| Noggin (fw): | 5′ AAA CAG CGC CTG AGC AAG A
(rv): 5′ AGC GGC TGC CTA GGT CAT T |
| BMP 4 (fw): | 5′ GCT TGA GTA CCC GGA GCG T
(rv): 5′ TGT TCT TCG TGA TGG AAA CTC CT |
| Wnt 3a (fw): | 5′ TGA GGC AAT GGT CAC CAG AT
(rv): 5′ CTT GTG GCA GAT GGG CTG TA |
Primers used in “conventional” RT-PCR assays
| MyoD (fw): | 5′ GGA GGA GCA CGC ACA CTT CT
(rv): 5′ CGC TGT AAT CCA TCA TGC CA |
| Myog. (fw): | 5′ CCA TCC AGT ACA TTG AGC GCC TA
(rv): 5′ GGG GCT CTC TGG ACT CCA TCT T |
| MRF4 (fw): | 5′ TCG TCG GAA AGC AGC TAC CCT
(rv): 5′ CTG GGG AGT TTG CGT TCC TCT |
| NKX 2.5 (fw): | 5′ CAA GTG CTC TCC TGC TTT CC
(rv): 5′ GAC AGG TAC CGC TGT TGC TT |
| vWF (fw): | 5′ CTC AGA GCT TCG GCG CAT CAC CAG
(rv): 5′ GAC AAA CAC CAC ATC CAG AAC CAT |
| MASH-1 (fw): | 5′ CTC GTC CTC TCC GGA ACT GAT G
(rv): 5′ CGA CAG GAC GCC CGC CTG AAA G |
| Cardiac TnI (fw): | 5′ ATG GAA CGA GAG GCA GAA GA
(rv): 5′ CCT TCT TCA CCT GCT TGA GG |
Supplementary Material
Acknowledgments
National Institutes of Health; grant HL064387 (Bioengineered Autologous Tissue (BEAT) Grant)
Footnotes
Dedicated to the memory of Stephanie Kaestner, whose many contributions and meticulous experimentation are greatly missed.
References
- Afrakhte M, Schultheiss TM. Construction and analysis of a subtracted library and microarray of cDNAs expressed specifically in chicken heart progenitor cells. Dev Dyn. 2004;230(2):290–8. doi: 10.1002/dvdy.20059. [DOI] [PubMed] [Google Scholar]
- Alvarez Martinez CE, Binato R, Gonzalez S, Pereira M, Robert B, Abdelhay E. Characterization of a Smad motif similar to Drosophila mad in the mouse Msx 1 promoter. Biochem Biophys Res Commun. 2002;291(3):655–62. doi: 10.1006/bbrc.2002.6502. [DOI] [PubMed] [Google Scholar]
- Angello JC, Stern HM, Hauschka SD. P19 embryonal carcinoma cells: a model system for studying neural tube induction of skeletal myogenesis. Dev Biol. 1997;192(1):93–8. doi: 10.1006/dbio.1997.8722. [DOI] [PubMed] [Google Scholar]
- Arai A, Yamamoto K, Toyama J. Murine cardiac progenitor cells require visceral embryonic endoderm and primitive streak for terminal differentiation. Dev Dyn. 1997;210(3):344–53. doi: 10.1002/(SICI)1097-0177(199711)210:3<344::AID-AJA13>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Bendall AJ, Abate-Shen C. Roles for Msx and Dlx homeoproteins in vertebrate development. Gene. 2000;247(1–2):17–31. doi: 10.1016/s0378-1119(00)00081-0. [DOI] [PubMed] [Google Scholar]
- Binato R, Martinez CE, Pizzatti L, Robert B, Abdelhay E. SMAD 8 binding to mice Msx-1 basal promoter is required for transcriptional activation. Biochem J. 2005 doi: 10.1042/BJ20050327. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen D, Zhao M, Mundy GR. Bone morphogenetic proteins. Growth Factors. 2004;22(4):233–41. doi: 10.1080/08977190412331279890. [DOI] [PubMed] [Google Scholar]
- Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162(1):156–9. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Coucouvanis E, Martin GR. BMP signaling plays a role in visceral endoderm differentiation and cavitation in the early mouse embryo. Development. 1999;126(3):535–46. doi: 10.1242/dev.126.3.535. [DOI] [PubMed] [Google Scholar]
- Edwards MKS, Harris JF, McBurney MW. Induced muscle differentiation in an embryonal carcinoma cell line. Mol Cell Biol. 1983;3(12):2280–86. doi: 10.1128/mcb.3.12.2280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrman LA, Yutzey KE. Lack of regulation in the heart forming region of avian embryos. Dev Biol. 1999;207(1):163–75. doi: 10.1006/dbio.1998.9167. [DOI] [PubMed] [Google Scholar]
- Fong SH, Emelyanov A, Teh C, Korzh V. Wnt signaling mediated by Tbx2b regulates cell migration during formation of the neural plate. Development. 2005;132(16):3587–96. doi: 10.1242/dev.01933. [DOI] [PubMed] [Google Scholar]
- Grepin C, Nemer G, Nemer M. Enhanced cardiogenesis in embryonic stem cells overexpressing the GATA-4 transcription factor. Development. 1997;124(12):2387–95. doi: 10.1242/dev.124.12.2387. [DOI] [PubMed] [Google Scholar]
- Grepin C, Robitaille L, Antakly T, Nemer M. Inhibition of transcription factor GATA-4 expression blocks in vitro cardiac muscle differentiation. Mol Cell Biol. 1995;15(8):4095–102. doi: 10.1128/mcb.15.8.4095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grover A, Rosentraus MJ, Sterman B, Snook ME, Adamson ED. An adhesion-defective variant of F9 embryonal carcinoma cells fails to differentiate into visceral endoderm. Dev Biol. 1987;120(1):1–11. doi: 10.1016/0012-1606(87)90098-4. [DOI] [PubMed] [Google Scholar]
- Habara-Ohkubo A. Differentiation of beating cardiac muscle cells from a derivative of P19 embryonal carcinoma cells. Cell Struct Funct. 1996;21(2):101–10. doi: 10.1247/csf.21.101. [DOI] [PubMed] [Google Scholar]
- Halevy O, Piestun Y, Allouh MZ, Rosser BWC, Rinkevich Y, Reshef R, Rozenboim I, Wieklinski-Lee M, Yablonka-Reuveni Z. Pattern of Pax7 expression during myogenesis in the posthatch chicken establishes a model for satellite cell differentiation and renewal. Dev Dyn. 2004;231:489–502. doi: 10.1002/dvdy.20151. [DOI] [PubMed] [Google Scholar]
- Heicklen-Klein A, Evans T. T-box binding sites are required for activity of a cardiac GATA-4 enhancer. Dev Biol. 2004;267(2):490–504. doi: 10.1016/j.ydbio.2003.09.042. [DOI] [PubMed] [Google Scholar]
- Hu G, Lee H, Price SM, Shen MM, Abate-Shen C. Msx homeobox genes inhibit differentiation through upregulation of cyclin D1. Development. 2001;128(12):2373–84. doi: 10.1242/dev.128.12.2373. [DOI] [PubMed] [Google Scholar]
- Ishimura A, Maeda R, Takeda M, Kikkawa M, Daar IO, Maeno M. Involvement of BMP-4/msx-1 and FGF pathways in neural induction in the Xenopus embryo. Dev Growth Differ. 2000;42(4):307–16. doi: 10.1046/j.1440-169x.2000.00514.x. [DOI] [PubMed] [Google Scholar]
- Jamali M, Karamboulas C, Rogerson PJ, Skerjanc IS. BMP signaling regulates Nkx2-5 activity during cardiomyogenesis. FEBS Lett. 2001a;509(1):126–30. doi: 10.1016/s0014-5793(01)03151-9. [DOI] [PubMed] [Google Scholar]
- Jamali M, Rogerson PJ, Wilton S, Skerjanc IS. Nkx2-5 activity is essential for cardiomyogenesis. J Biol Chem. 2001b;276(45):42252–8. doi: 10.1074/jbc.M107814200. [DOI] [PubMed] [Google Scholar]
- Kastner S, Elias MC, Rivera AJ, Yablonka-Reuveni Z. Gene expression patterns of the fibroblast growth factors and their receptors during myogenesis of rat satellite cells. J Histochem Cytochem. 2000;48(8):1079–96. doi: 10.1177/002215540004800805. [DOI] [PubMed] [Google Scholar]
- Komuro I, Izumo S. Csx: a murine homeobox-containing gene specifically expressed in the developing heart. Proc Natl Acad Sci U S A. 1993;90(17):8145–9. doi: 10.1073/pnas.90.17.8145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubo A, Shinozaki K, Shannon JM, Kouskoff V, Kennedy M, Woo S, Fehling HJ, Keller G. Development of definitive endoderm from embryonic stem cells in culture. Development. 2003;131:1651–1662. doi: 10.1242/dev.01044. [DOI] [PubMed] [Google Scholar]
- Ladd AN, Yatskievych TA, Antin PB. Regulation of avian cardiac myogenesis by activin/TGFbeta and bone morphogenetic proteins. Dev Biol. 1998;204(2):407–19. doi: 10.1006/dbio.1998.9094. [DOI] [PubMed] [Google Scholar]
- Lee H, Habas R, Abate-Shen C. MSX1 cooperates with histone H1b for inhibition of transcription and myogenesis. Science. 2004;304(5677):1675–78. doi: 10.1126/science.1098096. [DOI] [PubMed] [Google Scholar]
- Lincecum JM, Fannon A, Song K, Wang Y, Sassoon DA. Msh homeobox genes regulate cadherin-mediated cell adhesion and cell-cell sorting. J Cell Biochem. 1998;70(1):22–8. doi: 10.1002/(sici)1097-4644(19980701)70:1<22::aid-jcb3>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- Lough J, Barron M, Brogley M, Sugi Y, Bolender DL, Zhu X. Combined BMP-2 and FGF-4, but neither factor alone, induces cardiogenesis in non-precardiac embryonic mesoderm. Dev Biol. 1996;178(1):198–202. doi: 10.1006/dbio.1996.0211. [DOI] [PubMed] [Google Scholar]
- Marcel AG, van der Heyden Libert H, Defize K. Twenty one years of P19 cells: what an embryonal carcinoma cell line taught us about cardiomyocyte differentiation. Cardiovascular Research. 2003;58(2):292–302. doi: 10.1016/s0008-6363(02)00771-x. [DOI] [PubMed] [Google Scholar]
- Marvin MJ, Di Rocco G, Gardiner A, Bush SM, Lassar AB. Inhibition of Wnt activity induces heart formation from posterior mesoderm. Genes Dev. 2001;15(3):316–27. doi: 10.1101/gad.855501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBurney MW, Jones-Villeneuve EM, Edwards MK, Anderson PJ. Control of muscle and neuronal differentiation in a cultured embryonal carcinoma cell line. Nature. 1982;299(5879):165–7. doi: 10.1038/299165a0. [DOI] [PubMed] [Google Scholar]
- Monzen K, Shiojima I, Hiroi Y, Kudoh S, Oka T, Takimoto E, Hayashi D, Hosoda T, Habara-Ohkubo A, Nakaoka T, Fujita T, Yazaki Y, Komuro I. Bone morphogenetic proteins induce cardiomyocyte differentiation through the mitogen-activated protein kinase kinase kinase TAK1 and cardiac transcription factors Csx/Nkx-2.5 and GATA-4. Mol Cell Biol. 1999;19(10):7096–105. doi: 10.1128/mcb.19.10.7096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monzen K, Hiroi Y, Kudoh S, Akazawa H, Oka T, Takimoto E, Hayashi D, Hosoda T, Kawabata M, Miyazono K, Ishii S, Yazaki Y, Nagai R, Komuro I. Smads, TAK1, and their common target ATF-2 play a critical role in cardiomyocyte differentiation. J Cell Biol. 2001;153(4):687–98. doi: 10.1083/jcb.153.4.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monzen K, Nagai R, Komuro I. A role for bone morphogenetic protein signaling in cardiomyocyte differentiation. Trends Cardiovasc Med. 2002;12(6):263–9. doi: 10.1016/s1050-1738(02)00172-x. [DOI] [PubMed] [Google Scholar]
- Mummery CL, van Achterberg TA, van den Eijnden-van Raaij AJ, van Haaster L, Willemse A, de Laat SW, Piersma AH. Visceral-endoderm-like cell lines induce differentiation of murine P19 embryonal carcinoma cells. Differentiation. 1991;46(1):51–60. doi: 10.1111/j.1432-0436.1991.tb00865.x. [DOI] [PubMed] [Google Scholar]
- Naito AT, Akazawa H, Takano H, Minamino T, Nagai T, Aburatani H, Komuro I. Phosphatidylinositol 3-kinase-Akt pathway plays a critical role in early cardiomyogenesis by regulating canonical Wnt signaling. Circ Res. 2005;97(2):144–51. doi: 10.1161/01.RES.0000175241.92285.f8. [DOI] [PubMed] [Google Scholar]
- Nakamura T, Sano M, Songyang Z, Schneider MD. A Wnt- and beta -catenin-dependent pathway for mammalian cardiac myogenesis. Proc Natl Acad Sci U S A. 2003;100(10):5834–9. doi: 10.1073/pnas.0935626100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nascone N, Mercola M. An inductive role for the endoderm in Xenopus cardiogenesis. Development. 1995;121(2):515–23. doi: 10.1242/dev.121.2.515. [DOI] [PubMed] [Google Scholar]
- Odelberg SJ, Kollhoff A, Keating MT. Dedifferentiation of mammalian myotubes induced by msx1. Cell. 2000;103(7):1099–109. doi: 10.1016/s0092-8674(00)00212-9. [DOI] [PubMed] [Google Scholar]
- Pesce M, Scholer HR. Oct-4: Gatekeeper in the beginnings of mammalian development. Stem Cells. 2001;19:271–278. doi: 10.1634/stemcells.19-4-271. [DOI] [PubMed] [Google Scholar]
- Pera MF, Andrade J, Houssami S, Reubinoff B, Trounson A, Stanley EG, Ward-van Oostwaard D, Mummery C. Regulation of human embryonic stem cell differentiation by BMP-2 and its antagonist noggin. Cell Sci. 2004;117(Pt 7):1269–80. doi: 10.1242/jcs.00970. [DOI] [PubMed] [Google Scholar]
- Plageman TF, Jr, Yutzey KE. T-box genes and heart development: putting the “T” in heart. Dev Dyn. 2005;232(1):11–20. doi: 10.1002/dvdy.20201. [DOI] [PubMed] [Google Scholar]
- Rosenstraus MJ, Spadoro JP, Nilsson J. Cell position regulates endodermal differentiation in embryonal carcinoma cell aggregates. Dev Biol. 1983;98(1):110–6. doi: 10.1016/0012-1606(83)90339-1. [DOI] [PubMed] [Google Scholar]
- Rudnicki MA, Jackowski G, Saggin L, McBurney MW. Actin and myosin expression during development of cardiac muscle from cultured embryonal carcinoma cells. Dev Biol. 1990;138(2):348–58. doi: 10.1016/0012-1606(90)90202-t. [DOI] [PubMed] [Google Scholar]
- Schultheiss TM, Xydas S, Lassar AB. Induction of avian cardiac myogenesis by anterior endoderm. Development. 1995;121(12):4203–14. doi: 10.1242/dev.121.12.4203. [DOI] [PubMed] [Google Scholar]
- Schultheiss TM, Burch JB, Lassar AB. A role for bone morphogenetic proteins in the induction of cardiac myogenesis. Genes Dev. 1997;11(4):451–62. doi: 10.1101/gad.11.4.451. [DOI] [PubMed] [Google Scholar]
- Skerjanc IS, Petropoulos H, Ridgeway AG, Wilton S. Myocyte enhancer factor 2C and Nkx2-5 up-regulate each other’s expression and initiate cardiomyogenesis in P19 cells. J Biol Chem. 1998;273(52):34904–10. doi: 10.1074/jbc.273.52.34904. [DOI] [PubMed] [Google Scholar]
- Smith SC, Reuhl KR, Craig J, McBurney MW. The role of aggregation in embryonal carcinoma cell differentiation. J Cell Physiol. 1987;131(1):74–84. doi: 10.1002/jcp.1041310112. [DOI] [PubMed] [Google Scholar]
- Song K, Wang Y, Sassoon D. Expression of Hox-7.1 in myoblasts inhibits terminal differentiation and induces cell transformation. Nature. 1992;360(6403):477–81. doi: 10.1038/360477a0. [DOI] [PubMed] [Google Scholar]
- Soudais C, Bielinska M, Heikinheimo M, MacArthur CA, Narita N, Saffitz JE, Simon MC, Leiden JM, Wilson DB. Targeted mutagenesis of the transcription factor GATA-4 gene in mouse embryonic stem cells disrupts visceral endoderm differentiation in vitro. Development. 1995;121(11):3877–88. doi: 10.1242/dev.121.11.3877. [DOI] [PubMed] [Google Scholar]
- Sparrow DB, Kotecha S, Towers N, Mohun TJ. Xenopus eHAND: a marker for the developing cardiovascular system of the embryo that is regulated by bone morphogenetic proteins. Mech Dev. 1998;71(1–2):151–63. doi: 10.1016/s0925-4773(98)00010-0. [DOI] [PubMed] [Google Scholar]
- Toyofuku T, Hong Z, Kuzuya T, Tada M, Hori M. Wnt/frizzled-2 signaling induces aggregation and adhesion among cardiac myocytes by increased cadherin-beta-catenin complex. J Cell Biol. 2000;150(1):225–41. doi: 10.1083/jcb.150.1.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker AS, Al Khamis A, Sharpe PT. Interactions between Bmp-4 and Msx-1 act to restrict gene expression to odontogenic mesenchyme. Dev Dyn. 1998;212(4):533–9. doi: 10.1002/(SICI)1097-0177(199808)212:4<533::AID-AJA6>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Ueyama T, Kasahara H, Ishiwata T, Nie Q, Izumo S. Myocardin expression is regulated by Nkx2.5, and its function is required for cardiomyogenesis. Mol Cell Biol. 2003;23(24):9222–32. doi: 10.1128/MCB.23.24.9222-9232.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Heyden MA, Defize LH. Twenty one years of P19 cells: what an embryonal carcinoma cell line taught us about cardiomyocyte differentiation. Cardiovasc Res. 2003;58(2):292–302. doi: 10.1016/s0008-6363(02)00771-x. [DOI] [PubMed] [Google Scholar]
- Vidricaire G, Jardine K, McBurney MW. Expression of the Brachyury gene during mesoderm development in differentiating embryonal carcinoma cell cultures. Development. 1994;120(1):115–22. doi: 10.1242/dev.120.1.115. [DOI] [PubMed] [Google Scholar]
- Wilson V, Manson L, Skarnes WC, Beddington RS. The T gene is necessary for normal mesodermal morphogenetic cell movements during gastrulation. Development. 1995;121:877–886. doi: 10.1242/dev.121.3.877. [DOI] [PubMed] [Google Scholar]
- Yamaguchi H, Niimi T, Kitagawa Y, Miki K. Brachyury (T) expression in embryonal carcinoma P19 cells resembles its expression in primitive streak and tail-bud but not that in notochord. Dev Growth Differ. 1999;41(3):253–64. doi: 10.1046/j.1440-169x.1999.413427.x. [DOI] [PubMed] [Google Scholar]
- Yamashita JK, Takano M, Hiraoka-Kanie M, Shimazu C, Peishi Y, Yanagi K, Nakano A, Inoue E, Kita F, Nishikawa S. Prospective identification of cardiac progenitors by a novel single cell-based cardiomyocyte induction. FASEB J. 2005;19(11):1534–6. doi: 10.1096/fj.04-3540fje. [DOI] [PubMed] [Google Scholar]
- Yuasa S, Itabashi Y, Koshimizu U, Tanaka T, Sugimura K, Kinoshita M, Hattori F, Fukami S, Shimazaki T, Ogawa S, Okano H, Fukuda K. Transient inhibition of BMP signaling by Noggin induces cardiomyocyte differentiation of mouse embryonic stem cells. Nat Biotechnol. 2005;23(5):607–11. doi: 10.1038/nbt1093. [DOI] [PubMed] [Google Scholar]
- Zaffran S, Frasch M. Early signals in cardiac development. Circ Res. 2002;91(6):457–69. doi: 10.1161/01.res.0000034152.74523.a8. [DOI] [PubMed] [Google Scholar]
- Zhang H, Bradley A. Mice deficient for BMP2 are nonviable and have defects in amnion/chorion and cardiac development. Development. 1996;122(10):2977–86. doi: 10.1242/dev.122.10.2977. [DOI] [PubMed] [Google Scholar]
- Zhang H, Catron KM, Abate-Shen C. A role for the Msx-1 homeodomain in transcriptional regulation: residues in the N-terminal arm mediate TATA binding protein interaction and transcriptional repression. Proc Natl Acad Sci U S A. 1996;93(5):1764–9. doi: 10.1073/pnas.93.5.1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.